All plants, including those of the highest agricultural importance in the world such as rice, maize, soybeans and wheat, are affected by severe infections caused by pathogens such as bacteria, fungi, oomycetes and viruses (Nazarov et al., 2020; Velásquez et al., 2018). Infections caused by Magnaporthe oryzae in rice, Puccinia spp. in wheat and Fusarium spp. in all cereals, for example, contribute to losses in yield and quality of harvests globally (Almeida et al., 2019). Agronomic losses caused by pests and diseases are estimated to cover up to 40% of the world’s annual production (FAO, 2017). Incidences of plant diseases reduce food production and increase production costs, making them less accessible to the consumer (Ristaino et al., 2021; Savary et al., 2019). In addition, they reduce the diversity of species of, for example, beneficial insects and microbes (Gupta et al., 2022; van der Sluijs, 2020) and pose a risk to human health due to the indiscriminate use of pesticides for the control of plant pathogen populations (Rani et al., 2021).
The study of key factors that hinder or determine the development of plant diseases may help develop eco-friendly control methods that reduce chemical control (Thakur et al., 2020; Baker et al., 2020). The aim of this revision is to show the importance of effectors in phytopathology and to propose potential uses for the protection of plants. For this revision, we searched for articles with key words such as “protein effectors”, “effectors in plant-pathogen interactions”, “applications of effectors” and “effectoromics in pathogenic fungi” in the Google Scholar and PubMed databases. Articles from the past 15 years were selected, with a greater emphasis on the last 5 years, along with some older articles which are important references in the area of effectors.
Many effectors play roles in the suppression of immune responses in host plants; some effectors are hydrolytic enzymes, others are enzyme inhibitors, others modulate the microbiome of the host or protect the pathogen physically against enzymatic lysis (Rocafort et al., 2020; Schreiber et al., 2021; Snelders et al., 2022; Zhang et al., 2022).
The effectors are part of the molecular arsenal of microorganisms, both beneficial and pathogenic, that interact with the host plant or with other microorganisms (Castillo-Sanmiguel et al., 2020; Todd et al., 2022a). However, these molecules have been studied mostly in the context of plant pathogens, since they are key tools used by the phytopathogen to cause an infection and thereby obtain necessary nutrients from the host. They have been found in fungal (Carreón-Anguiano et al., 2020; Sperschneider et al., 2018), bacterial (Rufián et al. 2021), viral phytopathogens (Huang, 2021) and even larger organisms such as insects (Chen et al. 2019; Ray and Casteel, 2022) and nematodes (Figure 1A) (Verhoeven et al., 2023; Vieira and Gleason, 2019). The development of the omic technologies (genomics, transcriptomics and proteomics), as well as bioinformatics, has helped identify wide catalogues of effectors contained in the genomes of organisms (Carreón-Anguiano et al., 2022; Chen et al., 2021; Huang et al., 2022).
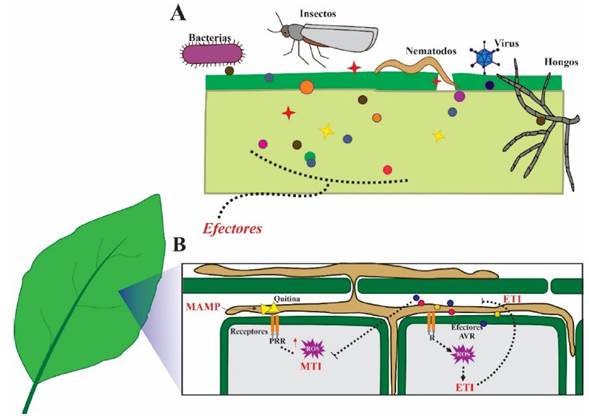
Figure 1 The pathogen-plant molecular interaction. A) Effectors, the molecular weapons of phytopathogens and pests. Bacteria, fungi, oomycetes, viruses, insects and nematodes secrete a plethora of effectors targeting the plant host, preventing their recognition by said host and blocking the host immune response to favor colonization. B.) On the other hand, the plants, in the first line of defense, recognize conserved molecules known as MAMPs (e.g., fungal chitin) that activate the defense mechanism called MTI. In the second line of plant defense, resistance proteins (R) recognize Avr effectors of the pathogen. This induces the hypersensitive response and localized cell death to prevent further infection (ETI). When R proteins are incapable of recognizing the Avr effector, effector-triggered susceptibility (ETS) ensues, and the disease is established.
