The Palmae family (Arecaceae) is classified into six subfamilies with 4,000 species and 200 genera worldwide (Ahmad et al., 2020). Palms present a morphological and ecological diversity, and they are usually found in regions with tropical, subtropical and Mediterranean climates. For the most part, palms are used for esthetic and ornamental purposes, but some species are also used for food (Howard, 2001). In the horticultural industry, ornamental palms represent 404 million dollars, that is, 10% of the total production (Hodges et al., 2011). In addition, from the ecological standpoint, these plants reduce environmental pollution and have a positive effect on the atmosphere (El-Juhany, 2010). Ornamental palms are affected by biotic factors, such as insects and diseases, and abiotic factors such as temperature, and soil nutrition and composition (Schmidhuber and Tubiello, 2007; Aldana et al., 2009). Different phytopathogenic fungi, bacteria, viruses and nematodes reduce the esthetic value of plants in different stages, causing symptoms that include wilting, blight, spot, root rot, stem and petiole rot, diamond scale and leaf sheath diseases, as well as wilting from Fusarium oxysporum (Broschat et al., 2014). Recent studies carried out on Chamaedorea cataractarum, C. seifrizii and C. costaricana reported some pathogens responsible for the diseases of these palms, as in Ganoderma spp., which causes stem base rot, a lethal disease with an enormous potential for spreading (Shakeel et al., 2020). Another one of the main pathogens in palms is Phytophthora palmivora, which causes the devastating disease called lethal palm bud rot (Broschat et al., 2014; Shakeel et al., 2020). Likewise, there have been reports of the leaf spot disease complex Helminthosporium, caused by a group of fungi (Drechslera setariae, Exserohilum rostratum and Phaeotrichoconis crotalariae) (Shakeel et al., 2020).
In Central America, the genus Chamaedorea is distributed in Guatemala, El Salvador, Honduras and in southern Mexico, as part of the montane cloud forest and tropical evergreen forest (Granados et al., 2004; López et al., 2005). In Chiapas, the genus Chamaedorea is found in depressions of the Sierra Madre de Chiapas and in the Lacandona jungle (Buda Arango et al., 2014) in altitudes ranging from 900 to 2300 masl (Martínez et al., 2011). For the case of the region known as the Frailesca, Chiapas, Chamaedorea quezalteca, commonly known as the camedor palm, is the most planted species for commercial use.
Chamaedorea quezalteca has been extracted since the 1940s to supply world flower markets, mainly in the United States, therefore it has been considered a non-timber yielding product (Pérez et al., 2012). This economic activity is feasible for small rural communities, since it does not require sophisticated technology (Evans, 1993). For many rural producers, this represents participating in a subsistence economy towards a market economy, which has meant the increase of their income.
Since 2020, in the Frailesca region, Chiapas, the camedor palm plantations have been affected by two foliar diseases foliar spot and anthracnose which reduce the production and quality of leaves, which has a direct impact on the incomes of producers. In Mexico there are currently no reports of leaf diseases related to the camedor palm (Chamaedorea quezalteca). Therefore, the aims of this research were to identify the causal agents, quantify the incidence and severity of the leaf diseases leaf spot and anthracnose in commercial camedor palm plantations.
Materials and methods
Gathering biological material. Twenty leaf samples of each of the leaf spot and anthracnose diseases were gathered from eight-year-old camedor palms located in the Sierra Morena (16° 08´51.6” LN - 93° 36´ 30.06” LW) and Plan de Ayala (15°90´47.22¨ LN-93°21´25.00¨ LW) ejidos of the municipal area of Villa Corzo, Chiapas. The samples were placed in plastic bags with paper towels, labeled and sent to the Laboratory of Biofertilizers and Bioinsecticides of the Universidad de Ciencias y Artes de Chiapas in Villa Corzo, and they were stored in a container with water until used.
Fungus isolation and purification. The camedor palm leaves with symptoms of leaf spot and anthracnose were dissected into pieces of 0.5 cm2 each, washed with 70% ethanol for 3 minutes and with 10% sodium hypochlorite for 10 min, then washed twice with sterile distilled water for 2 min. Ten pieces with each of the symptoms were planted in a potato dextrose agar (PDA) medium in triplicate and incubated at 28 °C, a 12:12 light/darkness photoperiod for 10 days; the mycelial growth of the fungi was monitored every 24 hours and purified by hyphal tipping and monosporic culture (Ruiz-Cisneros et al., 2017).
