Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.38 n.3 Texcoco Sep. 2020 Epub Nov 27, 2020
https://doi.org/10.18781/r.mex.fit.2006-1
Phytopathological Notes
Antagonistic bacteria for biospace control of roselle spot (Corynespora cassiicola) of Hibiscus sabdariffa
1 Laboratorio de Microbiología Molecular y Biotecnología Ambiental, , Facultad de Ciencias Químico Biológicas, Universidad Autónoma de Guerrero, Chilpancingo, Guerrero, México;
2 Laboratorio de Patometabolismo Microbiano, Facultad de Ciencias Químico Biológicas, Universidad Autónoma de Guerrero, Chilpancingo, Guerrero, México;
3 Departamento de Agronomía, Facultad de Ciencias Agropecuarias y Ambientales, Universidad Autónoma de Guerrero, Iguala de la Independencia, Guerrero, México;
4 Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, Zacatepec de Hidalgo, Morelos, México;
In recent years, Corynespora cassiicola induced spotted of roselle threatens calyx production. The objective was to determine if some bacterial strains inhibit the development of C. cassiicola in vitro and under biospace conditions. The inhibition of development was due to the dual confrontation of the bacteria: food (20), amphibian skin (41), tailings (22), air (seven) and rhizosphere (10) against the fungus. Plants of jamaican “Creole” variety were sown and artificially inoculated with C. cassiicola. From the beginning of symptoms of the disease, the selected bacterial strains with the highest percentage of inhibition were inoculated. AUDPC was evaluated. Only seven strains inhibited the development of the fungus in vitro from 62.7 to 100%: Klebsiella pneumoniae (M10-1 and M10-10), Acinetobacter lwoffii (A5), Sphingomonas paucimobilis (NF21), Serratia marcescens (M13ACD), S. liquefaciens (M8ACD) and Acinetobacter sp. (5H2). Regarding leaf severity, the M10-1 and M10-10 strains reduced AUDPC by 9.5 and 12.6%, respectively, followed by NF21 (AUDPC 14.5), the control registered the highest levels (AUDPC 45.7). For the severity in calyces, the AUDPC analyzes of all the strains exercised control by reducing severity levels (17.1-8.6 AUDPC).
Key words: biological control; bacterial; phytopathogenic fungus; in vitro
En años recientes, el manchado de la jamaica inducido por Corynespora cassiicola amenaza la producción de cálices. El objetivo fue determinar si algunas cepas bacterianas inhiben el desarrollo de C. cassiicola in vitro y bajo condiciones de bioespacio. La inhibición del desarrollo fue por confrontación dual de las bacterias: alimentos (20), piel de anfibios (41), jales mineros (22), aire (siete) y rizosfera (10) contra el hongo. Se sembraron plantas de jamaica variedad “criolla” y se inocularon artificialmente con C. cassiicola, a partir del inicio de síntomas de la enfermedad se inocularon las cepas bacterianas seleccionadas con mayor porcentaje de inhibición. Se evaluó la ABCPE. Solo siete cepas inhibieron del 62.7 al 100% el desarrollo del hongo in vitro: Klebsiella pneumoniae (M10-1 y M10-10), Acinetobacter lwoffii (A5), Sphingomonas paucimobilis (NF21), Serratia marcescens (M13ACD), S. liquefaciens (M8ACD) y Acinetobacter sp. (5H2). En relación a la severidad en hojas las cepas M10-1 y M10-10 redujeron el ABCPE en un 9.5 y 12.6%, respectivamente, seguido de NF21 (14.5 de ABCPE), el control registró los mayores niveles (45.7 de ABCPE). Para la severidad en cálices, los análisis del ABCPE de todas las cepas ejercieron control al reducir niveles de severidad (17.1-8.6 ABCPE).
Palabras clave: control biológico; bacterias; hongo fitopatógeno; in vitro
In Mexico, roselle (Hibiscus sabdariffa) is an economically important crop; it is cultivated for domestic consumption and for export. The state of Guerrero is the major roselle producer with 14,294 hectares cultivated, which represent approximately 70% of the national area (SIAP, 2019).
