The tomato (Solanum lycopersicum) is the second most consumed vegetable in the world and in Mexico it is the most important in the agri-food sphere (SADER, 2020). Clavibacter michiganensis subsp. michiganensis (Cmm) causes “wilt” and “bacterial canker” and it is the most destructive and recurring disease in the tomato crop. In Mexico, it causes an estimated economic loss of 40 million dollars a year (Borboa-Flores et al., 2009). Cmm is an international quarantine pathogen (EFSA, 2014; EPPO, 2016). Wilt and canker are more severe in indoor production systems, due to the environmental conditions and continuous farming practices that promote infections (Martínez-Castro et al., 2018; Yuqing et al., 2018). Cmm infects the xylem vessels and causes symptoms of wilting, marginal leaf chlorosis and cracks (canker) on the stem (Bae et al., 2015). It is disseminated mainly via seeds (Nandi et al., 2018), the use of infected seedlings, tools, soil and water (Tancos et al., 2013), contact with the guttation fluids between healthy and diseased plants (Sharabani et al., 2013a) and nutrient solutions in hydroponic crops (Huang and Tu, 2001).
The Cmm strains with the most virulence harbor the plasmids pCM1(27.4 kb) and pCM2 (70.0 kb), which codify for the production of polysaccharides (EPS) and extracellular enzymes (Dreier et al., 1997; Gartemann et al., 2008), which confer pathogenicity, different degrees of virulence and aggressiveness between isolated strains from different areas of the world (Wasserman et al., 2020; Yuqing et al., 2018), as well as the ability to adapt to new habitats (Nandi et al., 2018).
To date, no commercial varieties with complete resistance to Cmm have been found or other effective control measures, which causes severe epidemics (Nandi et al., 2018). In other countries, tomato varieties with greater tolerance to the disease have been identified (Eichenlaub and Gatermann, 2011). Wild forms of tomato have Cmm-resistant genes, although the inheritance of these genes is complex and express undesirable traits, such as small fruits and low yields during genetic breeding (Bae et al., 2015; Coaker et al., 2004). Diverse control strategies have been studied, including chemical control, mainly using antibiotics and copper-based compounds (de León et al., 2008; Milijašević et al., 2009), biological control (Nandi et al., 2018), the use of plant extracts (Muhammad et al., 2020), bacteriocins (Mirzaee et al., 2021) with a limited efficacy, in many cases due to the high variation in the aggressiveness and virulence of the pathogen, as well as its adaptation to certain genotypes and tomato production systems (Croce et al., 2016).
In the last five years, in Chignahuapan, Puebla, outbreaks of the disease were identified in greenhouse-grown tomato, and to date, there are no studies on the response to the infection by Cmm in the varieties planted in this region. Our hypothesis establishes that there are tomato varieties with a greater tolerance to infections caused by aggressive Cmm strains and that this pathogen is sensitive to commercial bactericides available in the region. The identification of tomato varieties with a greater tolerance to the infection by Cmm and the use of effective bactericides can contribute to a better management and reduce the economic damage caused by this disease. Due to this, the aims of this investigation were: i) to characterize and identify the more aggressive Cmm in Chignahuapan, Puebla, ii) to evaluate the tolerance in two phenological stages of 10 varieties of tomato to infection by the more aggressive Cmm in the greenhouse and the sensitivity in vitro of the bacteria to bactericides.
Materials and methods
Isolation of the pathogen and growth conditions
In 2019, stems and leaves were gathered in greenhouses from tomato plants of the Benedetti (19° 52’ 11.2” N 98° 02’ 10.7” W), Reserva (19° 52’ 12.9” N 98° 02’ 12.8” W) and Pai Pai (19° 52’ 17.4” N 98° 02’ 15.3” W) varieties with symptoms of wilt in the location of Chignahuapan, Puebla. The tissue samples (0.5 cm) were disinfested with sodium hypochlorite at 1% and washed three times with sterile distilled water. They were then placed in tubes with 3 mL of a sterile salt solution (0.85%) for 1 h. Out of this suspension, 20 µL were plated on nutrient agar culture medium (NA) (Sigma-Aldrich) and incubated at 28 °C for 48 h. The purified bacteria were morphologically identified according to Cmm (EPPO, 2016). The bacteria were preserved in a cryogenic culture at -80 oC in a Luria Bertani medium (Merck) and 40% of glycerol.