Morphological identification. Out of the 30 pieces of tissue planted in PDA, 10 fungi were isolated from each of the symptoms of leaf spot and anthracnose that were the first to emerge from the tissues after 48 hours. They were identified morphologically at a genus level via their reproductive structures observed under an optical microscope (Axiolab 5, Carl Zeiss) and with the aid of taxonomical keys (Barnett and Hunter, 2006; Watanabe, 2002).
Molecular identification. For the molecular identification, the fungus isolated from the leaves with symptoms of leaf spot and the anthracnose fungus were planted in Petri dishes with PDA covered with a sheet of sterile cellophane and incubated for 7 days at 28 ºC. The mycelium was harvested and frozen using liquid nitrogen; the DNA was then extracted following the protocol by Raeder and Broda (1989). The total DNA was used to amplify the internal transcribed spacer (ITS) of the18S of the rDNA using oligos ITS5 (5’-GGAAGTAAAAGTCGTAACAAGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) with the following conditions of amplification from an initial denaturing step at 94 ºC for 5 minutes, followed by 30 cycles (denaturation at 94 ºC for 30 s, annealing at 60 ºC for 30 s, extension at 72 ºC for 45 s) and a final extension of 72 ºC for 10 minutes, where the fragment may be from 710 to 850 pb, depending on the fungal species (White et al., 1990). The amplicons of the polymerase chain reaction (PCR) were sequenced by the Sanger method in an ABI (Applied Biosystems) sequencer. The sequences were compared with the NCBI data base using the de BLAST algorithm (Altschul et al., 1990).
Pathogenicity test. Koch’s postulates were carried out on fields under the shade in the facilities of the Universidad de Ciencias y Artes de Chiapas (UNICACH), using two-year old seedlings planted in 5 kg polyethylene bags and natural soil substrate from the same plantations. Both fungi isolated and previously identified were planted in a PDA medium at 28 ºC in a 12:12 light/darkness photoperiod for 15 days. The inoculation of Neopestalotiopsis sp. was carried out directly and consisted in taking the conidia from the acervuli with a hypodermic needle and inoculating the leaves of the plants via a small lesion in the epidermis (Rebollar et al., 2020), and for C. karstii, a conidial solution was used with 1x 106 spores mL-1 and 10 µL were inoculated. For both fungi, five plants were inoculated (two leaves per plant). The inoculated plants were sprayed with sterile distilled water, covered with plastic bags and were uncovered on the third day. They were kept under regular irrigation and observation until the symptoms of the diseases appeared. Samples were taken from the symptoms obtained and the inoculated fungi were reisolated, following the process described above. Five control plants were used with sterile water and the experiment was carried out in duplicate.
Field incidence and severity. To quantify the incidence of the leaf diseases in the camedor palm, a 1 ha, eight-year-old plot in the Sierra Morena ejido was chosen, a five-point sampling was carried out, 20 plants were labeled and sampled in each point, the number of plants with symptoms were quantified out of a total of 100, samples were taken every 2 months in a year in the same points and the same plants and in each one, the values of the five points were averaged. For the severity of the diseases, a pictographic scale of damages was created with values from 1 to 5, where 1 represents 0% damage; 2, 1-25% damage; 3, 26-50% damage; 4, 51-75% damage and 5, 76-100% damage (Figure 1). The percentage of damage was measured directly from each of the plants quantified in the incidence of the disease. The percentages of incidence and severity of the disease were converted into values of areas under the disease progress curve (AUDPC) (Pedroza and Samaniego, 2009).
Results and discussion
Morphological and molecular identification. Out of the leaves with symptoms of leaf spot, 10 fungal cultures were isolated and purified, which, based on morphological analyses formed on PDA medium, all were identified as Neopestalotiopsis sp., since they displayed characteristics of cottonlike filamentous fungi with elevated, initially white hyphae, acervuli that look like small black pustules with abundant spindle-shaped conidia with three central pigmented cells and hyaline basals (dimensions from 13.22 to 15.88 µm in length x 2.82 to 3.59 µm in width),with two to four apical appendages (measuring 5.99 x 8.49 µm in length x 0.67 x 1.21 µm in width) and a peduncle in the basal cells (Barnett and Hunter, 2006; Watanabe, 2002) (Figure 2). Structures with similar characteristics were reported by Rebollar et al. (2020) in strawberry crops and by Gerardo-Lugo et al. (2020) in mango crops: three central cells colored yellow and 3 apical appendages with a peduncle in the basal cells. However, the morphological characteristics of the phytopathogenic fungi, and particularly Neopestalotiopsis can vary and be modified by the environmental conditions and these are not reliable as a form of identification (Maharachchikumbura et al., 2011). Studies carried out by Acosta-González (2022) confirm that there are differences in the diameter of the final growth, as well as coloring, the texture of the mycelium, the production of acervuli, the elevation of the mycelium and the margins of the genus Neopestalotiopsis. Molecular identification was based on the analysis of the consensus sequence of gene 18S rDNA (ITS5/ITS4) that displayed a high similarity with Neopestalotiopsis sp. with a coverage of 99% and a percentage of similarity of 99.48%, the sequence was registered in the GenBank with accession number ON860658 (White et al., 1990).