In recent years, the disease known as spotted of roselle, induced by the fungus Corynespora cassiicola, has reached 100% incidence and caused the total loss of the crop (Ortega-Acosta et al., 2015; Ortega-Acosta et al., 2019) with severity values higher than 50% for leaves and calyces; at this level of severity, the commercial product (calyces) is usually discarded thus causing economic losses to the producers (Ortega-Acosta et al., 2019; Ortega-Acosta et al., 2020a). In addition to roselle, C. cassiicola causes severe diseases in diverse crops such as cotton (Gossypium hirsutum), soybean (Glycine max), rubber tree (Hevea brasiliensis), among others, and, worldwide, it has been isolated from more than 530 plant species (Nghia et al., 2008; Dixon et al., 2009; Fortunato et al., 2015).
Currently, conventional and alternative agrochemicals are used for controlling C. cassiicola (Vawdrey et al., 2008; Ortega-Acosta et al., 2019; Zhu et al., 2020). However, despite their effectiveness, the frequent use of these substances can cause serious environment and health issues (Hahn 2014; Ghosal and Hati, 2019; Weber and Hahn, 2019). For this reason, one of the alternatives for sustainably controlling plant diseases is the biological control with antagonistic microorganisms in order to limit the development of pathogens or reduce the severity of crop damages (Blakeman and Fokkema, 1982; Köhl et al., 2019).
In previous studies about biological control to reduce disease damages caused by C. cassicola, bacteria such as Pseudomonas flourescens, Bacillus subtilis, B. cereus and B. thuringiensis have been evaluated in cucumber, tomato and rubber trees (Romeiro et al., 2010; Rahman et al., 2010; Manju et al., 2019, Köhl et al., 2019). No studies about the use of biological controls for spotted of roselle caused by the fungus were found. Therefore, the objectives of this study were to determine the ability of bacterial strains to inhibit C. cassiicola growth in vitro and evaluate their control in leaves and calyces under biospace conditions.
For the study, 100 bacterial strains from the biobank of the Molecular Microbiology and Environment Biotechnology Laboratory, Autonomous University of Guerrero, were used; the strains were isolated from diverse environments: food (20), amphibian skin (41), tailings (22), air (7) and rhizosphere (10). For in vitro studies, the C. cassiicola CCHFR strain (GenBank: KM207768), reported by Ortega-Acosta et al. (2015), was used. All the bacterial strains were used in antagonistic in vitro tests based on dual confrontations to select only those able to inhibit the growth of the CCHFR strain; the bacteria were identified through micro- and macroscopic morphology using the Vitek2® automated system (Vargas et al., 2005) for their subsequent evaluation in biological control essays of spotted of roselle under biospace conditions.
The essay to determine the inhibition percent of mycelial development in vitro consisted of a mycelial fragment 1 cm2 in size obtained from a seven-day culture of C. cassiicola (CCHFR), which was placed in the middle of a Petri dish containing agar PDA culture medium (Potato- Dextrose-Agar Difco®); then, the bacteria were inoculated using 6 mm sterile filter paper disks impregnated with 20 µL of a bacterial suspension at a density of 1×108 UFC mL-1, placed at each cardinal point at a distance of 2.5 cm from the fungus (Petatán-Sagahón et al., 2011) and incubated at 25±2 °C for 21 days; disks with sterile distilled water were used as the negative control. The essays in triplicate were applied only to strains with >50% inhibition. Photographs of each essay were taken, and the total area of the mycelial development was obtained using the ImageJ v.1.8.0 software (Cuervo-Parral et al., 2011).
The percent inhibition of the mycelial growth was calculated using the following formula:
Where Dc: Mycelial growth of the control plates, and Dt: Mycelial growth in the Petri dishes treated (Durairaj et al., 2018).
Since in a preliminary essay under biospace conditions, the CCHFR strain had a low level of pathogenicity (data not shown), a sampling in the field was conducted at El Pericón, municipality of Tecoanapa, Guerrero, where roselle leaves with spotted of roselle symptoms were collected in September 2019, following the methodology described by Ortega-Acosta et al. (2015); a pathogenically active C. cassiicola strain called CCPER4 was isolated and identified.