The identification and characterization of effectors are extremely important activities, since they provide clues as to how phytopathogens infect their hosts, causing massive reductions in crop yields, threatening food security worldwide. The study of effectors is paving the way for their use in agriculture, providing an opportunity for the creation of new methods to control phytopathogens within integrated management systems.
Effectors and their characteristics
Effectors are defined as small molecules, generally secreted, which manipulate the structure and the function of the host cell, allowing the microorganism to establish an interaction with the host (Fabro, 2022; Langin et al., 2020). These molecules produce physical and physiological changes in the target organisms (the organisms upon which they act) and in some cases, on the same microorganisms that produce them (Cai et al., 2023; Figueroa et al., 2021; He et al., 2020). Most of the effectors known today are proteins (Carreón-Anguiano et al., 2020; Kanja and Hammond-Kosack, 2020; Sperschneider and Dodds, 2022), although secondary metabolites (Rangel and Bolton, 2022), and small RNAs have also been described (Yamankurt et al., 2020).
Most of the known effectors are not conserved among different organisms, therefore the in silico predicative identification approaches have been based on relatively wide structural criteria, mainly (a) the number of amino acids (usually less than 400 amino acids); b) the presence of a signal peptide, which increases the probability of the protein exiting the phytopathogen cells (Carreón-Anguiano et al., 2020; Sperschneider et al., 2018; Sperschneider and Dodds, 2022); c) the number or percentage of the amino acid cysteine, as many apoplastic effectors are rich in this amino acid; and (d) candidates are also selected based on the absence of transmembrane domains (Carreón-Anguiano et al., 2020). Other characteristics help refine their identification, such as the increase in their expression during the interaction of the phytopathogen with its host (Tao et al., 2020; Toruño et al., 2016). Likewise, in the amino acid sequences of the protein effectors, small conserved regions called motifs can be identified, such as RxLR, CHXC or LFLAK, common in oomycete effectors (Fabro et al., 2022).
Some effectors are codified by genes found in dispensable chromosomes (named as such because these chromosomes are not present in all the microorganism strains, unlike the indispensable ones) or in chromosomal regions rich in repetitions and with a scarce gene density (Peng et al., 2019). Noar and Daub (2016) analyzed the distribution of genes related to virulence in the fungus Pseudocercospora fijiensis and found that most are located in “dispensable” genomic regions, and using transcriptomic analysis, they found that these genes are expressed during the P. fijiensis infection of banana (Musa acuminata). Using EffHunter, an algorithm that integrates the canonical characteristics of the effectors (Carreón-Anguiano et al., 2020), 136 effectors were predicted for P. fijiensis; these effectors are distributed in dispensable genomic regions, as well as in the genomic regions found in all strains, known as the “core” genome.
The genes that codify effector proteins are under high evolutionary pressure, which results in a higher mutation rate in these genes than in other gene families. Consequently, polymorphisms are commonly found in the sequences of effectors shared among strains of the same species; such changes are related to the adaptation and virulence on the host (Kanja and Hammond-Kosack, 2020; Padilla-Ramos et al., 2018). Regarding the level of conservation, some effectors are found in related microorganisms, and others, in phylogenetically distant organisms. In these cases, orthologous proteins (proteins that are homologous proteins in sequence, having the same function in different organisms and originating from one the same ancestor) display little sequence similarity. A clear example is the effector Avr4, which is shared between species of the same class of Dothidiomycetes; Avr4 in P. fijiensis only displays 50.5% identity with its orthologous protein in the fungus Cladosporium fulvum (Hurlburt et al., 2018).
Even though the percentage of identity between the members of an effector family tends to be low, the current omic analyses show the presence of conserved domains and motifs in effector proteins. Some of the most frequently identified domains include LysM, ceratoplatanin, RNAase, necrosis inducing protein domains (NPP1 or NEP), CFEM, among others (Carreón-Anguiano et al., 2022; Outram et al., 2021; Zhao et al., 2020). Recent investigations reveal that a microorganism can contain hundreds of effectors (Carreón-Anguiano et al., 2020; Noar and Daub, 2016; Sperschneider et al., 2018), with different functions that are expressed in different moments (Noar and Daub, 2016; Toruño et al., 2016). Most of them interfere with signaling functions in the plant, the synthesis of phytoregulators or in plant defense mechanisms (Fabro 2022; Han yand Kahmann, 2019; Padilla-Ramos et al., 2018; Plett et al., 2020).