Figure 2 Symptoms of foliar spot in camedor palm leaves in the field, light brown oval-shaped spots with a black dot in the center, affecting the folioles of the leaf (A), marroon-white culture with cottonlike mycelia and abundant acervuli in the center (B), bright black acervuli with abundant conidia (C) and conidia with three pigmented cells in the center and hyalin basals, with apical appendages and basal pedicel (D) Neopestalotiopsis sp formed in PDA, symptoms presented four (E) and eight (F) days after inoculation, reisolated conidia produced in PDA from pathogenicity tests (E) and control plant (F).
For the symptom of anthracnose, Colletotrichum karstii was identified as the causal agent, and the identification was carried out from 10 frequent fungal isolations, which were characterized for being filamentous, for the cultures forming asexual reproduction sporodochia with pale maroon semi-spherical hyaline conidia, either simple or branched or erect (dimensions 6.47 to 8.59 µm in length x 2.69 to 3.31 µm in width), conidiophores 17.04 to 33.35 µm in height (Barnett and Hunter, 2006; Watanabe, 2002) (Figure 3). Similar characteristics to this fungus were reported in other crops such as strawberries in Brazil (Soares, 2021) and papaya crops in Mexico (Pacheco et al., 2022). The molecular identification was obtained by the amplification of gene 18S rDNA (ITS5/ITS4), which displayed a high level of similarity with Colletotrichum karstii with a coverage of 100% and a percentage of similarity of 99.83%; it was registered in the GenBank with accession number ON799261 (White et al., 1990).
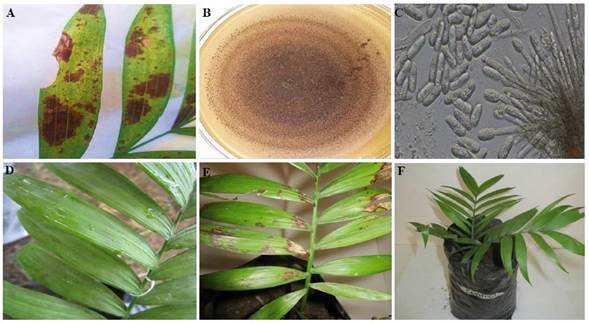
Figure 3 Symptom of anthracnose in camedor palm in the field, irregular black spots affect the leaf folioles (A), pale brown colony with abundant sporodochia (B), semi-spherical conidiophores and hyaline conidia (C) of Colletotrichum karstii formed in PDA, symptoms manifested after three (D) and 11 days (E) after the inoculation of the pathogenicity test and control plant (F).
Pathogenicity tests. The plants inoculated with Neopestalotiopsis sp. presented the first symptoms of leaf spot after four days, and after eight days, the symptoms of small, oval-shaped spots with a black dot in the middle were marked and defined, just like those observed in the field. The control presented no symptoms. The plants with symptoms were used to reisolate the pathogen and Neopestalotiopsis sp. was confirmed as the leaf spot disease (Figure 2). These results agree with those reported by Khoo et al. (2022), who found that Ixora chinensis plants inoculated with Neopestalotiopsis cubana presented symptoms after seven days, whereas mango plant inoculated with Neopestalotiopsis sp., and strawberry plants inoculated with Neopestalotiopsis rosae presented symptoms 10 days after inoculation (Gerardo-Lugo et al., 2020; Rebollar et al., 2020).
In the pathogenicity test with the fungus Colletotrichum karstii, the symptoms of anthracnose became apparent on the third day, with small undefined dark brown spots, and after 11 days, the irregular spots were well defined, reproducing the same symptoms displayed on the field; the fungus was reisolated and identified as C. karstii (Figure 3). According to reports by Ayvar-Serna et al. (2021), avocado fruits inoculated with the same pathogen displayed symptoms after five days, whereas Fernández-Herrera (2020) coincided that symptoms begin displaying three days after inoculation in Dendrobium nobile orchid plants, and seven days later in chili pepper plants (Saini et al., 2016).
