Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.38 n.1 Texcoco Jan. 2020 Epub Nov 27, 2020
https://doi.org/10.18781/r.mex.fit.1911-2
Phytopathological notes
Isolation, identification and characterization of antagonistic rhizobacteria to Sclerotium cepivorum
1 Centro Universitario de Ciencias Biológicas y Agropecuarias, Universidad de Guadalajara. CP.44600. Camino Ramón Padilla No. 2100. Nextipac, CP. 44600. Zapopan, Jalisco; México
2 Unidad de Biotecnología e Ingeniería Genética de Plantas, Centro de Investigación y de Estudios Avanzados-IPN, Unidad Irapuato. Km 9.6 Libramiento Norte Carretera Irapuato-León, CP. 36820. Irapuato Guanajuato, México.
White rot caused by the fungus Sclerotium cepivorum causes great economic losses in the genus Allium nationwide. In this work, three rhizobacteria with antagonistic effect on this fungus were isolated, identified and characterized. 656 bacteria were isolated in soil samples from the rhizosphere of onion plants (Allium cepa). And in vitro antagonism to S. cepivorum was also evaluated. 23 of these isolates showed antagonistic activity with inhibition halos greater than 5 mm; three of these were greater than 20 mm; these were selected and identified as Gram positive bacilli, belonging to the genus Bacillus, B. amyloliquefaciens and B. subtilis. These rhizobacteria showed enzymatic activity of 1-aminocyclopropane 1-carboxylate deaminase, production of indolacetic acid and siderophores, as well as NaCl tolerance (up to 7.5%). Temperatures of 24 and 37 °C and humidity (50, 75 and 100%) did not affect bacterial development. Of the cell-free extracts obtained in different growth phases, the highest antifungal activity on S. cepivorum was obtained with the stationary phase extracts (16 to 24 h of incubation). Due to the attributes of these rhizobacteria they could be considered as an alternative for the control of S. cepivorum in onion.
Keywords: White rot; Bacillus; siderophores; indolacetic acid; ACC; surviva
La pudrición blanca causada por el hongo Sclerotium cepivorum ocasiona grandes pérdidas económicas en el género Allium a nivel nacional. En este trabajo se aislaron, identificaron y caracterizaron tres rizobacterias con efecto antagonico sobre este hongo. Se aislaron 656 bacterias en muestras de suelo de la rizosfera de plantas de cebolla (Allium cepa). Y se evaluó el antagonismo in vitro a S. cepivorum. 23 de estos aislados mostraron actividad antagonica con halos de inhibición superiores a 5 mm; tres de estos fueron mayores a 20 mm, los cuales se seleccionaron, y se identificaron como bacilos Gram positivos, pertenecientes al género Bacillus, B. amyloliquefaciens y B. subtilis. Estas rizobacterias presentaron actividad enzimática de 1-aminociclopropano 1-carboxilato desaminasa, producción de ácido indolacético y sideróforos, tolerancia a NaCl (hasta 7.5%). Las temperaturas de 24 y 37 °C y humedad (50, 75 y 100%) no afectaron el desarrollo bacteriano. De los extractos libres de células obtenidos en diferentes fases de crecimiento, la mayor actividad antifungica sobre S. cepivorum se obtuvo con los extractos de la fase estacionaria (16 a 24 h de incubación). Por los atributos de estas rizobacterias podrían ser consideradas como una alternativa para el control de S. cepivorum en cebolla.
Palabras clave: Pudrición blanca; Bacillus; sideróforos; ácido indolacético; ACC; sobrevivencia
Sclerotium cepivorum is a fungus that causes the disease known as white rot in the genus Allium (onion, garlic, leek) (Castillo et al., 2016). This disease affects plants in any stage of development and its infection increases as the root system and the bulb grow; it presents sclerotia, which are stuctures of resistance, stimulated by sulfur compounds in the root exudates of the plant, such as aquil cysteine and sulfoxides, which are persistant and easily disseminated (Elshahawy et al., 2017). In Mexico, the disease was found for the first time in Zacatecas in 1990 (Reveles-Hernández et al., 2014). Sclerotium cepivorum causes low quality and yield, and therefore large economic losses. Several chemical products have been used to eradicate this pathogen, including tebuconazole, mancozeb and captan (Hussain et al., 2017). However, due to epidemiological characteristics of the pathogen, after the slcerotia establish themselves in the soil, they can remain there for up to 40 years (Reveles-Hernández et al., 2014). In addition, the inadequate use of fungicides has had a negative impact on the environment and its application is expansive their use is costly. As an alternative for the reduction of these products and to lessen the impact on the environment, native fungi and bacteria have been used to suppress white rot, thanks to their biocontrolling capacity (Vega-Celedón et al., 2016; Kumbhar et al., 2018) and biofertilizers with rhizobacteria (Moreno et al., 2018).
