The genus Sphaeralcea, which belongs to the Malvaceae family, is comprised of approximately 40 species mainly distributed in the western part of North America. More than half of these species are found in Mexico, with the most representative being Sphaeralcea angustifolia Cav. G. Don., commonly known as “vara de San José,” “hierba del negro,” or “tlixihuitl” in the Nahuatl language (Rzedowski and Rzedowski, 2005). This species is widely distributed (McVaugh, 2001). Andrade-Ceto (2009) reports that it has been used for its anti-inflammatory effect in diseases such as tonsillitis, bronchitis, conjunctivitis, rheumatism, bruises, and hemorrhoids. It is also used as a healing agent and for the treatment of gastrointestinal disorders such as diarrhea and dysentery (García-Rodríguez et al., 2012; Calzada et al., 2017) and osteoarthritis (Romero-Cerecero et al., 2013).
The Pucciniales (Basidiomycota) are a group of fungi that parasitize vascular plants, with complex life cycles and causing diseases known as rusts (Toome-Heller, 2016; Aime et al., 2018). There are currently an estimated 7,500 to 8,500 described species of Pucciniales, with the genus Puccinia causing the most economic losses (Toome-Heller, 2016) and comprising approximately 4,000 species. Due to their high host specificity, studying their evolution is complicated (Aime et al., 2018). Puccinia sherardiana (Horst, 2013; Demers et al., 2015) affects different species of the genera Sphaeralcea, Sidalceae, Althaea, Malvastrum, Alcea (Malvaceae). This rust is reported as microcyclic (Briere and Franc, 1998; Dugan and Nazaire, 2011) and produces symptoms and signs in the stem and leaves, such as the presence of dark brown telia surrounded by chlorotic halos. These symptoms have been reported in Sphaeralcea grossulariaefolia, S. munroana, and Sidalcea malviflora in the USA (Briere and Franc, 1998; Sampangi et al., 2010). The objectives of this study were to observe the life cycle of P. sherardiana in S. angustifolia, provide a detailed morphological description of the fungus, and confirm its pathogenicity.
From June to August in 2017 and 2018, S. angustifolia plants with sori were collected in the Santa Maria region (19°51’23.60” N, 98°44’57.02” W), Axapusco, State of Mexico. To describe the pathogen morphometrics, spermatia, aeciospores, teliospores, and sections of spermogonia, aecia, and telia were mounted separately in a drop of lactophenol with cotton blue on glass slides for observation under light microscopy. The morphology and size of 50 spores were determined at a magnification of 40X. The surface structures of the aeciospores and teliospores were observed directly using a scanning electron microscope (Carl Zeiss®). Telia and teliospores characteristics were compared with taxonomic keys and descriptions by Hotson (1934) and Arthur (1962).
To confirm the identity of the fungus, aeciospores were collected from an aecium of symptomatic plants in the field and DNA was extracted from them. The quality of the DNA was verified by electrophoresis on a 1% agarose gel stained with ethidium bromide and visualized under UV light using an M-26X transilluminator (UVP Ltd®, USA). The identity of the fungus was confirmed by amplifying part of the 28S ribosomal DNA gene (including D1 and D2 domains) using the LR0R and LR6 primers (Vilgalys and Hester, 1990, Aime et al., 2006). Each reaction mixture (50 μL) contained PCR Buffer 1X, 0.02 U µL-1 of DNA polymerase (Promega®, USA), 2.5 mM MgCl2, 0.2 mM dNTPs, 0.8 Μm of each primer, and 2 ng of template DNA. The PCR was carried out in a Bio-Rad C1000 thermocycler (Bio-Rad Laboratories®, USA) with initial denaturation at 95 ºC for 3 min, 35 cycles of 95 ºC for 90 s, 50 ºC for 60 s, 72 ºC for 90 s (alignment), followed by a final extension at 72ºC for 7 min. The amplified PCR products were purified using the QIAquick PCR purification kit (Qiagen®, USA) and sequenced at Macrogen (Seoul, Korea). Similar sequences deposited in the GenBank NCBI (National Center for Biotechnology Information) were obtained using the Basic Local Alignment Search Tool (BLAST) algorithm. The matrices were compiled using the MEGA 5.0.5 program and analyzed using Bayesian inference criteria. The alignments were performed online using the MAFFT 7 program with E-INS-i and FFT-NS-i strategies and default parameters.