Effectors and plant immunity (disease or health)
Plants have an innate immune system that responds to the presence of phytopathogens (Chang et al., 2022; Jones and Dangl 2006; Thordal-Christensen, 2020). Plants recognize conserved molecules called microbial-associated molecular patterns, or MAMP, and the recognition triggers the primary immune response (basal defense). The immunity activated by these MAMP involves the participation of receptors in the plant that recognize these molecular patterns (PRR) and trigger the MAMP-triggered Immunity (MTI). MAMP molecules include chitin and glucan in fungi, bacterial flagellin, among others (Alhoraibi et al., 2019; Zhou y Zhang, 2020). This detection takes place in the apoplast of plant cells and the plant defends itself by producing reactive oxygen species (ROS), antimicrobial compounds and hydrolytic enzymes. Phytopathogens respond by secreting effectors, which allow it to deactivate the immunity response or overcome the effects of the host’s defense mechanism. In their coevolution, plants have developed a second line of defense, the effector-triggered immunity (ETI), which involves the detection of avirulence effectors (Avr effectors) (Figure 1B). The proteins that recognize the Avr effectors are intracellular receptors known as resistance proteins (R), or cognates, and they play a very important part in genetic breeding programs (Ghislain et al., 2019; Li et al., 2020; Thordal-Christensen, 2020). All plants have a wide family of resistance genes; for example, in the genome of Arabidopsis thaliana, over 200 genes are predicted which codify leucine-rich repeat receptor-like protein kinases (LRR-RLK), one type of R gene family (Wu et al., 2016).
The interaction between the avirulence factors and the R proteins is a ubiquitous one in nature, but it was first described in the interaction between the biotrophic fungus Melampsora lini and the plant Linum usitatissimum (Flor, 1942). In the absence of the protein R or cognate, or in the presence of a protein R incapable of recognizing the effector, the ETI defense mechanism is not induced and instead, the effector promotes phytopathogen virulence (Jones and Dangl 2006; Todd et al., 2022a). This leads to effector-triggered susceptibility (ETS) and the expression of the Avr effector genes typically reaches its peak in the first stages of the infection. Conversely, when the Avr protein of the phytopathogen is recognized, an important phenomenon of ETI called the hypersensitive response (HR) is triggered. The HR is unfavorable for the phytopathogen since it produces localized cell death in the host at the site of infection; this prevents further pathogen invasion, keeping the plant healthy. This molecular interaction is key to the incompatible plant-pathogen interaction, which involves a resistant host and an avirulent phytopathogen that is unable to counteract the defense of the plant. On the contrary, the association of the phytopathogen with the susceptible host results in a compatible interaction through the ETS (He et al., 2020; Thordal-Christensen, 2020; Todd et al., 2022a).
Characterization of effectors
In silico identification of effectors in microbial genomes has resulted in tens or hundreds of effector candidates (Carreón-Anguiano et al., 2022; Sperschneider et al., 2018) which must be experimentally validated to determine if they are indeed true effectors.
Nicotiana benthamiana has been widely used as a model plant to transiently express the effectors and study the phenotype that results from this expression; its success as a model plant is due to its small size, fast growth and its ease of genetic manipulation. The most widely used method for functional analysis is “agroinfiltration”, which involves the transformation of Agrobacterium tumefaciens with an expression vector that contains the complete codifying region of the effector; later, the transformed bacteria is infiltrated in the N. benthamiana leaves (Figure 2). True effectors are identified because they generate a particular phenotype as a result of the HR. Most produce characteristic lesions on the leaf, between chlorotic and necrotic, due to their presence activating systemic acquired resistance (Jones and Dangl, 2006; Porras et al., 2022). In turn, other effectors, such as those from biotrophic phytopathogens, characteristically hinder the activation systemic acquired resistance and HR (Zhang et al., 2022). The success of N. benthamiana or Arabidopsis thaliana as model plants to identify phytopathogen effectors that do not infect them naturally has been described as non-host resistance (NHR). This form of resistance refers to the resistance displayed by a plant species against all the genetic variants of a non-adapted phytopathogen species (Wu et al., 2023).