The plant-growth-promoting rhizobacteria (PGPR) found in the rhizosphere promote growth and reduce the incidence of diseases (Glick, 2012; Hussain et al., 2017). These rhizobacteria are classified according to their mechanisms of action; some act on the development of the plant to increase the availability of nutrients with the production of indolacetic acid (IAA), the activity of the enzyme 1-aminocyclopropane-1-carboxylic acid deaminase (ACC deaminase) that regulates the ethylene levels by transforming ACC into α-ketobutyrate and ammonium, auxin-synthesizing phytohormones, among others (Glick, 2012; Esquivel-Cote et al., 2013). Others intervene in the reduction of the harmful effect of phytopathogens through the production of antagonistic substances (tetrasulfides, tiols, tiophenes and sulfur dioxide) and siderophores, that sequester the iron present in the medium, which will therefore be limited for the pathogen (Glick, 2012). This parameter has taken great interest, due to its potential use in the biological control of fungal and bacterial phytopathogens (Aguado-Santacruz et al., 2012). Likewise, PGPR’s are tolerant to salinity conditions and they avoid the stress caused by biotic and abiotic factors (Datta et al., 2011; Esquivel-Cote et al., 2013). thus, the aim of the present study was to evaluate in vitro the potential of rhizobacteria against Sclerotium cepivorum, responsible for white rot in onion.
Six soil samples were collected from the rhizosphere of onion plants in the farm located in Santa Anita, municipality of Tlaquepaque, Jalisco, Mexico. In order to obtain rhizobacterial isolations, we took 10 g of soil adhered to the plant root, diluted them in 90 mL of sterile distilled water, then carried out serial dilutions (10-7) and inoculated 1 mL (three final dilutions) in Petri dishes with agar nutritive (AN), and incubate at 37 °C for 24 h. The PGPR strains were selected and purified according to their morphology, typical of cultures, and preserved to avoid their antagonistic effect. In turn, the isolation of S. cepivorum was carried out following Vimard et al. (1986), from onion sclerotia affected by white rot inoculated in Sabouraud Dextrose Agar (SDA) and incubated at 20 °C. The development of mycelium and sclerotia was observed every day for 12 days (d) (Ortega-Aguilar et al., 2011; Rivera-Méndez et al., 2016).
The antagonism of rhizobacteria was the carried out on S. cepivorum. The fungus was inoculated in SDA broth and a mycelium disc, 5 mm in diameter was extracted and placed in the center of the Petri dish with the same medium, and incubated at 20 °C for 3 d. Next, four repetitions were inoculated in each dish with a streah of the same rhizobacteria around the mycelial disc belonging to the pathogen and incubated at 20 °C. The control was the fungal inoculation in the absence of rhizobacteria; the experiment was concluded when the control fungus totaly covered the Petri dish (Sarti and Miyazaki, 2013). The test was performed in duplicates; the inhibition zone of the rhizobacteria were measured in S. cepivorum and the percentage of inhibition was calculated considering the control as the radial growth of the fungus at 100% .
Moreover, the rhizobacteria with the greatest inhibition on fungus growth were characterized based on their plant growth promoting properties. This consisted of identification based on the colonial and tintorial morphology, and the metabolism by using the VITEX 2 system card (bioMérieuxMR), following manufacturer specifications. The functions of PGPR were characterized by evaluating the presence of the activity of the ACC deaminase, siderophores, tolerance to NaCl and the production of IAA. The enzyme activity was determined by inoculating the bacteria in a minimum salt medium (MM) supplemented with 0.3 g L-1 of ACC, and incubating at 37 °C for 24 h (Luna et al., 2013). In an AN we also determined the tolerance to salinity at different concentrations of NaCl (1.5 to 12%). The ACC desaminase activity and tolerance to NaCl (Sánchez et al., 2016) were recorded as positive in the bacteria that grew in this medium.