Plants of S. angustifolia were propagated under isolated conditions in a greenhouse. The plants were potted in polyethylene bags with a substrate comprised of agrolite, peat, and forest soil in a ratio of 20, 30, and 50%, respectively. The mixture was sterilized in an autoclave at 121 lb three times for 30 minutes each time. At six weeks of age, the plants were inoculated via leaf spray with a suspension of teliospores at a concentration of 1 × 104 teliospores mL-1, sourced from plants displaying symptoms of the pathogen. Inoculated plants were kept in greenhouse conditions, with an average temperature of 22 °C during the day and 18 °C at night, and relative humidity of 60%. The experiment was replicated twice.
In the field, symptoms began as light green circular spots on both the adaxial and abaxial surfaces of the leaves (Figure 1A and 1B). Small light brown dots subsequently developed in the center of the spots, corresponding to the formation of spermogonia. The spots then progressed to a light yellow color and irregularly increased in size, accompanied by the development of yellow aecia and aeciospores on the abaxial surface (Figure 1C, 1D, and 1E). Telia were then formed (Figure 1F), which gave rise to teliospores. Both telia and teliospores were exclusively observed on the abaxial surface, as well as on the stem (Figure 1G). Telia were surrounded by chlorotic halos, and lesions of brown color delimited by the chlorotic halo were observed on the adaxial surface (Figure 1H).

Figure 1 Symptoms and signs induced by Puccinia sherardiana in plants of Sphaeralcea angustifolia. A, B) Light green circular spots; C, D and E) Development of yellow aecia; F and G) Development of telia on leaf and stem; H) Telia delimited by a chlorotic halo.
The pycnia (spermogonia) were found to develop in small groups on opposite sides of the aecium. These structures were epiphyllous, subepidermal, and belonged to the V group type 4 classification (Cummins and Hiratsuka, 2003) light brown, located on opposite sides of the ecium, globose, 120-150 μm in diameter (Figure 2A).
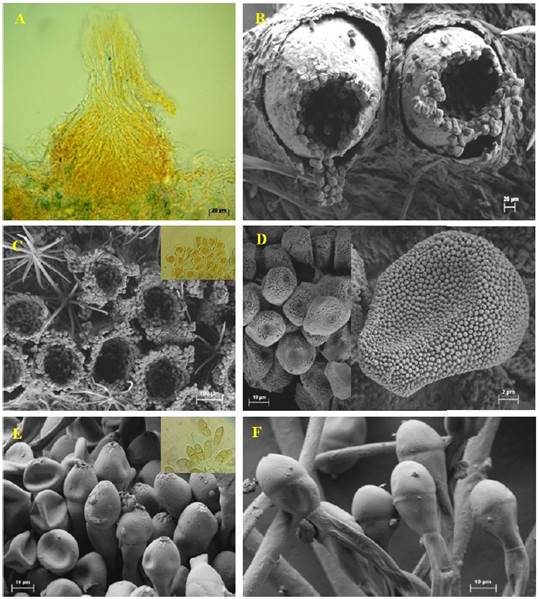
Figure 2 Reproductive structures of Puccinia sherardiana as seen by light microscopy (A) and scanning electron microscopy (B-F). A) Longitudinal section of a spermogonium. B-C) Eciospores. D) Eciospores. E-F) Teliospores.
The pycniospores (spermatia) were ellipsoidal to subglobose, hyaline to yellowish, and not septate, measuring 3.5 × 2.5 µm. The aecia were observed to be hypophyllous, cup-shaped, and clustered with a light brown color, measuring 219-230 µm in diameter (Figura 2B, 2C). The aeciospores were oblong to ellipsoidal, finely echinulate, 16.46 × 14.38 µm, and possessed a hyaline wall 1.5 µm thick, with the inner part appearing light brown or yellowish (Figura 2D). The telia found on stems were hypophyllous, cushion-shaped, verrucose, and regularly confluent, with a reddish-brown to dark brown color. Teliospores appeared mostly ellipsoidal to oblong, bicellular, and contained a central constriction with an apical pore. They averaged 15 × 42 µm and had smooth, dark brown or brown walls, measuring 0.5-1.5 µm thick at the sides and 1.5-3 µm apically (Figura 2E, 2F). The teliospore pedicels were usually yellowish, persistent, 105 µm long, and had smooth walls 1.5 µm thick. Puccinia sherardiana was identified as the rust based on morphological characteristics of the teliospores, according to previous studies (Arthur, 1962; Dugan and Nazaire, 2011; Demers et al., 2015). The observation period did not reveal the uredinial state.
