Fusarium oxysporum f. sp. cubense (Foc), causal agent of Fusarium wilt or musaceae Fusarium wilt, was reported for the first time in Australia, in 1874, and later in 1890, it was reported in Panama and Costa Rica (America); therefore, it is assumed that this pathogen spread from these two countries to the rest of this continent (Ashby, 1913; Fish, 1970). There are three races of this pathogen that affect plantains and bananas: i) Race 1 (FocR1), affects Musa textilis (abacá), bananas from the Gros Michel clones (AAA), Maqueño (AAB), Silk (AAB), Pome (AAB), Pisang Awak (ABB) and IC2 (AAAA); ii) Race 2 (FocR2), affects bananas from Bluggoe clones (ABB) and other AAAA genome hybrids; and iii) Tropical Race 4 (FocR4T) is an aggressive pathogen in Cavendish cultivars (AAA), such as Dwarf Cavendish, Grand Nain and Williams, as well as in other cultivars susceptible to FocR1 and FocR2 (Su et al., 1986; Ploetz, 2005). In Mexico this disease was reported for the first time in 1932 (Ploetz, 2018); Likewise, Florencio-Anastasio et al. (2022) determined through a phylogenetic analysis that four Foc isolates obtained from samples from the states of Puebla and Michoacan were grouped within Clade VI of the classification proposed by Fourie et al. (2009) and Karangwa et al. (2018). FocR1 has caused severe epidemics that impacted the banana industry in America, whose production was based on the Gros Michel variety, which caused the disappearance of most of the commercial plantations of this variety between 1950 and 1960, causing economic losses of up to $2.3 billion dollars (Dita et al., 2013). In the Ulua Valley (Honduras) 30,000 ha were lost from 1940 to 1960; while in Suriname and Quepos (Costa Rica) 4,000 and 6,000 ha were damaged in a period of eight and 12 years, respectively (Ploetz, 2000).
In Mexico, during the 1960s and early 1970s, close to 40,000 ha cultivated with the Gros Michel variety were destroyed; Only in 1994 there were around 4,000 ha of Manzano plantains, which were reduced to 1,260 ha, in 2004 (Orozco-Santos, 2013) and for the 2020 cycle, 1,690 ha had been established (SIAP, 2022). To counteract the economic losses caused by FocR1, the global solution consisted of replacing those plantations established with Gros Michel by cultivars of the Cavendish subgroup, which represent more than 99 % of export bananas (Dita et al., 2018). On the other hand, FocR4T was first reported in Taiwan in 1989 (Ploetz, 2018), by 2022 its distribution includes Australia, Burma, China, Colombia, the Philippines, India, Indonesia, Mayotte Islands (France), Israel, Jordan, Laos, Lebanon, Malaysia, Mozambique, Oman, Pakistan, Peru, United Kingdom, Thailand, Taiwan, Turkey, and Vietnam (Ploetz et al., 2018; Promusa, 2018; DOA, 2019; ICA, 2019; Aguayo et al., 2021; García-Bastidas et al., 2020; Özarslandan and Akgül, 2020; SENASA, 2021).
The presence of this pathogen in two countries of America (Colombia and Peru) increases the risk of its introduction to Mexico (Florencio-Anastasio et al., 2022). Although Cavendish group bananas are resistant to races 1 and 2 (Su et al., 1986), there are no resistant banana or plantain cultivars or chemicals effective against FocR4T (Cook, 2005). Likewise, some biological control schemes based on the use of antagonistic bacteria and fungi against Foc breeds have been proposed (Bubici et al., 2019); for example, the bacterium Burkholderia cenocepacia 869T2 presented 44.4% inhibition in vitro, and reduced the incidence of FocR4T in Cavendish bananas by 86.1% in the field (Ho et al., 2015). Pseudomonas fluorescens and P. fluorescens WCS417 also reduced (83.4 and 87.4%, respectively) the severity of FocR4T in Cavendish bananas in greenhouse. The use of biofertilizers based on pig manure in combination with Bacillus amyloliquefaciens NJN-6 reduced the incidence of FocR4T in greenhouse Cavendish bananas by 75% (Shen et al., 2015), while the same bacterium decreased the incidence of FocR4T by 68.5%. in the field (Xue et al., 2015). On the other hand, B. amyloliquefaciens W19 reduced the incidence of Foc by 42.8 and 44.4% in two Cavendish banana fields (Wang et al., 2016); In addition, B. subtilis N11 reduced the incidence of FocR4T by 82.1%, while the combination of Paenibacillus polymyxa SQR21 + Trichoderma harzianun T37 reduced the incidence of FocR4T by 64.3% in Cavendish banana (Zhang et al., 2011). The bacteria Paenibacillus sp. BSP.1.1, Serratia sp. AC35, Pseudomonas tolaasii P61, Bacillus pumilus BFIEST 4C, B. pumilus R44, B. pumilus A1, and Serratia liquefaciens CPA C53 inhibited mycelial growth in vitro of five Foc Race “1 or 2” isolates at 46.6, 26.6, 12.0, 11.9, 10.1, 9.7 and 4.9%, respectively (Florencio-Anastasio et al., 2022).