IAA production was determined with a colorimetric reaction using Salkowski’s reactive (Glickmann and Dessaux, 1995). The tests were carried out in triplicate on each of the selected isolates; the data were correlated with a standard IAA curve (0 to 30 ppm) and the concentration of IAA of the samples was quantified. The production of siderophores by the PGPR was determined using the chrome azurol S (CAS) medium, according to Schwyn and Neilands (Louden et al., 2011). Likewise, the survey of sensitivity to antibiotics was carried out using the method by Kirby Bauer (Bernal and Gúzman, 1984). Bacterial suspensions were prepared at a concentration of 1.5 × 107 colony forming units (CFU) mL-1 and inoculated in Petri dishes with Mueller Hinton agar. Next, a multidisc® (Bio-Rad) was placed with twelve antimicrobials and incubated at 37 °C for 24 h. The diameter of the inhibition area was measured and the resistance and sensitivity perfiles to the antibiotics were determined.
Furthermore, the bacterial kinetics was carried out for each selected isolate under axenic conditions: 1 mL of pre-inoculum of the bacteria was inoculated at a concentration of 1×108 CFU mL-1 in 50 mL of potato infusion, considered as the starting time (time 0). They were then incubated at 30 °C for a period of 32 h, during which, every 2 h, the absorbance was determined at 595 nm in a spectrophotometer (Jenway model 7305). Two aliquots were taken in each sampling time, one was used to determine the bacterial growth rate (CFU), placing 1 mL of the inoculant in AN dishes, which were incubated at 30 °C and the CFU were quantified every 4 h; the other aliquot was used to obtain Cell Free Extracts (CFE). To obtain the CFE the samples were centrifuged at 11,000 rpm for 10 min and filtered through a 0.22 µm nitrocellulose membrane. The antagonistic effect of the extracts were evaluated using the method of excavation in Petri dished with a SDA medium, according to Mitidieri (1998) as mentioned by Ariza and Sánchez (2012). This test was performed in triplicates in each one of the isolations in a specific obtained time and a control (only with PDA in the absence of CFE). Finally, we determined the survival of the rhizobacteria at different percentages of humidity and at different temperatures. Three treatments were formed with different percentages of humidity (50, 75 and 100%) in 10 g of soil, previously sterilized (autoclaved) and in triplicate; each treatment was inoculated using the Bacillus strains and placed at two different temperatures, 24 and 37 °C, to observe the survival of rhizobacteria to both temperatures and then incubated for 15 d (Arribalzaga, 2007).
The fungus Sclerotium cepivorum and 656 rhizobacteria were isolated from onion plants. The fungus was isolated and identified by its morphological traits described in the taxonomic codes of Sarmiento and Velandía (2013). Out of the isolated bacterial diversity, only 23 isolations presented antagonistic activity and only three of these were larger than 20 mm and the percentages of inhibition of the rhizobacteria against S. cepivorum were between 21 and 24% (Table 1). The three selected isolates were confirmed as spore-forming bacilli using the VITEX 2 system with the reactive card. The biochemical tests were confirmed to be two Bacillus subtilis strains (1 and 2), and the third one was confirmed to be B. amyloliquefaciens, with a reliability of 95%. These characteristics have been reported by other authors for the genus Bacillus (Calvo and Zuniga, 2010).
The antagonistic effect of the Bacillus isolations on S. cepivorum may be due to the possible production of antimicrobial molecules such as lipopeptides that have been reported in rhizobacteria (Sarti and Miyazaki, 2013; Villarreal-Delgado et al., 2018). In 2018, the Fungicide Resistance Action Comittee (FRAC), listed Bacillus subtilis and B. amyloliquefaciens as producers of antifungal substances classified in group F. They act in the transportation or synthesis of lipids, affecting the function and integrity of the cell membranes of pathogens (FRAC, 2018). On the other hand, Sánchez et al. (2016) reported that they are capable of producing iturines and phengicin that cause osmotic imbalances in spores and antifungal activity against filamentous fungi, along with surfactin, which inhibit the spore germination, excretion of antimicrobial enzymes such as β-glucosidases and proteases of fungi that cause leaf and root diseases (Da Silva et al., 2018). Some authors have proven the biocontrolling potential of B. subtilis in the control of the genus Fusarium (Mejía-Bautista et al., 2016), Rhizoctonia solani, Fusarium oxysporum, Sclerotium rolfsii (Paredes-Escalente et al., 2009), and other. In the case of B. amyloliquefaciens, it has antimicrobial capacity and it induces a defensive response in plants (Soto et al., 2018).