The vegetal material used consisted of three-month-old roselle plants of a “Creole” variety, which were sown in polyethylene pots (15×25 cm) using a mixture of soil and compost (1:1) as substrate. The plants were fertilized twice using a 45-30-20 of NPK formulation (Alejo, 2017). No chemical products were applied for controlling pests and diseases. To evaluate the biological control of C. cassiicola (CCPER4), only the seven bacteria which inhibited ≥50% mycelial development were used; each strain was considered as a treatment with six replications; each replication consisted of four pots with roselle plants (2 plants/pot) arranged in a completely randomized design, and the experiment unit consisted of two central/replication pots. For inoculation, a bacterial suspension of 1×108 UFC mL-1 was prepared and sprayed on three-month-old plants using a manual sprayer on the point of dripping. After 24 h, a suspension of 2×105 conidia mL-1 of C. cassiicola (CCPER4) was prepared and inoculated using a manual sprayer; all the plants were covered with transparent polyethylene bags for 72 h. For the positive control, roselle plants were sprayed only with C. cassiicola (CCPER4) conidia.
To estimate the severity of the disease induced by C. cassiicola (CCPER4) in each treatment, the plants were stratified in three levels (low, intermediate and high) (Villanueva-Couoh et al., 2005), and then four leaves or calyces per stratum were evaluated. To estimate the extent of disease severity, two diagrammatic scales were used for leaves: 0=0, 1= (>0-2 to 4), 2=(>4-7 to 12), 3= (>12-19 to 29), 4= (>29-42 to 57) and 5=(>57-70 to ≤100), and for calyces: 0=0, 1=(>0-3 to 5), 2=(>5-10 to 18), 3=(>18-30 to 46), 4=(>46-63 to 77) and 5=(>77-87 to ≤100) (Ortega-Acosta et al., 2016). The estimation of the severity was evaluated from October to November, for leaves, and from November to December for calyces. Four evaluations were made to leaves and calyces at six-day intervals, according to the phenological stage. The temperature and relative humidity within the biospace were estimated using an Extech® RHT10 digital hygrothermograph.
The data obtained from the antagonism in vitro tests were subjected to an analysis of variance (ANOVA) and a separation of means (Tukey, p=0.01). Using the estimated data of severity in roselle leaves and calyces, the Area Under the Disease Progress Curve (AUDPC) per treatment was estimated based on the method described by Campbell and Madden (1990), followed by an analysis of variance and a separation of means (Tukey, p=0.01). Statistical analyses were performed using SAS v 9.4 statistical software.
Of the 100 strains evaluated, seven that inhibited ≥50% of C. cassiicola (CCHFR) were selected. Of these, the strain Serratia marcescens (M13ACD) showed 100% inhibition (Figure 1, Table 1), and the strains of Acinetobacter lwoffii (A5), Klebsiella pneumoniae (M10-1 y M10-10), Sphingomonas paucimobilis (NF21), Serratia liquefaciens (M8ACD) and Acinetobacter sp. (5H2) had statistical differences compared to the control; these strains were selected for control essays under biospace conditions (Table 1).
The CCPER4 strain produced dense, compact and black-greyish mycelium, and straight-to-curve conidia with short light-brown pseudosepta that measured 57.0-308.2 x 10-20 µm (length x width, respectively), which are distinctive characteristics of C. cassiicola (Qi et al., 2011; Ortega-Acosta et al., 2020b).
The artificial inoculation of C. cassiicola (CCPER4) induced the spotted of roselle disease under biospace conditions. The estimated severity values of AUDPC for spots on roselle leaves and calyces allowed to determine an effect on disease reduction in all the bacterial treatments compared to the control treatment.