Therefore, the present investigation evaluated the in vitro effect of five antagonistic bacteria on the mycelial development of FocR2, as a model phytopathogenic fungus, given its presence in the country, to assess its potential application in future phytosanitary management schemes, in the event of a possible introduction of FocR4T to our country.
Phylogenetic analysis. To determine the race to which the isolates evaluated in the present study belong (Table 1), the same methodology reported by Florencio-Anastasio et al. (2022), phylogenetic analysis of the IGS (Intergenic spacers) region of the rDNA amplified with the primers PNFo and PN22 (Edel et al., 1995) was performed.
Table 1 Origin of the isolates of Fusarium oxysporum f. sp. cubense race 2, deposited in the collection of Laboratorio de Micología, del Centro Nacional de Referencia Fitosanitaria (DGSV-Senasica) (Florencio-Anastasio et al., 2022).
| Aislamientos | Municipio / Estado | Latitud | Longitud | No. de accesión |
|---|---|---|---|---|
| CNRF-MIC17188 | Hueytamalco, Puebla | 20.00857 | -97.24197 | MN702818 |
| CNRF-MIC17189 | Hueytamalco, Puebla | 20.00882 | -97.2419 | MN702819 |
| CNRF-MIC17190 | Hueytamalco, Puebla | 20.00928 | -97.24209 | MN702820 |
| CNRF-MIC17191 | Tacámbaro, Michoacán | 19.21827 | -101.45857 | MN702821 |
| CNRF-MIC17192 | Villa de Tututepec de Melchor Ocampo, Oaxaca | 16.04554 | -97.70423 |
FocR2 isolates. The fungi isolates were donated by Centro Nacional de Referencia Fitosanitaria del Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA). Which were isolated from banana plantations in Hueytamalco, Puebla (MIC17188; MIC17189; MIC17190), Tacámbaro, Michoacán (MIC17191) and Villa de Tututepec de Melchor Ocampo, Oaxaca (MIC17192), from samples of individual plants collected by the National Program Phytosanitary Epidemiological Surveillance (PVEF) for surveillance and timely detection of FocR4T. The collected samples consisted of 5 x 1.5 cm fragments of vascular bundles from banana plants with yellowing symptoms.
Evaluated bacterial strains. For bioassay 1, the bacterial strains Bacillus subtilis (BASU), B. sonorensis (BASO), B. velezensis (BAVE) were used, which were provided by Laboratorio de bacterias, Centro Nacional de Referencia Fitosanitaria del SENASICA (Table 2) to evaluate its effect on the mycelial growth of three FocR2 isolates (MIC17188, MIC17191 and MIC17192) (Table 1). On the other hand, in bioassay 2, the bacterial strains Paenibacillus sp. BSP 1.1 and Serratia sp. AC 35, which were provided by Laboratorio de Microbiología, Postgrado de Edafología, Colegio de Postgraduados, Campus Montecillo, to determine their influence on the mycelial growth of the FocR2 strain CNRF-MIC17188 (accession number MN702818).
