Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.37 n.2 Texcoco May. 2019 Epub Sep 30, 2020
https://doi.org/10.18781/r.mex.fit.1902-5
Phytopathological notes
Ultrastructural changes in blastospores of Taphrina caerulescens in the presence of a susceptible and non-host species
1 Ciencias Biológicas, Departamento de Microbiología,
2 Departamento de Disciplinas Agrícolas. Universidad Autónoma de Aguascalientes, Avenida Universidad #940, Ciudad Universitaria, CP. 20131, Aguascalientes, Aguascalientes. México,
3 Centro de Bachillerato Tecnológica Agropecuaria, Calvillo, Aguascalientes.
Taphrina caerulescens is a fungal pathogen of oak trees, it causes the disease leaf blister in 50 reported Quercus spp. In 2015, the fungus was isolated for the first time from Quercus eduardii, an oak species endemic to the Sierra Fria of Aguascalientes, Mexico. The objective of this study was to document the changes that occur in the inoculum prior to infection of its host as it changes from saprophytic to parasitic. Two host plant species were used: Q. eduardii and Q. potosina and one non-host species Pittosporum tobira. Portions of leaf tissue 24 h, 48 h and 72 h post inoculation with a suspension of T. caerulescens conidia were analysed with a scanning electron microscope. Moderate budding was observed after 24 h on Quercus samples; after 48 h increased budding on both Quercus spp. and the formation of blastospores significantly smaller than the original inoculum was observed on the Quercus eduardii samples. Formation of germ tubes was verified after 48 h on Quercus eduardii. The germinative tubes were observed growing randomly towards the stomata. No significant changes were observed on the surface of the non-host samples. There is no other report of these smaller blastospores in T. caerulescens.
Key words: conidia; germ tubes; infection; Quercus spp.; leaf blister
Taphrina caerulescens es un hongo fitopatógeno de encinos, que causa ampollas en hojas de 50 especies de Quercus. En 2015, el hongo se aisló por primera vez de Quercus eduardii, especie de encino endémica de la Sierra Fría de Aguascalientes, México. El objetivo de este estudio fue documentar los cambios que se producen en el inóculo fúngico antes de la infección de su hospedante a medida que cambia de saprófito a parásito. Se utilizaron dos especies de plantas hospedantes: Q. eduardii y Q. potosina, y una especie no hospedante, Pittosporum tobira. En el microscopio electrónico de barrido se analizaron muestras de hojas 24 h, 48 h y 72 h post inoculación con una suspensión de conidios de T. caerulescens. Se observó una gemación moderada de conidios después de 24 h en muestras de Quercus; después de 48 h la gemación incrementó en las dos especies de Quercus y se observó la formación de blastosporas significativamente más pequeñas, que el inóculo original en las muestras de Quercus eduardii. La formación de tubos germinativos de las blastosporas pequeñas se comprobó después de 48 h en Quercus eduardii. Los tubos germinativos se observaron creciendo de manera aleatoria hacia las estomas. No se observaron cambios significativos en las muestras no hospedantes. No existe otro reporte de blastosporas pequeñas en T. caerulescens.
Palabras claves Conidio; tubos germinativos; infección; Quercus spp.; ampollas de las hojas
The genus Taphrina is a poorly studied ascomycete that belongs to the class Taphrinomycetes. The order Taphrinales includes two families (Protomycetaceae and Taphrinaceae), eight genera and 140 species. Taphrina contains only parasites of vascular plants and causes deformations of plant tissues (Rodrigues and Fonseca, 2003; Fonseca and Rodrigues, 2011).
Taphrina caerulescens is the plant pathogenic fungus responsible for the disease oak leaf blister. Once the young leaves are successfully infected, it stimulates hypertrophy and hyperplasia of the host cells. This abnormal cell growth eventually leads to the formation of raised, irregular lesions on the infected leaves, these lesions can measure from a few millimetres in diameter or deform the entire leaf surface. Oak leaf blister results in an accelerated rate of necrosis and premature leaf senescence and death of infected leaves (Taylor and Birdwell, 2000; Horst, 2008).
In 2015 symptoms of the disease leaf blister caused by T. caerulescens were observed severely affecting Quercus spp. in the Sierra Fría of Aguascalientes, Mexico. This was the first time that the disease was reported in Mexico (Moreno-Rico et al., 2015).