However, the effect was differential on leaves depending on the inoculated bacteria (p=0.01), while in calyces, all showed the same effect on the decrease of severity compared to the control treatment (p=0.01). For example, in leaves, the treatments inoculated with the strains M10-1 and M10-10 (K. pneumoniae) had the lowest severity values with 9.5 and 12.6 of AUDPC, while the strains NF21 (S. paucimobilis), A5 (A. lwoffii), 5H2 (Acinetobacter sp.), M13ACD (S. marcescens) and M8ACD (S. liquefaciens) reduced the severity of AUDPC with values of 14.5 to 30.9, and the control treatment had the greatest severity with 42.1 of AUDPC (Table 1).
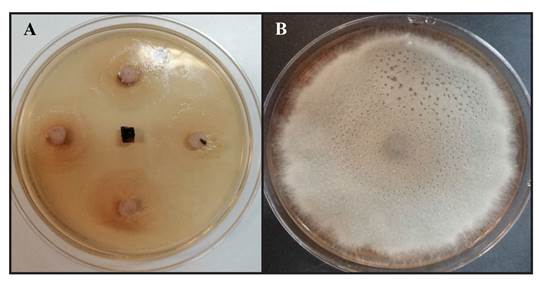
Figure 1. Antagonistic activity in PDA culture medium after 21 days in a culture medium at 27±2 °C. A= Serratia marcescens strain (M13ACD) in C. cassiicola. B= Control treatment (C. cassiicola- CCHFR).
Table 1. Origin and identification of strains with ≥50% antagonistic capacity of inhibition in vitro against C. cassiicola and their effect on severity reduction of spots on roselle leaves and calyces under biospace conditions in Iguala, Guerrero, Mexico.
| Cepa | Origen | Identificaciónw | Inhibición (%) in vitro | Severidad en hojas (ABCPEz) | Severidad en cálices (ABCPE) |
|---|---|---|---|---|---|
| 5H2 | Aire | Acinetobacter sp. | 69.4dy | 26.9bc | 13.6b |
| A5 | Aire | A. lwoffii | 62.7e | 18.8bcd | 9.1b |
| M10-1 | Jales mineros | K. pneumoniae | 66.6ed | 9.5d | 15.0b |
| M10-10 | Jales mineros | K. pneumoniae | 75.2c | 12.6d | 13.7b |
| NF21 | Jales mineros | S. paucimobilis | 80.9b | 14.5cd | 13.1b |
| M13ACD | Piel de anfibios | S. marcescens | 100a | 30.7b | 17.1b |
| M8ACD | Piel de anfibios | S. liquefaciens | 68.0d | 30.9b | 8.6b |
| Controlx | --- | --- | --- | 45.7a | 42.1a |
w Genus and species identified.
x Positive control treatment in trials under biospace conditions.
y Means in the same column followed by the same letter are not significantly different according to Tukey’s test (*= P> 0.01).
z Area Under the Disease Progress Curve.
On the other hand, in calyces, there was a significant decrease in severity in all the bacterial strains with AUDPC values between 8.6 and 17.1 and were statistically similar but different compared to the control with 42.1 of AUDPC (Table 1). The temperature and relative humidity conditions during the evaluations were 28 °C and 67%, respectively.
Based on the evaluation of the 100 bacterial strains, seven had an effect on inhibiting the development of C. cassiicola (CCHFR) in vitro, and significantly reduced the severity of spotted of roselle under biospace conditions. The results indicate that the studied bacteria have the potential to be evaluated in further studies as biological control agents of spotted of roselle under field conditions and inoculum natural pressure.
Under in vitro conditions, S. liquefaciens (M8ACD) inhibited 68% of C. cassiicola (CCHFR) and 100% of S. marcescens (M13ACD), and was the best under in vitro conditions (Table 1); inhibition can be possibly due to the surfactants (serrawatina type) produced by the strains (Shekhar et al., 2015).
Regarding severity on leaves, S. marcescens (M13ACD) and S. liquefaciens (M8ACD) had 30.7 and 30.9 of ABCPE, respectively. These results suggest that the Serratia spp. strains had a better effect in vitro and that this result is similar to that reported by Sabu et al. (2017), who stated that the mentioned genus is able to control Pythium myriotylum in vitro, as well as other pathogens in ginger crops (Zingiber officinale). However, the effect exerted under greenhouse conditions was significantly lower compared to the other strains used (Mustafa et al., 2019).