Table 2 Origin of the antagonistic bacteria evaluated against Fusarium oxysporum f. sp. cubense race 2.
| Bacteria | Clave asignada | Origen de las bacterias |
|---|---|---|
| Bacillus subtilis | BASU | SENASICA |
| Bacillus sonorensis | BASO | SENASICA |
| Bacillus velezensis | BAVE | SENASICA |
| Paenibacillus sp. | BSP 1.1 | COLPOS |
| Serratia sp. | AC 35 | COLPOS |
Bioassay 1. In vitro inhibition of bacteria against three isolates of FocR2. For this bioassay, the antagonistic effect of the bacterial strains: BASU, BASO, BAZE (Table 1) on the mycelial growth of the isolates MIC17188, MIC17191 and MIC17192 (Table 2) was evaluated using the technique proposed by (Pineda-Mendoza et al., 2019). The bacteria were reactivated in nutrient agar (Merck®). Four treatments were established (three bacteria plus the control) with four repetitions each. At the ends of the Petri dishes with PDA medium (Potato Dextrose Agar, Merck®), a streak (5 cm line) of the seven-day-old bacterial strains was placed. The Petri dishes were incubated in the dark at 28 ± 2 °C for 24 h; Subsequently, 7 mm diameter agar disks of each FocR2 isolate were placed, which were removed from the margins of the seven-day-old fungal cultures and placed on the opposite side of each Petri dish, 5 cm from each other. distance from bacterial growth. The control consisted of only placing fungal growth disks in PDA culture medium. The dishes were incubated again in dark at 28 ± 2 °C; at 3, 7, 10 and 14 days after sowing, the radius of mycelial growth of each fungal isolate was measured, four measurements of fungal growth were taken with a vernier, to calculate the percentage of inhibition by utilizing the reported formula was used by Landa et al. (1997):
Percentage of inhibition
Where, r is the growth radius of the fungus in the presence of the bacteria and R is the growth radius of the fungus without the bacteria (control).
Bioassay 2. In vitro inhibition of Paenibacillus sp. (BSP 1.1) and Serratia sp. against the FocR2 isolate MIC17188 at three pre-inoculation times. In this bioassay, the inhibitory effect in vitro of the bacterial strains Paenibacillus sp. (BSP 1.1) and Serratia sp. (AC 35) together and separately (Table 1) against the FocR2 isolate MIC17188 (Table 2) was evaluated, in previous studies, this isolate presented a higher in vitro growth rate (Florencio-Anastasio et al., 2022), three different pre-inoculation times at 2, 4 and 7 days of pre-inoculation (DPI) in the culture medium (pre-inoculation means to the seeding of the bacteria in the Petri dish prior to seeding the isolates of the fungus, in order to allow the bacteria to produce and secrete antifungal metabolites). The same inoculation procedure described in Bioassay 1 was used. A 7 mm disk of the fungus was placed in the center of the Petri dish and 5-cm streak of the bacterial strains were placed at opposite ends, in such a way that the bacterial growth was initially separated 3 cm from the fungus and 6 cm from each other. Four replicates were established for each treatment, including the control which consisted of a 7 mm disk of the fungus, and the Petri dishes were incubated again in the dark at 28 ± 2 °C. The radius of mycelial growth of the isolation of the fungus was measured at 2, 4, 6 and 7 days.
Experimental design and statistical analysis. For each trial, a completely randomized experimental design was established and they were performed separately, to avoid possible cross effects due to volatile compounds produced by antagonistic bacteria. The inhibition data were analyzed with the statistical package SAS for Windows (SAS Institute Inc, 2002), performing an analysis of variance and Tukey’s mean comparison test (α ≤ 0.05).
Phylogenetic analysis. BLAST analysis of the consensus sequences of the isolates MIC17188, MIC17189, MIC17190 (Hueytamalco, Puebla) and MIC17191 (Tacámbaro, Michoacán) confirmed the identity of Fusarium oxysporum f. sp. cubense race 2 with 100 % cover and grouped with the race within Clade VI proposed by Fourie et al. (2009) and Karangwa et al. (2018) (Figure 1).
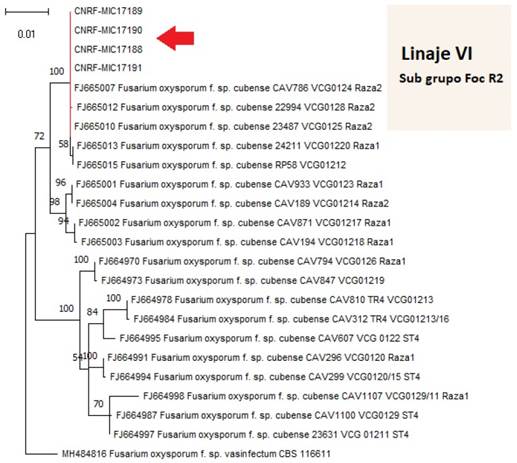
Figure 1 Phylogenetic analysis of four isolates of Fusarium oxysporum f. sp. cubense (MIC17188, MIC17189, MIC17190, MIC17191) from Puebla and Michoacan, Mexico.