Table 1. Biochemical and physiological bacterial properties related with the growth promotion of PGPR of three onion isolates.
| Características | B. subtilis-1 | B. amyloliquefaciens | B. subtilis-2 |
|---|---|---|---|
| Antagonismo (Halos de inhibición en mm)x | 21 ± 0.5b | 23 ± 0.2a | 20 ± 0.6b |
| Porcentaje de inhibición a S. cepivorum (%)x | 22.2 ± 0.5b | 24.2 ± 0.2a | 21.3 ± 0.6b |
| Actividad de ACC desaminasay | Positiva | Positiva | Positiva |
| Tolerancia a NaCl (1.5 a 7.5%)y | Positiva | Positiva | Positiva |
| Producción de Sideróforos (mm)z | 18 ± 0.2a | 15 ± 0.1b | 10 ± 0.1c |
x Values of antagonisms in an average of eight repetitions ± standard deviation,
y Positive = activity or growth,
z Values of production of siderophores in an average of three repetitions ± standard deviation. Different letters in each row represent statistical differences (one way ANOVA, p≤0.05).
The three isolations presented ACC deaminase activity that favors plant growth due to its ability to transform ACC into α-ketobutirate and ammonium (Glick, 2014), as well as to act against pathogens (Latif et al., 2016) and tolerate levels of biotic and abiotic stress (Vurukonda et al., 2016), tolerating concentrations of NaCl of up to 7.5% (1280 mM) (Table 1). This indicates its adaptation to stress caused by salinity (Mahmood et al., 2014); in addition, these Bacillus strains favor water use efficiency (Esquivel-Cote et al., 2013), since they can exert an influence on stress reduction and on the regulation of ethylene biosynthesis (Glick, 2014) with the enzymatic division of the ACC (Jayakumar et al., 2018).
For IAA production, Bacillus subtilis-1 was significantly higler (Figure 1). Luna et al., 2013 reported a production of indoles between 2.3 and 6.8 mg L-1 in four strains of Bacillus. This variation depends on the type of host and Kumar et al. (2015) mention that the genus Bacillus spp. Is a producer of IAA and siderophores. The three Bacillus isolates produced siderophores (Table 1), and this production has been widely documented in Bacillus subtilis and B. amyloliquefaciens (Villarreal-Delgado et al., 2018). These metabolites form an iron complex in which protein receptors specifically recognize each bacterial species; this reduces the availability of the mineral in the soil and a competition takes place for iron, which is lethal for pathogenic bacteria (Glick, 2012), which relates to a defense mechanism in the plants against phytopathogens (Ahemad and Kibret, 2013), as shown in strains evaluated against S. cepivorum in the study. The antimicrobial siderophores are synthesized by lipopeptide of the groups of surfactin, phengicin and iturine, through the non-ribosomal synthetase peptide enzyme. The attachment of these lipopeptide to the membrane causes depolarization, translocation and attacks on the intracellular components, inducing resistance against the pathogen (Jayakumar et al., 2018). These rhizobacteria increase the iron available in the natural habitat (Tejera-Hernández et al., 2011) and helps the plant absorb this mineral to constitute a growth-enhancing mechanism (Gouda et al., 2018).
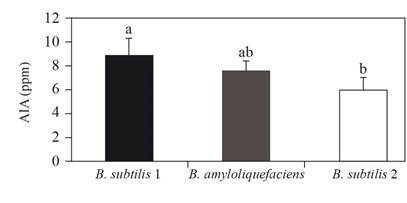
Figure 1. Production of IAA (ppm) with the addition of tryptophan by Bacillus isolates. Values in the average of three repetitions ± standard deviation. Different letters represent statistical differences (one way ANOVA p≤0.05).
Regarding the sensitivity test, the three isolations of the genus Bacillus displayed a sensitivity profile to 30 µg of the antibiotics cephalothin, amikacin, chloramphenicol, ceftriaxone, and resistant to penicillin (10 U). This sensitivity test represents the metabolic activity of each of the bacteria affected by different factors that exert an influence on the promotion of growth and antifungal activity. This ability to transfer the genetic material of the bacterium leads to additional natural resistance mechanisms against certain diseases (Gutiérrez et al., 2017). Antibiotics are currently being applied to the production of crops that may have a negative repercussion on bacterial growth, formation of biofilm, production of indoles and the survival of bacteria in plants (Klein et al., 2017).