Agressiveness of Clavibacter michiganensis subsp. michiganensis
The Cmm isolates (CP_Cmm-1 to CP_Cmm-12) were individually inoculated in three var. Reserva tomato seedlings in a greenhouse in a phenological stage of four true leaves. The inoculation was carried out by cutting the apical leaf with scissors soaked (Thyr, 1968) in a cellular suspension with 3 x 108 UFC mL-1 based on the pattern of turbidity of the McFarland standard (McFarland, 1907). The appearance of wilt symptoms in inoculated plants was recorded for 13 days and the most aggressive isolation was selected (the Cp_Cmm-1 strain in this study) based on the period of incubation (PI), which was considered as the time (days) between inoculation and the appearance of symptoms in the inoculated plants. The control plants were inoculated with sterile distilled water. From the internal tissues of the stem of the plants with symptoms of wilt, bacteria with the same phenotypical characteristics of the inoculated strain were reisolated and identified as Cmm using PCR.
Characterization and identification of Clavibacter michiganensis subsp. michiganensis
Isolation CP_Cmm-1 was characterized according to the protocols described by Schaad et al. (2001). In addition, the API20 E (Biomerieux, Durhan, NC, U.S.A.) systems were used, along with the commercial kit DAS-ELISA (Double Antibody Sandwich Enzyme Linked Immunosorbent Assay) (Agdia® Inc, SRA 44000/0096) with specific antibodies for Cmm. Likewise, a suspension was infiltrated, with 3 x 108 UFC mL-1 of the CP_Cmm-1 strain on the abaxial surface of tobacco (Nicotiana tabacum) and four o’clock plants (Mirabilis jalapa) leaves.
For identification by PCR, the DNA of the CP_Cmm-1 strain was taken from individual colonies with a 72 h growth at 28 °C in NA medium with a PureLink Genomic DNA Kits (Invitrogen Life Technologies, Carlsbad, CA, U.S.A.), following the protocol by the manufacturer. Using PCR, plasmid pCM2 was amplified, which harbors the specific fragment of the gene pat-1 (serine protease) of 614 pb of Clavibacter michiganensis subsp. michiganensis with the primers CMM5F-5´GCGAATAAGCCCATATCAA 3´ and CMM6R-5´ CGTCAGGAGGTCGCTAATA 3´ and the PCR conditions described by Dreier et al. (1997). The PCR was carried out in a thermal cycler (C1000 Touch TM Thermal Cycler) and the amplified fragments were sequenced in the Institute of Biotechnology of the UNAM (IBT). The sequences were compared in the GenBank of the National Center of Biotechnology Information (NCBI), using the algorithm Blastx (Altschul et al., 1990) of the National Center of Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/Blast).
Inoculation of Clavibacter michiganensis subsp. michiganensis in tomato varieties
This study was carried out as a factorial in a randomized complete block design with four repetitions; the first factor used 10 tomato varieties: 1) Benedetti, 2) Reserva, 3) Malvia, 4) USATX12227, 5) Sahel, 6) Pai-Pai, 7) Súper óptimo, 8) Nápoles 9) Sv4401, and 10) Tobalá. After the germination of the seed, the seedlings were transplanted in black plastic bags (2 kg), with a mixture of peat moss, tierra vega and tepezil (2:1:1) and were kept in the greenhouse with a relative humidity of >70 and temperatures between 30 and 33 oC. The plants were irrigated on a daily basis and fertilized twice a week with the Steiner nutrient formulation (Steiner, 1984).