Regarding K. pneumoniae strains (M10-1 and M10-10), these were the species with the greatest ability to reduce severity on leaves (Table 1), but in calyces the effect was similar to that of the other bacterial isolates. In this regard,, Dey et al. (2019) evaluated the effect of a Klebsiella sp. strain on in vitro and in vivo control of root rot in black bean crops (Vigna mungo), and determined a good control of the disease, and for this reason, the authors recommend its use as a biocontrol agent.
On the other hand, A. lwoffii (A5) inhibited 62.7% of C. cassiicola growth in vitro, which had an effect similar to that exerted by K. pneumoniae strains. However, A. lwoffii reduced the disease severity in leaves with 18.8 of AUDPC, which was statistically similar to the effect exerted by strains of K. pneumoniae (M10-1 and M10-10), S. marcescens (M13ACD) and S. liquefaciens (M8ACD). In calyces, the severity caused by A. lwoffii showed 9.1 of AUDPC, which was similar to the other bacterial treatments (Table 1). In this regard, Trotel-Aziz et al. (2008) mentioned that this bacterial genus is able to induce resistance in grapevine plants (Vitis vinifera) and that this is a way to prevent Botrytis cinerea colonization and, consequently, reduces damage. Under in vitro conditions, S. paucimobilis (NF21) showed 80.9% inhibition. Regarding severity in leaves and calyces, the ABCPE values were 14.5 and 13.1, respectively. Medina-De la Rosa et al. (2016) mentioned that this species had an antagonistic effect on Fusarium sp.
The Acinetobacter sp. (5H2) strain inhibited 69.4% of C. cassiicola and the AUDPC value for severity in leaves was 26.9, which was similar to that of S. liquefaciens (M8ACD); the AUDPC value for severity in calyces was 13.6, which was statistically similar to the other bacterial treatments. These results are in agreement with those reported by other authors, who mention that the use of bacteria exerts control on diseases caused by C. cassiicola (Romeiro et al. 2010; Manju et al., 2019).
No reports of the bacterial species evaluated as biological control of C. cassiicola agents were found, and, therefore, this research shows the first evidences of biological control of C. cassiicola, the causal agent of spotted of roselle in Guerrero, by using and applying bacterial species such as K. pneumoniae, A. lwoffii, S. paucimobilis, S. marcescens and S. liquefaciens, isolated from diverse environments. For this reason, future studies could focus on the evaluation of these bacteria under inoculum natural pressure of the spotted of roselle disease, and also integrate other sustainable control strategies such as the removal of weed reservoirs of C. cassiicola (Hernández-Morales et al., 2018), rotation with chemical alternatives (Ortega-Acosta et al., 2019), among others, in order to decrease the impact of the damage caused by the pathogen in roselle cropping.
For the study, seven bacteria were isolated from air, tailings, and amphibian skin with in vitro antagonistic potential against C. cassiicola, the causal agent of spotted of roselle. The strains of M13ACD (S. marcescens) and NF21 (S. paucimobilis) were the most effective to inhibit the development of C. cassiicola in vitro with 100 and 80.9% inhibition, respectively. Under biospace conditions, M10-1 and M10-10 (K. pneumoniae), were the most effective in reducing the severity of spotted of roselle with AUDPC values of 9.5 and 12.6, compared to the control treatment (45.7 of AUDPC). In calyces, the seven strains selected decreased the levels of disease severity with AUDPC values of 8.6 to 17.1, while the control treatment had the highest levels of severity with an AUDPC value of 42.1.
Acknowledgments
The authors wish to thank Consejo Nacional de Ciencia y Tecnología (CONACYT) for the economic support provided through the scholarship number 883268 and National Mobility’s support, and to Dr. Miguel Ángel Catalán and Dra. Ma. Elena Moreno Godínez for their assistance during the project.