In the case of bacterial kinetics, each evaluated isolation displayed similar behaviors in their bacterial development; a phase of constant latency up to 4 h increased its logarithmic development until after 15 h, and finally, the stationary phase continued until after 24 h; this behavior depends on the bacterial strain. The metabolites generated in the stationary phase displayed an inhibiting effect on the fungus, the ELC of a time of 16 hours generated an inhibition of 25% and the 20 and 24 hour extracts displayed a greater effect of up to 35%. This may be related to reports on the genus Bacillus, which, as a part of its metabolism, released toxic products and forms fungal active metabolites such as gramidicin, which were effective in vitro in the control of 23 different phytopathogens (Rodríguez et al., 2017). Some authors have shown that the ELC of Bacillus presented antagonism on Xanthomonas campestris pv. campestris (Da Silva et al., 2018) and Fusarium oxysporum (Rodríguez et al., 2017). Both the isolates and the CFE of the rhizobacteria in the study are related to their antagonistic effect, depending on the metabolic characteristics evaluated and the active metabolites produced against the fungus studied.
Finally, in the case of the study of the survival of the rhizobacteria under different conditions, they proved that in a temperature of 24 °C with a humidity of 50%, B. subtilis presented the highest percentage of survival with 57.5% and the lowest B. amyloliquefaciens with 42.9% (Figure 2). The three rhizobacteria showed that in most of the conditions evaluated, there are no significant differences in the survival rates with regard to temperature and humidity.
These result suggest a good adaptation of these rhizobacteria to the conditions of growth temperature in vitro of the fungus. Some authors mention that there is a great diversity of bacteria able to survive in different environments. Temperature may affect the cell in terms of structure, vital processes of microorganisms, growth rate, chemical composition and enzymatic activity (Calvo and Zúñiga, 2010). However, under the conditions evaluated in this investigation, a similar behavior was observed in the genus Bacillus, and this is related to the characteristics provided by the endospores, resistance structures that survive certain conditions of humidity and temperature, and also promote transcription factors involved in the sporulation process, which favores the process of adaptation and the ability of microbial antagonism (Gouda et al., 2018; Soto et al., 2018).
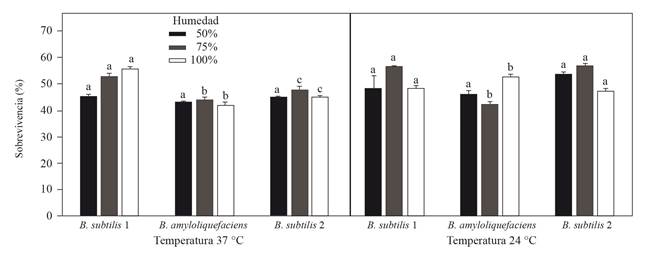
Figure 2. Survival of rhizobacteria to different percentages of humidity (50, 75 and 100 %) and temperatures of 24 and 37 °C. Values in the averages of three repetitions ± standard deviation. Different letters in the same conditions of temperature and humidity represent statistical differences (Kruskal-Wallis p≤0.05).
In conclusion, the rhizobacteria of the genus Bacillus presented in this study and the compounds generated in the stationary phase displayed in vitro antifungal activity against S. cepivorum, along with the functional and metabolical characteristics of PGPR such as ACC activity, tolerance to NaCl (up to 7.5%), bacterial kinetics and survival. They show biologically active strains that have an effect on the pathogens control and the ability to promote plant growth-promoting substances. According to the above, they are a sustainable alternative for the reduction of the impact caused by negative effects to the use of agrochemicals, avoiding high production costs, soil degradation and environmental pollution, along with biofertilizers in the control of S. cepivorum in onion plantations.