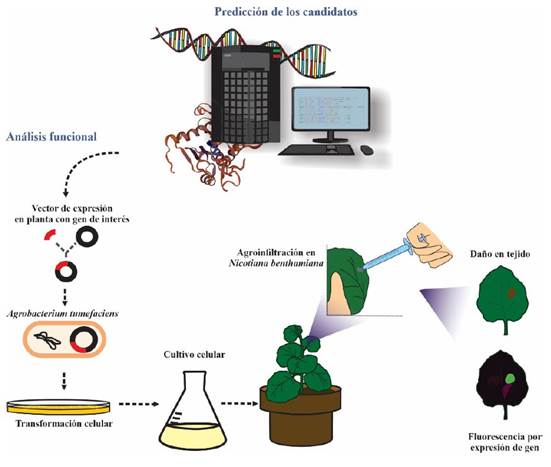
Figure 2 Effector functional analysis workflow. Effectors are first identified through bioinformatics, and some are later selected for functional analysis. The full-length coding sequence is cloned in Agrobacterium tumefasciens, then inoculated in leaves of the model plant, Nicotiana benthamiana. Effectors can be recognized by the plant receptors resulting in lesions on the leaves produced by the hypersensitive response. However, biotrophic effectors are identified because they can suppress the hypersensitive response in plants that have been made to express a particular R protein; for their characterization, the effector recognized by this R protein is co-agroinfiltrated with the putative biotrophic-associated effector.
Several techniques are currently in use to explain the function of effectors. The most common techniques interfere with their expression, such as the generation of “knock out” mutants, which eliminates the gene from the pathogen’s genome, “knock down” or gene silencing using RNA interference (RNAi), which does not eliminate the gene, but interferes post-transcriptionally on the mRNA, preventing its correct translation, and finally, gene editing with CRISPR/Cas9 (Kanja and Hammond-Kosack, 2020). By interrupting the gene’s function, the evaluation determines whether the virulence in a susceptible host is lost (or reduced) to prove that the candidate is a true effector, and a possible protein of interest in phytopathology (Kanja and Hammond-Kosack, 2020).
Other analysis to characterize the effector involves cloning it as a chimeric protein, fusing the codifying region of the effector with a fluorescent protein. This helps determine its subcellular location, that is, whether it is apoplastic or if the protein localizes to intracellular organelles, which helps later locate their target proteins (Camborde et al., 2022; Tang et al., 2022). Identifying and characterizing effectors continue to be challenging tasks. Despite their studies having started 80 years ago (Flor, 1942), by 2020, the functions of only 150 true fungal effectors were known (Carreón-Anguiano et al., 2020), although the number has increased significantly and to date, more than 300 effectors have been discovered (Nur et al., 2021; Sperschneider and Dodds, 2022).
Functions of the effectors
Effectors participate in all plant-microbe interactions; in negative interactions with phytopathogens as well as in interactions between plants and beneficial microorganisms, such as mycorrhizae (Plett et al., 2020; Kang et al., 2020), and recently, they were also discovered in microbe-microbe interactions (Snelders et al., 2020; Snelders et al., 2021). Regarding phytopathogens, the effector functions reflect their lifestyles; for example, biotrophic phytopathogens require a live host to complete their infection cycle, whereas necrotrophic phytopathogens require dead tissue to obtain their nutrients. On the other hand, hemibiotrophic phytopathogens are a combination of the previous two; they obtain their nutrients from live tissue first and from dead tissue at the end of their infection cycle. While the effectors of biotrophic organisms usually work by blocking the host’s immune response; the effectors from necrotrophs trigger the host’s defense mechanism, but in an uncontrolled manner that is intense and not localized, inducing host cell death. Hemibiotrophic phytopathogens initially produce effectors that suppress the immune response and cell death, but later, in the necrotrophic phase, they secrete effectors that induce host cell death (Castillo-Sanmiguel et al., 2022; Jones and Dangl, 2006; Thordal-Christensen, 2020; Todd et al., 2022a) (Figure 3). For example, in Botrytis cinerea, the effector BcNEP1 shows strong expression at the initial phase of the infection, whereas effectors BcSSP2 and BcNEP2 are expressed later on (Zhu et al., 2022). Similarly, in Colletotrichum spp., a genus of hemibiotrophic fungi, there are effectors that participate specifically in the stage of biotrophy, whereas others facilitate necrotrophy, inducing cell death (Ono et al., 2020; Tsushima et al., 2021).