REFERENCES
Alejo, JA. 2017. Jamaica (monocultivo). Pp: 23-29. In: Moctezuma, LG., González, HA., Romero, SME., Pérez, MR., Castillo, MCR. (Eds.). Agenda Técnica Agrícola de Guerrero. INIFAP. 116 p. https://vun.inifap.gob.mx/VUN_MEDIA/BibliotecaWeb/_media/_agendas/4130_4827_Agenda_T%c3%a9cnica_Guerrero_2017.pdf [ Links ]
Blakeman, JP. and Fokkema, NJ. 1982. Potential for biological control of plant diseases on the phylloplane. Annual Review of Phytopathology 20:167-92. https://doi.org/10.1146/annurev.py.20.090182.001123 [ Links ]
Campbell, CL. and Madden, LV. 1990. Introduction to Plant Disease Epidemiology. Jonh Wiley & Sons. New York, USA. 532p. https://doi.org/10.1017/S00074853000 [ Links ]
Cuervo-Parral, JA., Ramírez-Suerol, M., Sánchez-López, V. and Ramírez-Lepel, M. 2011. Antagonistic effect of Trichoderma harzianum VSL291 on phytopathogenic fungi isolated from cocoa (Theobroma cacao L.) fruits. African Journal of Biotechnology 10(52):10657-10663. https://doi.org/10.5897/ajb11.1333 [ Links ]
Dey, S., Dutta, P. and Majumbar, S. 2019. Biological control of Macrosphomina phaseolina in Vigna nungo L. by endophytic Klebsiella pneumoniae HR1. Jordan Journal of Biological Sciences 12(2): 210-227. http://jjbs.hu.edu.jo/files/vol12/n2/Paper%20number%2014.pdf [ Links ]
Dixon, LJ., Schlub, RL., Pernezny, K., Datnoff, LE. 2009. Host specialization and phylogenetic diversity of Corynespora cassiicola. Phytopathology 99(9): 1015-1027. https://doi.org/10.1094/PHYTO-99-9-1015 [ Links ]
Durairaj, K., Velmurugan, P., Park, JH., Chang, WS., Park, YJ., Senthilkumar, P., Choi, KM., Lee, JH. and Oh, BT. 2018. An investigation of biocontrol activity Pseudomonas and Bacillus strains against Panax ginseng root rot fungal phytopathogens. Biological Control 125:138-146. https://doi.org/10.1016/j.biocontrol.2018.05.021 [ Links ]
Fortunato, AA., Debona, D., Bernardeli, AMA. and Rodrigues, FA. 2015. Changes in the antioxidant system in soybean leaves infected by Corynespora cassiicola. Phytopathology 105: 1050-1058. https://doi.org/10.1094/PHYTO-10-14-0283-R [ Links ]
Ghosal, A. and Hati, A. 2019. Impact of some new generation insecticides on soil arthropods in rice maize cropping system. The Journal of Basic and Applied Zoology 80(6). https://doi.org/10.1186/s41936-019-0077-3 [ Links ]
Hahn, M. 2014. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. Journal of Chemical Biology 7(4):133-141. https://doi.org/10.1007/s12154-014-0113-1 [ Links ]
Hernández-Morales, J., Ochoa-Martínez, DL., Ortega-Acosta, SÁ. and Vega-Muñoz, R. 2018. Survey on alternative hosts of Corynespora cassiicola, the cause of the leaf and calyx spot, in the surroundings of roselle fields in Mexico. Tropical Plant Pathology 43: 263-270. https://doi.org/10.1007/s40858- 017-0206-9 [ Links ]
Köhl, J., Kolnaar, R. and Ravensberg, WJ. 2019. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Frontiers in Plant Science 10:845. https://doi.org/10.3389/fpls.2019.00845 [ Links ]
Manju, MJ., Sadananda, M., Santhosh, HM., Roopa, S., Patil, TH., Shankarappa, VI., Benagi, I. and Idicula, P. 2019. Evaluation of different fungi toxicants against Corynespora cassiicola causing Corynespora Leaf Fall (CLF) disease of rubber [Hevea brasiliensis Muell. Arg.,] International Journal of Current Microbiology and Applied Sciences 8(2): 1640-1647. https://doi.org/10.20546/ijcmas.2019.802.193 [ Links ]