Literatura Citada
Aguado-Santacruz, GA., Moreno-Gómez, B., Jiménez-Francisco, B., García-Moya, E. y Preciado-Ortiz, RE. 2012. Impacto de los sideróforos microbianos y fitosideróforos en la asimilación de hierro por las plantas: una síntesis. Revista de Fitotecnia Mexicana 35 (1):9-12 http://www.redalyc.org/articulo.oa?id=61023295002 [ Links ]
Ahemad, M. and Kibret, M. 2013. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. Journal of King Saud Universityz-Science 26:1-20. Doi:10.1016/j.jksus.2013.05.001 [ Links ]
Ariza, Y. y Sánchez, L. 2012. Determinación de metabolitos secundarios a partir de Bacillus subtilis con efecto biocontrolador sobre Fusarium sp. Nova 10(18): 1794-2470. http://www.scielo.org.co/pdf/nova/v10n18/v10n18a01.pdf [ Links ]
Arribalzaga, EB. 2007. Interpretación de las curvas de supervivencia. Revista Chilena de Cirugía 59(1):75-83. Doi:10.4067/S0718-40262007000100013 [ Links ]
Calvo, P. y Zuñiga, D. 2010. Caracterización fisiológica de cepas de Bacillus spp. aisladas de la rizosfera de papa (Solanum tuberosum). Ecología Aplicada 9(1): 31-39. http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1726-22162010000100004 [ Links ]
Castillo, H., Rojas, RR. y Villalta, M. 2016. Actividad antagonista de Gliocladium sp. contra Sclerotium cepivorum. Tecnología en Marcha 57-54. Doi:10.18845/tm.v29i7.2706 [ Links ]
Bernal, RM. y Guzman, UM. 1984. Antibriograma de discos. Normalización de la técnica de Kirby Bauer. Biomedica 4(3-4). https://doi.org/10.7705/biomedica.v4i3-4.1891 [ Links ]
Da Silva, SR., Moutinho, LB., Dos Santos, RD., Vasconcelos-Rodrigues, IS., Talamini, V., Fernandes, FM. and Fernades, MRP. 2018. Using antagonistic soil bacteria and their cell-free filtrates to control the black rot pathogen Xanthomonas campestris pv. campestris. Journal of Phytopathology 166:494-501. Doi:10.1111/jph.12709 [ Links ]
Datta, M., Palit, R., Sengupta, C., Pandit, MK. and Banerjee, S. 2011. Plant growth promoting rhizobacteria enhance growth and yield of chilli (Capsicum annuum L.) under field conditions. Australia. Journal of Crop Science 5(5):531-536. http://www.cropj.com/banerjee_5_5_2011_531_536.pdf [ Links ]
Elshahawy, IE., Saied, NM., Abd-El-Kareem, F. and Morsy, AA. 2017. Field application of Sclerotial micoparasites as biocontrol agents to Stromatinia cepivora, the cause of onion White rot. Journal of Plant Pathology 99(2):391-401. Doi:10.4454/jpp.v99i2.3888 [ Links ]
Esquivel-Cote, R., Gavilanes-Ruiz, M., Cruz-Ortega, R. y Huante, P. 2013. Importancia agrobiotecnológica de la enzima ACC desaminasa en rizobacterias. Una revisión. Universidad Autónoma de México. Revista de Fitotecnia Mexicana 36(3):251-258. http://www.scielo.org.mx/pdf/rfm/v36n3/v36n3a10.pdf [ Links ]
FRAC-Fungicide resistace action committee. 2018. Code List©. pp.1-14. http://www.phi-base.org/images/fracCodeList.pdf (consulta, noviembre 2018). [ Links ]
Glick, RB. 2012. Plant growth promoting bacteria: Mechanisms and applications. Hindawi publishing corporation, Scientifica ID-963401 p.15. Doi:10.6064/2012/963401 [ Links ]
Glick, RB. 2014. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiological Research 169:30-39. Doi:10.1016/j.micres.2013.09.009 [ Links ]
Glickmann, E. and Dessaux, Y. 1995. A critical examination of the specificity of the Salkowski reagent for synodic compounds produced by phytopathogenic bacteria. Applied and Environmental Microbiology 61(2):793-796. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1388360/pdf/hw0793.pdf [ Links ]