The CP_Cmm-1 strain was inoculated in the apical leaf of each tomato variety (n=10) by cutting with scissors soaked in a cellular suspension (Thyr, 1968) adjusted with 3 x 108 UFC mL-1 using the McFarland standard and confirmed by counting in a plate in an NA medium after 72 h at 28 oC (Klement et al., 1990). The second factor contemplated two phenological stages in independent treatments for the development of 1) five true leaves, 2) 10 true leaves. Each variety was inoculated in 10 plants (repetitions). The inoculation of all varieties was simultaneous and was carried out in a timeframe of 60 min. The control plants were inoculated with sterile distilled water. The appearance of symptoms was registered every day for 30 days after inoculation (dai). In order to evaluate the severity, an arbitrary visual scale was designed, which went from 0 to 4, where 0= healthy plant, 1= leaf wilt, 2= epinasty, leaf chlorosis, 3= stem vessel colonization and 4=canker in stem and internal colonization of the fruit. The variable analyzed was time (number of days to severity degree 4) with the interaction tomato variety-phenological stage-development of symptoms. The data were analyzed using the statistical program R version 4.0.5 (Avello and Seisdedo, 2017). From the internal tissue of the stem of each variety, the inoculated bacteria was reisolated and identified using PCR.
Sensitivity in vitro to bactericides
Eight commercial bactericides were evaluated in the concentration recommended on the label, in a stock with 25 mL of water: 1) Streptomycin sulfate + Oxytetracycline + Tribasic copper sulfate monohydarte, 1.5 g (Cuprimycin 500) (Industrial Engineering); 2) Streptomycin + Oxytetracycline, 0.2 g (Bactrol 2x) (Industrial Engineering); 3) Oxytetracycline Hydrochloride, 0.5 g (Agricultural Cuprimicin 5%) (Industrial Engineering); 4) Streptomycin + Oxytetracycline, 1 g (Agricultural Bactiomycin) (AgriBest); 5) Ammonium Quaternary, 3.5 mL (Quatz IV) (Alterna Agro); 6) Kasugamycine, 0.17 mL (Kasumin) (Gowan Mexicana); 7) Streptomycin sulfate, 0.2 g (Cuprimicin 17) (Industrial Engineering); 8) Gentamicin sulfate + Oxytetracycline Hydrochloride, 1.5 g (Final Bacter) (FormulAbagro of Mexico).
The test for in vitro sensitivity against the CP_Cmm-1 strain was carried out using the method described by Jung et al. (2014), modified: The Clavibacter inoculant was obtained from a pure 48 h culture at 28 °C in nutrient agar medium, and afterwards, followed by the preparation of an aqueous solution with 1 x 108 UFC mL-1. Sensitivity was evaluated on 96-well polystyrene microplates (Thermo Scientific™); each 80 µL well with a nutrient broth medium was added 10 µL of Clavibacter inoculant and 10 µL of the bactericidal solution; the control consisted of the inoculation of Clavibacter in a nutrient broth without bactericide. The microplates were incubated at 28 °C and the absorbance was registered at an optic density (DO) of 600 nm after 0, 12, 24, 48, 60, 72 and 84 h of growth in a Multiskan Ascent V1.24 354-01843 Version-1.9.1 reader. The sensitivity to the bactericides by Cmm was determined by the lowest value of DO in the co-cultivation of the pathogen with the bactericide in regard to the control. A factorial design was carried out with repeated measurements using a mixed model in which experimental blocks and units were random; the model was adjusted with a correlation function without structure between times, the variance heterogeneity between treatments in the model was contemplated, and all treatments were evaluated in triplicate. The data of the interaction of Cmm with the bactericide were analyzed with the InfoStat statistical program, version 1.5 (Infostat Group 2003).
Results and discussion
Characterization and identification of Clavibacter michiganensis subsp. michiganensis
The 12 Cmm isolations incubated in tomato var. Reserva caused wilt, although the period of incubation (PI) was different between the isolations. The symptoms of wilt in the inoculated seedlings were observed in a range of 5 to 12 dai. The CP_Cmm-1 isolation from Clavibacter michiganensis subsp. michiganensis (isolated from the var. Reserva) displayed greater aggressiveness, causing wilt 5 dai (PI), hence its selection for this study. In control plants, no symptoms were observed to develop. These results coincide with reports by Tancos et al. (2013), who displayed differences in virulence between strains of Cmm. Other studies identified haplotypes of Cmm with greater virulence and aggressiveness in some tomato-producing areas than in others and they suggest the existence of types of sequences that adapt to certain regions, tomato genotypes, weather conditions and production systems (Croce et al., 2016; Martínez-Castro et al., 2018; Thapa et al., 2017). This diversity may be caused by the introduction of new strains of Cmm of a different origin, mainly due to seed imports (Yuqing et al., 2018). The phenotypic characterization of the CP_Cmm-1 strain displayed the growth of yellow-orange colonies in a YDC medium, Gram positive, not motile, glucose oxidative metabolism, negative oxidase, which produced acid from mannose although not from mannitol, ribose, sorbitol and inulin, hydrolyze esculin but not casein, use acetate and sodium succinate as carbon sources; these characteristics were previously reported for Clavibacter michiganensis subsp. michiganesis (EPPO, 2016; Schaad et al., 2001).