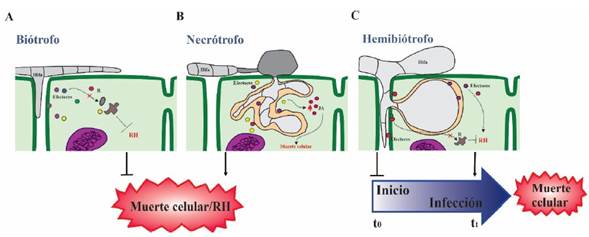
Figure 3 The biological function of effectors according to the trophic lifestyles of different kinds of phytopathogens. A) In biotrophs, the effectors prevent the hypersensitive response and suppress plant defense, keeping the plant host alive. Examples of biotrophs are the fungi Ustilago maydis, Blumeria graminis and Puccinia triticina. B) Necrotrophs release effectors that induce cell death through necrosis, phagocytosis, etc. Examples of necrotrophs are the fungi Botrytis cinerea and Alternaria brassicicola, and the bacterium Rhizobium radiobacter. C) Hemibiotrophs have both kinds of effectors; initially they use effectors expressed in the biotrophic stage which prevent cell death by HR; but towards the end of their life cycle, they use necrotrophic-associated effectors that induce the host cell death. Examples of hemibiotrophs are the fungi Colletotrichum graminicola, Cladosporium fulvum, the oomycete Phytophthora infestans, and the bacterium Pseudomonas syringae.
The most widely studied effectors are apoplastic (extracellular) anduntil the last decade, “extracellular” was part of the definition of effectors (Carreón-Anguiano et al., 2020), since it was believed that all effectors were extracellular. However, intracellular effectors that act in the cytoplasm and organelles have been described in recent times and are gaining ground in effectoromics (Sperschneider and Dodds, 2022; Tariqjaveed et al., 2021; Todd et al., 2022b). Apoplastic effectors tend to be small proteins with enzymatic activity that degrade cell walls, or expansins, that relax them; others are protease inhibitors or inhibitors of the recognition of the phytopathogen by the plant (Fabro, 2022; He et al., 2020; Langin et al., 2020), among other functions. Inside the host cells, the intracellular effectors vary in their location and biological functions; most of their targets in the host are proteins with important functions in plant immunity (Thordal-Christensen, 2020). The targets in the host are usually proteases, components of the ubiquitin-proteasome system, proteins involved in transcription, receptors and proteins involved in biosynthesis pathways and signaling mediated by phytoregulators that regulate plant defense (Fabro 2022; Han and Kahmann, 2019). Table 1 shows some examples of characterized effectors. The reader may expand this catalogue of effectors by consulting the revisions by Kanja and Hammond-Kosack (2020), Todd et al., (2022a, 2022b) and Zhang and collaborators (2022), among others.
Table 1 Examples of characterized effectors and their functions in the host.