Medina-De la Rosa, G., López-Reyes, L., Carcaño-Montiel, MG., López-Olguín, JH., Hernández-Espinosa, MA. and Rivera-Tapia, JA. 2016. Rhizosphere bacteria of maize with chitinolytic activity and its potential in the control of phytopathogenic fungi. Archives of Phytopathology and Plant Protection 49 (12): 310-321. https://doi.org/10.1080/03235408.2016.1201345 [ Links ]
Mustafa, S., Kabir, S., Shabbir, U. and Batool, R. 2019. Plant growth promoting rhizobacteria in sustainable agriculture: from theoretical to pragmatic approach. Symbiosis 78: 115-123. https://doi.org/10.1007/s13199-019-00602-w [ Links ]
Nghia, NA., Kadir, J., Sunderasan, E., Puad, AM., Malik, A. and Napis, S. 2008. Morphological and inter simple sequence repeat (ISSR) markers analyses of Corynespora cassiicola isolates from rubber plantations in Malaysia. Mycopathologia 166: 189-201. https://doi.org/10.1007/s11046-008-9138-8 [ Links ]
Ortega-Acosta, SÁ., Mora-Aguilera, JA., Velasco-Cruz, C., Ochoa-Martínez, DL., Leyva-Mir, SG. and Hernández-Morales, J. 2020a. Temporal progress of roselle (Hibiscus sabdariffa L.) leaf and calyx spot disease (Corynespora cassiicola) in Guerrero, Mexico. Journal of Plant Pathology. https://doi.org/10.1007/s42161-020-00550-1 [ Links ]
Ortega-Acosta, SA., Ochoa-Martínez, DL., Hernández-Morales, J. and Palemón-Alberto, F. 2020b. Morphological and genetic characterization of Corynespora cassiicola isolates obtained from roselle and associated weeds. Mexican Journal of Phytopathology 38(1): 62-78. https://doi.org/10.18781/r.mex.fit.1909-2 [ Links ]
Ortega-Acosta, SA., Velasco-Cruz, C., Hernández-Morales, J., Ochoa-Martínez, DL. and Hernández-Ruiz, J. 2016. Diagrammatic logarithmic scales for assess the severity of spotted leaves and calyces of roselle. Mexican Journal of Phytopathology 34(3): 270-285. https://doi.org/10.18781/r.mex.fit.1606-6 [ Links ]
Ortega-Acosta, SA., Ochoa-Martínez, DL., Leyva-Mir, SG., Velasco-Cruz, C., Mora-Aguilera, JA. y Hernández-Morales, J. 2019. Control químico del manchado de hojas y cálices de jamaica en Guerrero, México. Summa Phytopathologica, 45(1): 38-43. https://doi.org/10.1590/0100-5405/182006 [ Links ]
Ortega-Acosta, SA., Hernández-Morales, L., Ochoa-Martínez, D. and Ayala-Escobar, V. 2015. First report of Corynespora cassiicola causing leaf and calyx spot on roselle in Mexico. Plant Disease 99(7): 1041. https://doi.org/10.1094/pdis-04-14-0438-pdn [ Links ]
Petatán-Sagahón, I., Anducho-Reyes, MA., Silva-Rojas, HV., Arana-Cuenca, A., Tellez-Jurado, A., Cárdenas-Álvarez, IO. and Mercado-Flores, Y. 2011. Isolation of bacteria with antifungal activity against the phytopathogenic fungi Stenocarpella maydis and Stenocarpella macrospora. International Journal of Molecular Sciences 12(9): 5522-5537. https://doi.org/10.3390/ijms12095522 [ Links ]
Qi, Y-X., Zhang, X., J-J, P., Liu, X-M., Lu, Y., Zhang, H., Hui-Qiang, Z., YanChao, L. and Yi-Xian, X. 2011. Morphological and molecular analysis of genetic variability within isolates of Corynespora cassiicola from different hosts. European Journal of Plant Pathology 130:83-95. https://doi.org/10.1007/s10658-010-9734-6 [ Links ]