The phenotype of the CP_Cmm-1 strain coincided by 97% with the API20 E system, and it was positive by serology with the DAS-ELISA system. Using PCR, the fragment of gene pat-1 (serine protease) of 614 pb of plasmid pCM2 of Clavibacter michiganensis subsp. michiganensis was amplified with primers CMM5F and CMM6R. The gene pat-1 (serine protease) confers pathogenicity into Cmm and is an important virulence determinant for the production of extracellular polysaccharides, cellulases, glucanases and other enzymes, which induce diseases in tomato via the degradation of the cell wall in the xylem vessels, causing the symptom of wilt (Eichenlaub and Gatermann, 2011; Wassermann et al., 2020). The comparison of the sequence in the GenBank of the amplified PCR product of the Cp_Cmm-1 strain displayed a 96.6% identity with C. michiganensis subsp. michiganensis NCPPB 382 plasmid pCM2 (accession AM711866.1). The infiltration of the Cp_Cmm-1 strain in the abaxial surface of tobacco and four o’clock leaves induced a hypersensitivity reaction, which coincides with reports by Alarcón et al. (1998) and Lu et al. (2015), who proved the induction of hypersensitivity in these same plant species with the expression of the protein ChpG with virulent Cmm strains. The results of the identification of the Cp_Cmm-1 strain comply with the official protocol for the characterization and identification of Cmm in symptomatic tomato samples, which suggests the combination of biochemical, serological, molecular and pathogenicity tests, according to EPPO (2016).
Response of tomato to Clavibacter michiganensis subsp. michiganensis
The response of the 10 tomato varieties inoculated with the Cp_Cmm-1 strain was different. The inoculation technique of cutting the petiole with scissors soaked in the inoculant is considered quick and adequate for the evaluation of tolerance to the systemic infection by Cmm (van Steekelenburg, 1985). The symptoms and severity of the disease were different throughout time and dependent on the phenological stage (age of the plant and number of leaves). In all varieties but one, the appearance of symptoms was quicker and more severe in the inoculated plants with five than with 10 true leaves. This suggests that the genetic characteristics and the phenological stage of each variety are important factors for the expression of virulence by Cmm. These results suggest that the lower the plant development, the higher the susceptibility and severity of the infection caused by this pathogen will be.
The symptoms in both phenological stages (five and 10 leaves) during and at the end of the evaluation (30 dai) included all the degrees of severity (leaf wilt, chlorosis, vascular infection, canker in the stem and colonization of the fruit) in all varieties. However, the results of the statistical analysis with the interaction of the factors variety of tomato-phenological stage -development of symptoms indicated that the Sahel variety was significantly different to the rest (*= p≤0.05) with the higher average of days (13.7 and 14.5) for the appearance and development of all the symptoms, both in the five leaf and the 10 true leaf stages, respectively. Likewise, this variety displayed the lowest severity of symptoms, suggesting a higher tolerance to the infection by Cmm in both phenological stages than the rest of the varieties. By contrast, varieties Sv4401, Nápoles and Súper óptimo were highly susceptible in the five-leaf stage. Among these, variety Sv4401 displayed the highest susceptibility and severity out of all varieties in the two phenological stages (five and 10 leaves), with the lowest average (5.5 and 9 days, respectively) for the appearance and development of the symptoms (Table 1). In addition, the plants of this variety displayed lower heights, abundant bacterial exudate in petioles, stems with severe canker, and an extensive internal colonization of the fruit.