| Efector | Organismo | Actividad Biológica | Localización de la proteína | Rol de virulencia/Patogenicidad | Diana vegetal | Referencia |
|---|---|---|---|---|---|---|
| AVR 2 | Cladosporium fulvum | Induce RH; inhibidor de proteasa | Apoplasto | Inhibe Rcr3 y otras proteasas | Cf-2 | Ali y Bakkeren 2011y Selin et al., 2016z |
| AVR 4 | C. fulvum | Induce RH; Unión a quitina | Apoplasto;célula fúngica, pared de quitina | Protege contra quitinasas | Cf-4 | Ali y Bakkeren 2011y Selin et al., 2016z |
| AVR 9 | C. fulvum | Induce RH; Inhibidor de carboxypeptidasa | Apoplasto | Desconocido | Cf.9 | Ali y Bakkeren 2011y Selin et al., 2016z |
| BcSSP2 | Botrytis cinerea | Efector citotóxico; Induce muerte celular | Apoplasto | No esencial para la patogenicidad | Desconocido | Zhu et al., 2022 |
| PaMissP10b | Pisolithus albus | Interactua con la S-adenosylmetionina Descarboxilasa | Citoplasma | Modifica la ruta de biosintesis de poliaminas | Desconocido | Plett et al., 2020 |
| PWL 1 | Magnaporthe oryzae | Proteína hidrofilica rica en glicina | Complejo interfacial biotrófico | Desconocido | Desconocido | Ali y Bakkeren 2011y Selin et al., 2016z |
| PWL 2 | M. oryzae | Proteína hidrofilica rica en glicina | Citoplasma | Desconocido | Desconocido | Ali y Bakkeren 2011y Selin et al., 2016z |
| PWL 3 | M. oryzae | Proteína hidrofilica rica en glicina | Probablemente apoplasto | No funcional | Desconocido | Ali y Bakkeren 2011y |
| AVR 3 (SIX 1) | Fusarium oxysporum f. sp. lycopersici | Desconocido | Xilema | Requerido para la total virulencia | I-3 | Ali y Bakkeren 2011y Selin et al., 2016z |
| AVR 4 (SIX2) | F. oxysporum f. sp. lycopersici | Desconocido | Xilema | Requerido para la total virulencia | Desconocido | Ali y Bakkeren 2011y Selin et al., 2016z |
| MfAVR4 | Pseudocercospora fijiensis | Peritrofina-A con unión a quitina, inducción de RH | Probablemente apoplasto | Protección del hongo contra quitinasas | Cf-4 y Hcr9 | Ali y Bakkeren 2011y Selin et al., 2016z |
| RxLR30 | Phytophthora brassicae | Efector de la familia RXLR | Probablemente apoplasto | Inhibe la secreción de antimicrobianos mediado por vesículas | RABA GTPase | Tomczynska et al., 2018 |
| MoCDIP6 | M. oryzae | Induce Muerte Celular | No reportado | Induce necrosis en hojas | Relacionados con Patogénesis (PR): OsCHT1, OsCHT3, OsNac4, OsPR1B | Guo et al., 2019 |
| PstCEP1 | Puccinia striiformis | Induce HR / muerte celular programada | Citoplasma | Responde a altas temperaturas | Desconocido | Tao et al., 2020 |
| PTTG08198 (CFEM) | P. triticina | Aumenta muerte celular | No reportado | Promueve acumulación de ROS | Desconocido | Zhao et al., 2020 |
| BLN08 | Bremia lactucae | Efector de la familia WY | Mitocondria | Induce muerte cellular en lechuga | Desconocido | Wood et al., 2020 |
| VdAMP3 | Verticillium dahliae | Suprime la respuesta inmune / Induce necrosis y senescencia | Xilema | Manipulación del microbioma | Desconocido | Snelders et al., 2021 |
| XopL | Xanthomonas oryzae | Contrarresta la autofagia en el hospedero | Citoplasma | Se une y degrada al componente SH3P2 de la vía de autofagia | SH3P2 | Leong et al., 2022 |
| MeTCTP | Meloidogyne enterolobii | Suprime la inmunidad vegetal | Citoplasma | Se une al calico e impide su aumento en el citosol | Unión directa al calcio | Guo et al., 2022 |
| MiMSP32 | M. incognita | Interactúa con una enzima involucrada en la síntesis de jasmonato; suprime la inmunidad vegetal | Citoplasma | Promueve la susceptibilidad, contribuye a la virulencia | 12-oxofitodienoato reductasa 2 (OPR2) | Verhoeven et al., 2022 |
| Al6 | Apolygus lucorum | Suprime la inmunidad vegetal y permite que el insecto se alimente | Apoplasto, citoplasma | Usa la glutation peroxidasa para evitar acumulación de especies reactivas de oxígeno | Glutation peroxidasa | Dong et al., 2023 |
| βC1 | Virus del rizado amarillo de la hoja del tomate | Reduce la actividad de terpeno sintasa. | Núcleo | Disminuye producción de volátiles, provoca mayor atracción a la planta del insecto Bemisia tabaci y mejora el desempeño de éste | PIF y MYC2 | Ray y Casteel, 2022 |
y, z review
Through their effectors, microorganisms can manipulate the synthesis of the phytoregulators: jasmonate (JA), salicylate (SA) and ethylene (ET) to their benefit (Alhoraibi et al., 2019; Chini et al., 2018; Langin et al., 2020). For example, the effector Cmu of the fungus Erysiphe quercicola chorismate mutase activity, an enzyme that inhibits the synthesis of salicylic acid in the host (He et al., 2021). The effector VdIsc1, of Verticillium dahliae, isochorismatase activity, which also interferes in the synthesis of salicylic acid (Zhu et al., 2017), whereas the effector RipAB, of Ralstonia solanacearum, interferes with the signaling regulated by salicylic acid (Qi et al., 2022). These examples highlight the importance of inhibiting the synthesis of this phytoregulator, which participates in signaling and the defense of the plant. In the mycorrhiza Laccaria bicolor, the effector MiSSP7 interacts with the repressor proteins PtJAZ5 and PtJAZ6 of the jasmonic acid signaling pathway, preventing the degradation of these repressor proteins, thus blocking the transcription of defense genes regulated by jasmonic acid, which helps establish a mutualism between the mycorrhiza and the host (Plett et al., 2014). Other effectors affect the physiology of the host to create an ideal environment for colonization; the effector AvrE, from Pseudomonas syringae regulates the levels of abscisic acid in the cells to induce stomatal closure, thus increasing water levels in the plant tissue (Hu et al., 2022).