Bioassay 1. In vitro inhibition of bacteria against three isolates of Foc R2. The BASU bacterial strain was the one that caused the greatest inhibition of mycelial development of the three FocR2 isolates, with an average of 55.1%, 14 days after the confrontation. On the other hand, the BAVE and BASO strains showed an average inhibition percentage of 45.8 and 21.7%, respectively (Table 3; Figures 2 and 3).
Table 3 Comparative fungal inhibitions (average of the three strain isolates of Fusarium oxysporum f. sp. cubense race 2) obtained from fungal confrontations with three bacteria, 14 days after evaluation.
| Bioensayo 1 | Inhibición fúngicaz (%) |
|---|---|
| Bacillus subtilis (BASU) | 55.1 a |
| Bacillus velezensis (BAVE) | 45.8 b |
| Bacillus sonorensis (BASO) | 21.7 c |
z Values estimated from the control fungal growth data for the corresponding bioassay. Identical letters are not significantly different (Tukey, α ≤ 0.05), n=4.
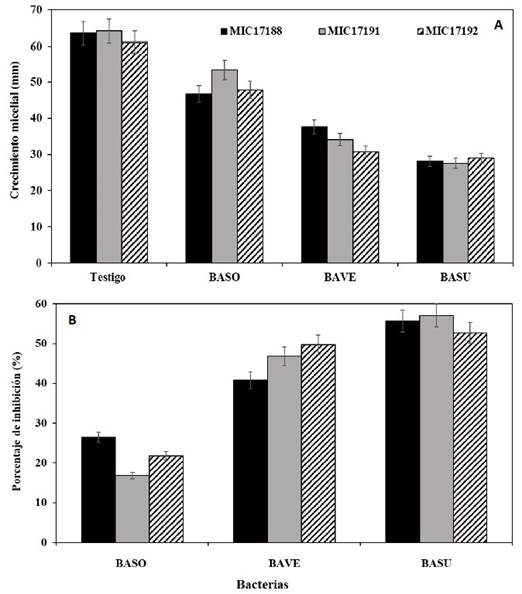
Figure 2 In vitro inhibition of Bacillus subtilis (BASU), B. sonorensis (BASO), and B. velezensis (BAVE) against the isolates MIC17188, MIC17191, and MIC17192 of Fusarium oxysporum f. sp. cubense race 2, after 14 days of evaluation. A) Mycelial growth, and B) Percentage of inhibition. Means+Standard error. n=4.

Figure 3 In vitro mycelial growth of the isolates MIC17188, MIC17191 and MIC17192, of Fusarium oxysporum f. sp. cubense race 2 in the presence of Bacillus subtilis (BASU), B. sonorensis (BASO) and B. velezensis (BAVE) bacteria, at 3, 7, 10 and 14 days of evaluation. Means + Standard error. n=4.
Bioassay 2. In vitro inhibition of Paenibacillus sp. (BSP 1.1) and Serratia sp. against the FocR2 isolate MIC17188 at three pre-inoculation times. After 7 days of evaluation, the bacterial strain Paenibacillus sp. BSP 1.1, by itself, had the greatest inhibitory effect on the mycelial growth of the MIC17188 isolate at 4 and 7 DPI of 75.9 and 80.1%, respectively, followed by the combination of Paenibacillus sp. BSP 1.1 + Serratia sp. AC35 whose inhibition was 52.7 and 75.5%, respectively; While the bacterial strain Serratia sp. AC35 presented inhibition values of 46.8 and 52.3% on the same pre-inoculation dates (Table 4; Figure 4).
Table 4 Inhibitory effect of Paenibacillus sp. (BSP 1.1), Serratia sp. (AC 35) and its combination, against Fusarium oxysporum f. sp. cubense race 2 (MIC17188), with seven days of bacterial pre-inoculation and seven days of confrontation.
| Tratamiento | Inhibición fúngica (%)* |
|---|---|
| BSP 1.1 | 80.15 a |
| BSP 1.1 + AC 35 | 75.51 b |
| AC 35 | 52.31 c |
*Estimated value from the fungal growth of the control of the corresponding bioassay. Identical letters in the column are not significantly different (Tukey, α ≤ 0.05). n=3.