These results are similar to those obtained in other investigations. In greenhouse trials, Stüwe and Tiederman et al. (2013) identified tomato genotypes that display a higher severity of symptoms caused by Cmm in contrast to others under the same experimental inoculation conditions. Likewise, the results of our study display the difficulty of finding tomato varieties which are tolerant to the infection caused by Cmm. In a study in China, five out of 25 tomato genotypes were catalogued as moderately resistant and none had a high or complete resistance to infection by Cmm (Yuqing et al., 2018). Meanwhile, Kabas et al. (2018), out of 78 genotypes, classified 55% as very susceptible, 39% as susceptible, 4% as moderately resistant and only 2% as tolerant to infection by Cmm.
Table 1 Interaction of variety, phenological stage, days of appearance of symptoms and severity in 10 tomato varieties inoculated with the CP_Cmm-1 strain of Clavibacter michiganensis subsp. michiganensis.
| Inoculación etapa fenológica cinco hojas verdaderas | Inoculación etapa fenológica 10 hojas verdaderas | ||
|---|---|---|---|
| Variedad Jitomate | Media días desarrollo de síntomas | Variedad Jitomate | Media días desarrollo de síntomas |
| Sahel | 13.75 az | Sahel | 14.50 az |
| Tobalá | 10.50 b | Malvia | 14.00 b |
| Benedetti | 10.50 b | USA12227 | 12.75 c |
| Reserva | 10.25 bc | Benedetti | 12.75 c |
| Malvia | 10.00 c | Reserva | 12.50 c |
| Pai-Pai | 9.75 h | Tobalá | 12.50 c |
| USA12227 | 9.75 h | Súper óptimo | 11.75 d |
| Súper óptimo | 8.75 i | Pai-Pai | 11.50 de |
| Nápoles | 7.50 j | Nápoles | 11.25 e |
| Sv4401 | 5.50k | Sv4401 | 9.00 f |
Test: LSD Fisher Alfa=0.05 DMS= 0. 44431. Error: 0.0985 gl: 57.
z Means with a common letter are not significantly different (*= p≤0.05).
Diverse factors explain the severity of the symptoms caused by the Cmm infection among different tomato genotypes, one of which is the age of the plant. Nandi et al. (2018) and Sharabani et al. (2013b) showed that some tomato varieties that become infected in late development stages can be asymptomatic or develop slow wilt. However, in early infections, the bacteria multiply in the xylem vessels and forms biofilms (Lelis et al., 2014), which help it colonize with high densities and cause a systemic infection that originates severe wilt symptoms, stem canker, vascular discoloring and infection of the fruit (Bae et al., 2015; Chalupowicz et al., 2012; Tancos et al., 2013). In this study, these same symptoms were observed in the most susceptible varieties with the inoculation of the Cp_Cmm-1 strain (Figure 1).
Systemic infection in tomato varieties
The systemic infection by Cmm produces the symptoms of leaf wilting and chlorosis, damage in the xylem and infection of the fruit. In this investigation, the 10 varieties inoculated with the Cp_Cmm-1 strain developed all these symptoms, except the Sahel variety. The difference in the severity of the symptoms was evident between the variety with the highest tolerance and the most susceptible ones (Figure 1). The development of the syndrome is related to the infection of highly virulent Cmm strains (Chalupowicz et al., 2017), suggesting that the Cp_Cmm-1 strain, used in this study, could be found in this group.

Figure 1 Severity of symptoms developed 30 dai with Clavibacter michiganensis subsp. michiganensis (strain CP_Cmm-1) in tomato varieties with greater tolerance and susceptibility: A) control, B and C) chlorosis and wilting in leaves, D) systemic infection in the stem xylem, E) canker on the stem, and F) internal fruit colonization.
The systemic infection, leaf wilt and chlorosis, stem canker and fruit infection were more severe in the susceptible varieties of Súper óptimo, USATX12227 and Sv4401, as opposed to the variety with the highest tolerance (Sahel), in which all symptoms were notoriously less severe and there was no fruit infection in both phenological stages. In the susceptible varieties, the stem xylem displayed symptoms ranging from the discoloration of vascular tissues between yellow, light brown and dark brown to the degradation of the stem pith (Figure 1).