Microorganisms also secrete effectors that promote the synthesis or mimic phytoregulators. For example, the necrotic phytopathogen Lasiodiplodia mediterranea produces an analog of jasmonic acid, the ester lasiojasmonate A (LasA). LasA can be converted to jasmonyl-isoleucine (JA-Ile), a powerful activator of jasmonic acid signaling and inducer of cell death, facilitating the necrotrophy phase of this pathogen (Chini et al., 2018).
Perspectives of effectoromics in agriculture
The interest in effectoromics and its importance in agrobiotechnology have grown considerably in the last decade, and it is currently a priority area of investigations surrounding phytopathogen-host interactions. Some reports have shown that some effectors may, in the future, be used as bioproducts to induce plant defense responses. For example, the effector MSP1 of the hemibiotrophic fungus Magnaporthe oryzae was expressed in the bacteria Escherichia coli and when 0.1μM of the recombinant protein was applied on rice seedling leaves, the plant’s defense response was boosted and infection was avoided (Wang et al., 2016). Recently, the effectors MoCDIP6 and MoCDIP7 were reported in this same fungus; following a similar process, the treated plants displayed no symptoms of necrosis or wilting, and they began to express genes related to resistance. When a virulent strain of M. oryzae was inoculated, plants developed less and smaller lesions in comparison with the control plants (Guo et al., 2019). In Fusarium oxysporum in interaction with tobacco plants, the effector FocCP1 induced the expression of genes related to salicylic acid signaling. When FocCP1 was applied on tobacco plants followed by inoculation with the tobacco mosaic virus, the treated plants developed less symptoms than the control (Li et al., 2019). This is a promising line of investigation, since it offers an eco-friendly approach to disease control in comparison with commercial pesticides, although there are currently few investigations that aim to explore the agro-biotechnological use of effectors. The majority of investigations focus on elemental aspects such as their structure, function or cell location.
Vleeshouwers and collaborators (2011) pioneered the use of effectors in potato crops for the selection of resistant germplasm in genetic breeding programs. Phytophthora infestans effectors have been used to select potato germplasm, in which resistance genes were identified that were useful in the development of improved varieties. Nowadays, introducing resistance genes into susceptible germplasm is one of the most promising applications in genetic breeding programs (Chen et al., 2022; Ji et al., 2022; Ochola et al., 2020). Recombinant effector proteins have also been used to identify susceptible plants. The hypothesis is that when susceptibility genes are mutated, plants will have a more durable resistance in comparison with that mediated by resistance genes (R) (Campos et al., 2021; Garcia-Ruiz et al., 2021; Koseoglou et al., 2022; Ribeiro et al., 2022).
Due to the lack of effector conservation, the development of effectoromics has been slow and difficult. However, with the progress made in high throughput analyses, effectoromics is currently under development and has lots to offer agriculture (Li et al., 2021; Van de Wouw and Idnurm, 2019). Consequently, it is necessary to have robust prediction methods, as well as large-scale effector characterization protocols that help identify effectors with crucial functions to infections and that can protect the plant, at least against the phytopathogen that produces it, or preferably protect the plant against several phytopathogens at a time.