Figure 4 In vitro inhibition of the bacteria Paenibacillus sp. (BSP 1.1) and Serratia sp. (AC35) against the isolate MIC17188 of Fusarium oxysporum f. sp. cubense at three times of bacterial inoculation (two, four and seven days). A) Mycelial growth, and B) Percentage of inhibition. After seven days of evaluation. Means + Standard error. n=3.
The in vitro inhibition capacity of the BASU, BAVE and BASO bacterial strains considered in this work had not been previously determined against any pathogen; these strains inhibited the mycelial growth of three FocR2 strains, by 40.87% in average, being BASU (55.1%) and BAVE (45.8%) the most effective, while BASO showed the least effectiveness (21.7%). There are reports indicating that several bacterial strains of B. subtilis exert in vitro inhibition towards F. oxysporum and at the same time reduce the incidence of FocR1 when inoculated into vitroplants of banana variety red banana (AAA) under greenhouse conditions either alone or in combination with Pseudomonas fluorescens Pf1 (Rubio-Tinajero et al., 2021; Kavino and Manoranjitham, 2018). Similarly, the effectiveness of B. subtilis has been evaluated in in vivo trials, based on its ability to reduce the incidence of F. oxysporum in Dioscorea villosa tubers (Khan et al., 2017). Some species of B. velezensis have shown some effectiveness in reducing the incidence of F. oxysporum in banana seedlings, under greenhouse conditions (Cao et al., 2018), and especially the incidence of FocR4T in plants of Cavendish banana var. Brazil (Huang et al., 2019). This agrees with that report from Segura-Mena et al. (2021) who mention that the use of beneficial/antagonistic bacteria are part of soil management practices to reduce the incidence and severity of diseases caused by Fusarium.
The results obtained suggest that B. subtilis and B. sonorensis could potentially be evaluated in greenhouse and later in the field, to be considered in the future as part of a management program against FocR2 and against FocR4T, before an eventual introduction of it; either through bacterization practices of plantain vitroplants before their establishment in the field or in the preparation of biofertilizer formulations that could be applied once the crop is established in the field, as has already been reported by Kavino and Manoranjitham (2018) and Huang et al. (2019).
Regarding the inhibition of mycelial growth of FocR2 MIC17188, a previous study showed that the bacterial strains Paenibacillus sp. BSP1.1 and Serratia sp. AC35 exerted an inhibition of 45 and 27% when pre-inoculated one day before the fungal confrontation (Florencio-Anastasio et al., 2022); in the present work, this inhibition increased to 80.1 and 52.3%, respectively, when the bacteria were pre-inoculated in vitro, seven days before the confrontation, which suggests that the longer the bacteria have to establish themselves in the culture medium, the greater the inhibitory effect. This gives consistency to what was suggested by Caballero-Hernández (2011) when recommending the protection of banana plants with antagonistic bacteria applied seven days before the inoculation of the pathogen.
Furthermore, the strain Paenibacillus sp. BSP1.1 has also been effective in inhibiting the mycelial growth of Rhizoctonia sp. (Pineda-Mendoza et al., 2019), this bacterial strain is attractive for its biotechnological use as part of an integrated management program for the biocontrol of Foc, due to the high percentage of inhibition against five isolates of Foc Race “1 or 2” (Florencio-Anastasio et al. 2022). In addition, this bacterium produces auxins and indoles, and solubilizes phosphates, which is why it is considered a good promoter of plant growth and improves seed germination and the growth of Capsicum annum plants (Angulo-Castro et al., 2018; Pineda-Mendoza, 2015; Pineda-Mendoza et al., 2019). Therefore, Paenibacillus sp. BSP1.1 can potentially be used in biological control programs as a “bacterization practice” one week before its establishment in the field to induce greater protection in plantain and banana vitroplants.
Conclusions
The bacterial strain Paenibacillus sp. BSP 1.1, by itself, had the greatest inhibitory effect on the mycelial growth of the FocR2 isolate MIC17188 when pre-inoculated at seven days.
The inhibitory effect of the combined inoculation of the bacterial strains Paenibacillus sp. BSP 1.1 and Serratia sp. AC35 was lower compared to the single inoculation of the Paenibacillus sp. BSP 1.1.











 text in
text in 