Gouda, S., Kerry, RG., Das, G., Paramithiotis, S., Shin, HS. and Patra, JK. 2018. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiological Research 206:131-140. Doi:10.1016/j.micres.2017.08.016 [ Links ]
Gutiérrez, CO., Navarro, ILF., Loeza, LPD., Del Río, ROG. y Jiménez, MR. 2017. Perfiles de resistencia a antibióticos y metales pesados en Pseudomonas aeruginosa potencialmente patógenas aisladas de agua de uso agrícola. Nova Scientia 9(19):97-112. Doi:10.21640/ns.v9i19.957 [ Links ]
Hussain, W., Elzaawely, AA., El Sheery, NI., Ismail, AA. and El-Zahaby, HM. 2017. Biological control of onion white rot disease caused by Sclerotium cepivorum. Environment, Biodiversity & soil Security 1:101-107. Doi:10.21608/JENVBS.2017.1547.1008 [ Links ]
Jayakumar, AK., Krishna, A., Mohan, M., Nair, CI. and Radhakrishnan, EK. 2018. Plant growth enhancement, disease resistance,1. and elemental modulatory effects of pant probiotic endophytic Bacillus sp FcL. Probiotics Antimicrob Proteins 1-9. Doi:10.1007/s12602-018-9417-8 [ Links ]
Klein, JM., Loper, JE. and Stockwell, VO. 2017. Influence of endogenous plasmids on phenotypes of Pantoea vagans Strain C9-1 associated whit epiphytic fitness. Journal of Plant Pathology 99:81-89. Doi:10.4454/jpp.v99i0.3914 [ Links ]
Kumar, GP., Kumar De, T. and Kanti, MT. 2015. Production and etabolism of indole acetic acid in root nodules and simbiont (Rhizobium undicola) isolated from root nodule of aquatic medicinal legue Neptunia oleracea Lour. Journal of Botany 1-11. Doi:10.1155/2015/575067 [ Links ]
Kumbhar, VR., Mane, SR., Birajdar, GM., Bansode, SA., Swami, CS. and Bhale, UN. 2018. Physicochemical characterization and papulation dynamics of mycoflora in infected rhizosphere soil of onion white rot caused by Sclerotium cepivorum. Internationa Journal of current Microbiology and Applied Sciences 7(8):3771-3780. Doi:10.20546/ijcmas.2018.708.384 [ Links ]
Latif, KA., Ahmed, HB., Elyassi, A., Ali, S., Al-Hosni, K., Hussain, J., Al-Harrasi, A. and In-Jung, L. 2016. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solarium lycopersicum. Electronic Journal of Biotechnology 19(3).58-64. Doi:10.1016/j.ejbt.2016.02.001 [ Links ]
Louden, CB., Haarman, D. and Lynne, MA. 2011. Use of blue agar CAS assay for siderophore detection. Journal of Microbiology & Biology Education 12(1):51-53. Doi:10.1128/jmbe.v12i1.249 [ Links ]
Luna, ML., Martínez, PR., Hernández, IM., Arvizu, MSM. y Pacheco, AJR. 2013. Caracterización de rizobacterias aisladas de tomate y su efecto en el crecimiento de tomate y pimiento. Revista Fitotecnia Mexicana 36(1):63-69. http://www.scielo.org.mx/pdf/rfm/v36n1/v36n1a7.pdf [ Links ]
Mahmood, NS., Ahmad, M., Zahir, AZ., Javaid, A. and Ashraf, M. 2014. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnology advances 32(2):429-448. Doi:10.1016/j.biotechadv.2013.12.005 [ Links ]
Mejía-Bautista, MA., Reyes-Ramírez, A., Cristóbal-Alejo, J., Tun-Suárez, M. y Borges-Gómez, LC. 2016. Bacillus spp. en el control de marchitez causada por Fusarium spp. en Capsicum chinense. Revista Mexicana de Fitopatología 34(3):208-222. Doi:10.18781/R.MEX.FIT.1603-1. [ Links ]
Moreno, RA., García, MV., Reyes, CJL., Vázquez, AJ. y Cano, RP. 2018. Rizobacterias promotoras del crecimiento vegetal: una alternativa de biofertilización para la agricultura sustentable. Revista Colombiana de Bioctecnología 20(1): 68-83. Doi:10.15446/rev.colomb.biote.v20n1.73707 [ Links ]
Ortega-Aguilar, BL., Alarcón, A. and Ferrera-Cerrato, R. 2011. Effect of potassium bicarbonate on fungal growth and sclerotia of Sclerotium cepivorum and its interaction with Trichoderma. Revista Mexicana de Micología 33:53-61. http://www.redalyc.org/articulo.oa?id=88319884007 [ Links ]