The molecular mechanisms related to the response of the tomato plant to the infection by Cmm are complex and many aspects are not completely elucidated. Stüwe and Tiedemann et al. (2013) observed a greater occlusion of the xylem vessels and less tyloses in tomato varieties susceptible to Cmm. Likewise, Eichenlaub and Gatermann (2011) showed that the temperature, variety, plant age and concentration of the inoculant had an influence on the period of incubation and the severity of the disease. The systemic infection by Cmm was related to a high population density (>108 UFC g-1 tissue) on the infected plant (Gartemann et al., 2003), the production of pectate lyase, cellulases and xylanase enzymes that degrade the xylem vessels and cause canker in the stem (Chalupowicz et al., 2012; Gartemann et al., 2008), the induction of proteins related to pathogenesis (PR) by the plant (Savidor et al., 2012), as well as the concentration of ammonium and carboxylic acids in the fluid of the xylem in certain tomato varieties (Bialczyk et al., 2004; Yadeta et al., 2013), response of the plant to concentrations of ethylene induced by the infection of Cmm (Balaji et al., 2008) and antimicrobial compound α-tomatine produced by the plant, which affects the growth of Cmm (Eichenlaub and Gatermann, 2011).
Previous studies with wild tomato suggest that vascular morphology is an important factor in tolerance to Cmm (Coaker et al., 2004). Many of the above factors could explain the higher tolerance of the Sahel variety identified in this investigation, which stood out for the lower severity of vascular infection, stem canker and having no apparent fruit infection. Due to this, future investigations may delve further in the interaction of this variety with Cmm and the genetic bases of the tolerance observed in this study.
Infection in tomato variety fruits
When cutting the fruit transversally, differences were observed in the aggressiveness and severity of the internal colonization and infection of the fruit with more tolerance (Sahel) where the symptoms of wilt and canker were less severe and without any internal colonization of the fruit. On the other hand, the susceptible varieties (Súper óptimo, Nápoles and Sv4401) displayed a greater severity in the symptoms of wilting, damage to the xylem, canker in the stem and an extensive internal colonization of the pathogen in the fruit. The internal tissue in these fruits displayed a discoloration of vascular tissue and degradation of the pith (Figure 1).
These same colonization patterns in fruits are similar to those observed in other investigations (Tancos et al., 2013). The infection of fruits is a result of the extensive colonization of Cmm in the xylem of the plant, where it is dispersed until it penetrates the peduncle and it infects fruits and seeds (de León et al., 2011; Eichenlaub and Gartemann, 2011). In this investigation, in the fruits with a high internal colonization rate in the most susceptible varieties, no external symptoms were observed, making them apparently adequate for marketing. However, they represent an efficient source of inoculant for later growth seasons (Nandi et al., 2018), mainly via the infected seed (de León et al., 2011).
From the internal tissue of the stem with symptoms of degradation and necrosis of the xylem in each variety, we re-isolated, in pure cultivations, cultures with the same morphological characteristics as Cmm. The identity of the inoculated Cp_Cmm-1 strain was confirmed by PCR with the amplification of plasmid pCM2 with the protocol previously described (Figure 2).
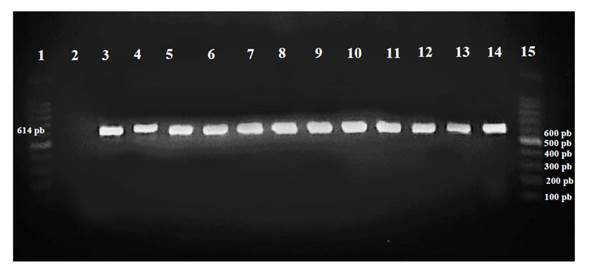
Figure 2 Amplification of the plasmid pCM2 -1 by PCR of the Cp_Cmm-1 strain inoculated in 10 tomato varieties. Lines: 1: 100pb molecular marker, 2: control (-), 3: control (+) 14: control (+), 4: Benedetti, 5: Reserva, 6: Malvia, 7: USATX12227, 8: Sahel, 9: Pai-Pai, 10: Súper óptimo, 11: Nápoles, 12: Sv4401, 13: Tobalá and 15: 100pb molecular marker.