Field incidence and severity. During the sampling period on the field for the disease caused by Neopestalotiopsis sp., the incidence and severity were observed to have an intensity that depended on the weather and management during the progress of the disease (Figure 4). For the month of June, an incidence of 12% was recorded (AUDPC=732), corresponding to one of the driest months of summer sampling, whereas in August, corresponding to the humid season due to rains and the highest anthropogenic activity due to the trimming of palm leaves, the highest value (45%) was presented (AUDPC= 2790). For severity, a similar behavior to incidence was displayed, in which the driest month was April, with a severity of 25% (AUDPC =1525), whereas the rainiest and most humid month was October, with 50% (AUDPC = 3355) (Figure 4). In this sense, it has been reported that in the leaf spot disease caused by Neopestalotiopsis in eucalyptus leaves, lesions are most severe when the interaction of the pathogen and the plant are exposed to prolonged humidity periods (Belisario et al., 2019). According to reports by Maharachchikumbura et al. (2011), incidence and severity are related to the thickness of the leaf and the stage of maturation of leaves, since the hyphae penetrate the spaces between the cells of leaved when they are in a state of maturity, therefore their susceptibility also depends on the thickness of the cuticle and on the production of phytotoxins such as pestalopyrones, hydroxypestalopyrones and pestalocides. It is worth pointing out that, although a minimum incidence of 12% and severity of 25%, palms cannot be marketed, since the commercial part of the camedor palm is comprised of its leaves, and the slightest damage they have stops them from complying the quality standards established by the market, according to De los Santos (2005), in the market report for camedor palm in Mexico.
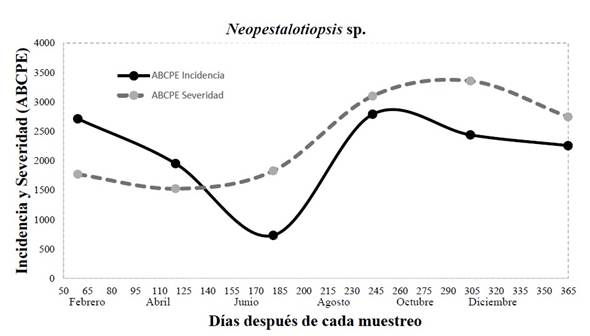
Figure 4 Incidence and severity (area under the disease progress curve) of leaf spot caused by Neopestalotiopsis sp. in eight-year-old commercial camedor palm plantations in the year 2021.
Regarding the behavior of the disease caused by Colletotrichum karstii in the month of June, an incidence of 13% was displayed (AUDPC= 793), which corresponds to a hot season and the start of the rainy season in the region, whereas the highest value was registered in December, with 37% (AUDPC= 2257), a season with cool temperatures and a high relative humidity (Figure 5). Regarding severity, the disease had a similar behavior to that of incidence, where the highest value was recorded in June, with 35% (AUDPC= 2135), and in August to October, when temperatures are cooler with a high relative humidity due to heavy rainfalls, severity was 65% (AUDPC= 4030) and 75% (AUDPC = 4575) respectively (Figure 5). In this sense, environmental factors are important for the dissemination and progress of the disease, mostly relative humidity and rainfall play a part in the epidemiology of Colletotrichum karstii in citrus orchards (Mayorking et al., 2019). Studies performed by Velho et al. (2014) mention that the incidence and severity caused by this pathogen can defoliate apple trees and it is favored by high levels of humidity and high temperatures. Likewise, Colletotrichum and Pestalotiopsis have been reported as potential fungi that cause leaf spots in palm, where the disease is disseminated by spores that are easily scattered by wind and rain (Tariq et al., 2015).
Conclusions
According to the morphological, molecular characterizations and pathogenicity tests, Neopestalotiopsis sp. has been concluded to be the causal agent of leaf spots and Colletotrichum karstii is responsible for the anthracnose disease in camedor palm. The progress of the incidence and severity of these diseases depend on the factors of relative humidity cause by rains and by agronomic management, which reduces palm quality and production. Therefore, this is the first report in Mexico on these pathogens related to the plantation of camedor palm (Chamaedorea quezalteca).











 text in
text in 