Rahman, MZ., Khanam, H., Ueno, M., Kihara, J., Honda, Y. and Arase, S. 2010. Suppression by red light irradiation of Corynespora leaf spot of cucumber caused by Corynespora cassiicola. Journal of Phytopathology 158: 378-381. https://doi.org/10.1111/j.1439-0434.2009.01632.x [ Links ]
Romeiro, RS., Lana, R., Macagnan, D., Garcia, F. and Silva, H. 2010. Evidence that the biocontrol agent Bacillus cereus synthesizes protein that can elicit increased resistance of tomato leaves to Corynespora cassiicola. Tropical Plant Pathology 35(1): 011-015. https://doi.org/10.1590/s1982-56762010000100002 [ Links ]
Sabu, R., Aswani, R., Jishma, P., Jasim, B., Mathew, J. and Radhakrishnan, EK. 2017. Plant growth promoting endophytic Serratia sp. ZoB14 protecting ginger from fungal pathogens. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences. https://doi.org/10.1007/s40011-017-0936-y [ Links ]
Shekhar, S., Sundaramanickam, A. and Balasubramanian, T. 2015. Biosurfactant producing microbes and their potential applications: a review. Critical Review in Environmental Science and Technology 45:1522-1554. https://doi.org/10.1080/10643389.2014.955631. [ Links ]
SIAP. 2018. Estadística de Producción Agrícola. Sistema de Información Agrícola y Pesquera. http://infosiap.siap.gob.mx/gobmx/datosAbiertos_a.php. [ Links ]
Trotel-Aziz, P., Couderchet, M., Biagianti, S. and Aziz, A. 2008. Characterization of new bacterial biocontrol agents Acinetobacter, Bacillus, Pantoea and Pseudomonas spp. Mediating grapevine resistance against Botrytis cinerea. Environmental and Experimental Botany 64: 21-32. https://doi.org/10.1016/j.envexpbot.2007.12.009 [ Links ]
Vargas-Jordá, L., Vila, A., Lanza, A., Bonvehi, P., Nazar, J., Mikietuk, A., Labat, R. y Smayevsky, J. 2005. Utilidad del sistema VITEK en la identificación bacteriana y estudios de sensibilidad antimicrobiana Acta Bioquímica Clínica Latinoamericana 39 (1): 19-25. [ Links ]
Vawdrey, LL., Grice, KRE and Westerhuis, D. 2008. Field and laboratory evaluations of fungicides for the control of brown spot (Corynespora cassiicola) and black spot (Asperisporium caricae) of papaya in far north Queensland, Australia. Australasian Plant Pathology 37: 552-558. https://doi.org/10.1071/AP08055 [ Links ]
Villanueva-Couoh, E., Sánchez-Briceño, MA., Cristóbal-Alejo, J., Ruiz-Sánchez, E. y Tún-Suárez, J. M. 2005. Diagnóstico y alternativas de manejo químico del tizón foliar (Alternaria chrysanthemi Simmons y Crosier) del crisantemo (Chrysanthemum morifolium Ramat.) kitamura en Yucatán, México. Revista Mexicana de Fitopatología 23(1): 49-56. https://www.redalyc.org/pdf/612/61223107.pdf [ Links ]
Weber, RWS. and Hahn, M. 2019. Grey mould disease of strawberry in northern Germany: causal agents, fungicide resistance and management strategies. Applied Microbiology and Biotechnology 103(4):1589‐1597. https://doi.org/10.1007/s00253-018-09590-1 [ Links ]
Zhu, J., Zhang, L., Li, T., Ma, D., Gao, Y., Mu, W. and Liu, F. 2020. Baseline sensitivity of Corynespora cassiicola to metconazole and efficacy of this fungicide. Crop Protection 130: 105056. https://doi.org/10.1016/j.cropro.2019.105056 [ Links ]
Received: June 05, 2020; Accepted: August 02, 2020











 text in
text in 