Paredes-Escalante, JE., Carrillo-Fasio, JA., García-Estrada, RS,, Allende-Molar, R., Sañudo-Barajas, JA. y Valdez-Torres, JB. 2009. Microorganismos antagonistas para el control del complejo de hongos causantes de la rabia del garbanzo (Cicerarietinum L.) en el Estado de Sinaloa, México. Revista Mexicana de Fitopatología 27(1):27-35. http://www.redalyc.org/articulo.oa?id=61211414004 [ Links ]
Reveles-Hernández, M., Velázquez-Valle, R., Reveles-Torres, LR. y Cid-Ríos, JA. 2014. Guía para la producción de cebolla en Zacatecas. Folleto Técnico No. 62. Campo experimental Zacatecas, CIRNOCINIFAP, Calera, Zac., México. 40. http://www.zacatecas.inifap.gob.mx/publicaciones/prodCebolla.pdf [ Links ]
Rivera-Méndez, W., Zúñiga-Vega, C. y Brenes-Madriz, J. 2016. Control biológico del hongo Sclerotium cepivorum utilizado Trichoderma asperellum en el cultivo de ajo en Costa Rica. Tecnología en Marcha 41-50. Doi:10.18845/tm.v29i7.2704 [ Links ]
Rodríguez GCA, Bultrago JE, Betancurt AD y Lara CR. 2017. Actividad antagonista de Bacillus frente a Fusarium oxysporum: un aporte a la agricultura sostenible. Revista Nova 3:9-19. http://revistas.sena.edu.co/index.php/rnova/article/view/1515/1691 [ Links ]
Sánchez LDB, Pérez PJV y David HHA. 2016. Efecto de la PGPB sobre el crecimiento Pennisetum clandestinum bajo condiciones de estrés salino. Revista Colombiana de Biotecnología 18(1):65-72. Doi:10.15446/rev.colmb.biote.v18n1.50413 [ Links ]
Sarmiento, GA. y Velandía, MJ. 2013. Evaluación de hongos y bacterias aislados de gallinaza en biocontrol de Sclerotium cepivorum Berk. Ciencia y Agricultura 10(2):37-43. Doi.10.19053/01228420.2839 [ Links ]
Sarti, GC. y Miyazaki, SS. 2013. Actividad antifúngica de Extractos crudos de Bacillus subtilis contra fitopatógenos de Soja (Glycine max) y efecto de su coinoculación con Bradyrhizobium japonicum. Agrociencia 47(4):373-383. http://www.scielo.org.mx/pdf/agro/v47n4/v47n4a6.pdf [ Links ]
Schwyn, B. y Neilands, JB. 1987. Ensayo químico universal para la detección y determinación de sideróforos. 160(1):47-56. Doi.10.1016/0003-2697(87)90612-9 [ Links ]
Soto, CF., Tramón, PC., Aqueveque, MP., and de Bruijn, J. 2018. Antagonist microorganisms that inhibit the development of post-hasrvest pathogens in lemons (Citrus limon L.). Chilean Journal of Agricultural & Animal Science 34(2):173-184. Doi:10.4067/S0719-38902018005000406 [ Links ]
Vega-Celedón, P., Canchignia, MH., González, M., y Seeger, M. 2016. Biosintesis de ácido indol-3-acético y promoción del crecimiento de plantas por bacterias. Cultivos Topicales. 37: 33-39. Doi:10.13140/RG.2.1.5158.3609 [ Links ]
Villarreal-Delgado, MF., Villa-Rodríguez, ED., Cira-Chávez, LA. y Estrada-Alvarado, MI. 2018. El género Bacillus como agente de control biológico y sus implicaciones en la bioseguridad agrícola. Revista Mexicana de Fitopatología 36(1):95-130. Doi:10.18781/R.MEX.FIT.1706-5 [ Links ]
Vimard, B., Leggett, ME. and Rahe, JE. 1986. Rapid isolation of Sclerotia of Sclerotium cepivorum from muck soil by sucrose centrifugation. The American Phytopathology Society 76(4):465-467. http://www.apsnet.org/publications/phytopathology/backissues/Documents/1986Articles/Phyto76n04_465.PDF [ Links ]
Vurukonda, SS., Vardharajula, S., Shrivastava, M. and SkZ, A. 2016. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiological Research 184: 13-24. Doi:10.1016/j.micres.2015.12.003 [ Links ]
Received: November 04, 2019; Accepted: December 14, 2019











 text in
text in 