Symptoms and signs on leaves were observed 15 days after greenhouse inoculation. Ecia were observed in formation at this time, which subsequently gave rise to eciospores after rupturing the leaf epidermis. The formation of telia and teliospores was observed five days later. The characteristics of the teliospores were observed and confirmed to be consistent with those of P. sherardiana, completing Koch’s Postulates.
The sequence generated in this study was deposited in the GenBank database under accession number MN967778.1. Phylogenetic analysis was conducted using the partial sequence of the 28S gene and the Bayesian inference criterion. The analysis showed a posterior probability value of 0.99 with sequences of P. sherardiana isolates BPI871783 and BPI102198 (Figure 3).

Figure 3 Bayesian tree generated from alignment of partial 28S gene sequences of Puccinia species. Bayesian posterior probability values above 0.50 are shown at the nodes. Pucciniosira pallidula isolate BPI863541 was used as an outgroup.
Puccinia sherardiana has been reported infecting various species within the family Malvaceae in the United States (Arizona, California, Colorado, Nevada, Oregon, Washington, Idaho, Nebraska, New Mexico, Texas, Utah, Wyoming, Montana, and North Dakota) infecting different species of the genera Alcea, Malvastrum, Sidalcea and Sphaeralcea, within the family of the family Malvaceae (Arthur, 1962; Briere and Franc, 1998; Dugan and Nazaire, 2011; Demers et al., 2015). In Mexico, the presence of P. sherardiana was reported by Arthur (1962), but information about the collection site or host was not provided. Demers et al. (2015) identified this rust in two specimens (origin unknown) of Sphaeralcea sp. intercepted in El Paso, Texas, from goods entering from the border with Mexico. Morphometrically, teliospore characteristics of P. sherardiana identified on S. angustifolia were similar to those reported by Arthur (1962), Briere and Franc (1998), Dugan and Nazaire (2011), and Demers et al. (2015), but differences were found in this study. The teliospores were narrower (15.8 µm) than the size reported (27-30 µm) by previously mentioned authors for different hosts, such as 30.85 µm in Sphaeralcea grossulariaefolia and S. munroana, 21-25 µm in Sidalcea malviflora, and 18-30 µm in Alcea rosea. Briere and Franc (1998) reported the presence of telia on both sides of the leaf in S. grossulariaefolia and S. munroana. Dugan and Nazaire (2011) observed telia primarily on the adaxial side of leaves of Sidalcea malviflora, whereas in this study, they were only found on the abaxial side. Under greenhouse conditions, foliar symptoms were successfully reproduced by inoculating six-week-old S. angustifolia plants with P. sherardiana teliospores for a period of 15 days, with an average temperature of 22 °C during the day and 18 °C at night. Briere and Franc (1998) completed Koch’s postulates in a period of 13 days using 8-week-old plants and temperatures of 20 °C and 15 °C during the day and night, respectively. This work provides the first description of the morphometric characteristics of the pycnia and pycniospores and reports the aecial phase, along with a description of the aecia and aeciospores. It presents the first evidence for a possible reclassification of P. sherardiana as a demicyclic rust, rather than microcyclic, as previously reported by Arthur (1962), Briere and Franc (1998), and Dugan and Nazaire (2011), which stated that the rust lacked the aecial and uredinial phases. However, it is still unknown whether it does or does not present the uredinial part.
Conclusions
P. sherardiana is reported for the first time in Axapusco, State of Mexico. Based on pathogenicity tests, it has been demonstrated that healthy plants inoculated with this rust can develop symptoms and signs similar to those observed in the initial phase. The present study aims to provide insights into the life cycle of P. sherardiana, specifically focusing on the various stages involved in infecting Sphaeralceae angustifolia.











 text in
text in 