Current landscape of effectoromics in Mexico
Effectoromics is an emerging field in Mexico. The first investigations in which Mexican scientists participated focused on the identification of effectors in the oomycetes P. infestans during the infection of tomato and Phytophthora capsici in a non-host interaction in Nicotiana spp. (Zuluaga et al., 2015; Vega-Arreguín et al., 2017). These investigations identified a diversity of effectors, including IpiO and SNE1 during the biotrophic phase of infection, as well as PiNPP1.1 during the necrotrophic phase. The RXLR, CRN and NPP effector families, common in oomycetes, were also identified (Zuluaga et al., 2015). In turn, the Nicotiana species displayed resistance against P. capsici. The analysis identified that resistance is mediated by the gene I2R, which recognizes the protein effector PcAvr3a1 in the phytopathogen (Vega-Arreguín et al., 2017).
In phytopathogenic fungi, the identification of effectors in P. fijiensis, the fungus that causes black Sigatoka in banana and plantains, is being addressed. Initial analysis identified 136 canonical effectors, that is, they display all the classic characteristics of effectors (secreted, small size, high cysteine content) (Carreón-Anguiano et al., 2020). In order to contribute to world fungal effectoromics, Carreón-Anguiano et al. (2022) created an algorithm, WideEffHunter, which can identify non-canonical effectors, and found that the canonical effectors compose approximately 10% of the effectoromes of fungi and oomycetes. The identification of global effectoromes is expected to help identify new effector families, a greater number of effectors that share homology in different organisms, and new motifs and domains in protein effectors (Carreón- Anguiano et al., 2022; Todd et al., 2022b).
Other Mexican investigations have focused on non-phytopathogenic organisms. Guzmán-Guzmán et al. (2017) bioinformatically identified 233 effectors in Trichoderma virens, T. atroviride and T. reesei proteomes, where 16 effectors from T. virens and T. atroviride were selected for characterization. They found that some effectors are expressed during fungal colonization of A. thaliana, whereas others are expressed when they confront the phytopathogenic fungus Rhizoctonia solani. Among the Trichoderma effectors, hydrolases have been found along with hydrophobins, cerato-platanins, and effectors with CFEM or LysM domains (Ramírez-Valdespino et al., 2019; Romero-Contreras et al., 2019). Interestingly, a class II hydrophobin, tvhydii1, is overexpressed in T. virens in the presence of the phytopathogen R. solani; the mutants that lose their tvhydii1 function lose part of their ability to colonize plant roots, whereas its overexpression increases colonization (Guzmán et al., 2017).
Recently, Báez-Astorga et al. (2022) reported the action mechanism of the biocontrol agent Bacillus cereus, which is able to inhibit in vitro Fusarium verticillioides, a phytopathogen that causes ear and root rot in maize. F. verticillioides secretes the effector Fv-cmp which has protease activity and digests types A and B chitinases of the plant. On the other hand, B. cereus secretes the effectors ChiA and ChiB with chitinase activity which, in vitro, act upon the F. verticillioides conidia and prevent them from germinating and developing into hyphae. This is due, in particular, to effector ChiB with the domain CBM 2, which helps it adhere strongly to the fungal cell wall, displaying greater activity than ChiA.
Although there are very few investigations in Mexico in the area of effectoromics, these investigations show promising results in the realm of biotechnological applications. Research on effectors in Mexico may expand to study phytopathogenic bacteria, as well as insect pests and nematodes. It is worth mentioning that the interest in these areas has grown in recent years worldwide, but the number of investigation groups is still limited, thus representing an opportunity for Mexican research to contribute to this niche in effectoromics.
Conclusions
Effectors are extremely important for the establishment of biological interactions; within the range of interactions in which they are found, the plant-pathogen interaction is the most studied. The first study was in the L. usitatissimum- M. lini interaction in the 1940s, and although currently there are great advances in effectoromics, knowledge is still limited. Consequently, it has become necessary to expedite the prioritization of effectors for their characterization, since hundreds of them are identified for each organism during in silico analysis.
The identification and characterization of effectors crucial to phytopathogen virulence could be key to the development of new methods to manage diseases in agriculture, based on effectors. The identification of target proteins in the host is incipient; among these target proteins, there are possible resistance proteins with genes that can be used for plant protection. Undoubtedly, the effectors of microorganisms represent opportunity niches that must be understood in order to use them for the benefit of society and world food security.











 text in
text in 