Sensitivity in vitro to bactericides
The results of the sensitivity of the Cp_Cmm-1 strain to the evaluated bactericides displayed significant differences (*= p≤0.05) with the Streptomycin sulfate + Oxytetracycline + tribasic copper sulfate monohydrate (Cuprimycin 500) and Kasugamycin (Kasumin) formulations on the reduction of cell growth. The DO values of the co-cultivation of Clavibacter with these bactericides were significantly lower than in the control, where normality was verified using the Shapiro-wilks test. The percentage of cell growth of Cmm decreased 19 and 18 % with the treatments with streptomycin sulfate + oxytetracycline + tribasic copper sulfate monohydrate (Cuprimycin 500) and kasugamycin (Kasumin) respectively, 84 h after interaction (Figure 3).
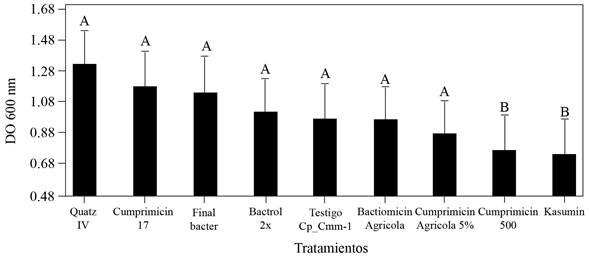
Figure 3 Sensitivity in vitro to Clavibacter michiganensis subsp. michiganensis strain CP_Cmm-1 bactericides. Interaction of the co-cultivation with bactericides after 84 h at 28 o C. Readings of the density of cell growth, absorbance 600 nm (DO). Means with a common letter are not significantly different (*= p≤0.05).
The results coincide with those by de León et al. (2008); Milijašević et al. (2009) and Werner et al. (2002), who reported that copper sulphate, oxytetracycline and kasugamycin is effective against Cmm and reduced the severity of foliar symptoms and damage to the fruit (Theodoro and Maringoni, 2000). Nowadays, the use of bactericides in agriculture is limited, the use of coppers and antibiotics, mainly streptomycin and oxytetracycline, are only authorized for some crops in many countries, and the generation of resistance by Cmm has been proven (Lyu et al., 2019; Valenzuela et al., 2019). Kasugamycin is only used in agriculture and its spectre is reduced (Sundin et al., 2016). Likewise, other investigations have displayed that kasugamycin reduces the populations of vascular bacterial pathogens (Dias et al., 2019; McGhee and Sundin, 2011). Streptomycin has been used to control bacterial diseases in Mexico, reducing the secondary dispersion of inoculant (Félix-Gastélum et al., 2012). The best Cmm control strategy in tomato is based mainly on strict preventive measures and cultural practices that include the use of less susceptible varieties, certified seed, healthy seedlings, the disinfestation of tools and greenhouses, and the use of bactericides that reduce the secondary inoculant of the pathogen (de León et al., 2011; EFSA, 2014; Sundin et al., 2016). The results of this investigation suggest that the combined use of the Sahel variety, kasugamycin, along with the above measures could be a useful strategy to reduce the damage caused by Cmm.
Conclusions
There is variation in the aggressiveness between the strains of Clavibacter michiganensis subsp. michiganensis in Chignahuapan, Puebla. The CP_Cmm-1 strain is the most aggressive of the strains isolated from the three tomato varieties. This strain was identified as Clavibacter michiganensis subsp. michiganensis using biochemical, serological methods and PCR, and it is sensitive to kasugamycin. No tomato varieties out of the 10 planted in this location are resistant to infection by this pathogen. Most varieties are more susceptible to infection in the initial phenological stages of the plant. Among these, there are varieties with a greater tolerance to susceptibility and infection. The Sahel variety is the most tolerant to infection in different phenological stages of the plant. The Sv4401, Nápoles and Súper óptimo varieties are the most susceptible to infection. Out of these, Sv4401 is highly susceptible in different phenological stages. The use of the Sahel variety and kasugamycin could reduce the damage caused by this pathogen.











 text in
text in 