All members of the genus Taphrina are dimorphic with parasitic and saprophytic phases. During the parasitic phase, Taphrina spp. infects leaves at bud break (Rossi and Languasco, 2007). The infective phase are the blastospores (also called conidia), which originate from the ascospores as a result of budding. In T. caerulescens it is not common to observe ascospores within the asci (Nagao and Katumoto, 1998; Fonseca and Rodrigues, 2011). Generally, the blastospores are spherical to ovoid, uninucleate and haploid and once formed they continue to divide, but, if they come into contact with a suitable host it will form a germ tube and infect the host tissue via the stomata (Nagao and Katumoto, 1998; Taylor and Birdwell, 2000).
The objective of this study was to observe the changes that occur in the inoculum (conidia/blastospores) prior to infection of the leaf host as it changes from saprophytic to parasitic of a new isolate found infecting an endemic oak species of Mexico.
The Sierra Fria national park has an area of 1419 km² and rises to an altitude of approximately 2450 meters. The park is located between the following Latitudes: N: 21° 52’ 45” - 23° 31’ 17” and Longitudes W: 102° 22’ 44” - 102° 50’ 53”. Quercus spp. make up the largest community of trees. The Sierra Fria is characterized as having a sub humid temperate climate (Arriaga et al., 2000).
Leaf tissue of Quercus eduardii bearing mature asci of Taphrina caerulescens was fastened to petri dish lids over potato dextrose agar (PDA) (potato extract 4 g, dextrose 20 g, agar 20 g, distilled water 1.0 L, final pH, 5.6±0.2) (Nagao and Katumoto, 1998; Taylor and Birdwell, 2000). Spores were discharged onto the PDA medium within 24 h. The colonies that formed were transferred to fresh media and incubated at 20 °C. The resulting colonies grew as yeast-like cells and exhibited frequent budding. No mycelial development was observed, characteristics consistent with previous reports: yeast-like cells that exhibited frequent budding, with colonies opaque to pale pink in colour, turning a darker shade of pink with age. They were circular with entire margins at the base and viscid in consistency, having a smooth, glistening appearance (Nagao and Katumoto, 1998; Taylor and Birdwell, 2000; Rodrigues and Fonseca, 2003).
The seeds were washed multiple times, first, with tap water containing tween 80% for 5 min, then with a 70% ethanol solution for 45 secs immediately after they are to be rinsed three times with distilled sterilized water and then immersed in a 1.0% sodium hypochlorite solution for 20 min and finally rinsed three more times in distilled sterilized water (Lindsey et al., 2017). The seeds were germinated under aseptic conditions and then transferred to 1.0 L clear sterile containers. To ensure that the soil samples were free from other pathogens it was autoclaved at 121 °C at 1.1 atm (approx. 16 lbs in-1; 1.137 kg cm-1) for a minimum of 15 min, for three consecutive days.
Two inoculation methods were used. The first of these tests was carried out using a modification of the detached leaf method according to Rossi et al. (2006), who realized similar test with Taphrina deformans with leaves of Prunus persica. Two days after the leaves emerged from the buds, these were detached from plants and sterilized in a 1.0% sodium hypochlorite solution for 60 secs, they were then rinsed three times in sterilized distilled water before being placed onto a moist sterile filter paper (Whatman 90 mm diameter) in a sterilize plastic petri dish 90 x 15 mm of the brand Interlux, the leaves were then inoculated on the abaxial or lower surface. A total of five leaves of each plant species were placed in four petri dishes, three of the petri dishes with leaves were inoculated with the suspension of T. caerulescens and the other sample was inoculated with pure sterilized water. This was repeated three times.
The second method the entire plant was used. The plants were grown in one L sterile plastic transparent containers and were approximately six - month-old. Although the plants were grown in aseptic conditions they were treated with the fungicide Copper oxychloride (Cupravit®, Bayer Crop science) according to manufacturer’s instructions, 30 days before tests were carried out. For each experiment four containers containing five plants each were used, three of which were inoculated with the inoculum suspension and the forth with pure sterilized water. The experiment was repeated three times.
The inoculum of T. caerulescens was obtained from an actively growing 14-days isolate which was cultured on PDA médium at 20 °C. The blastospores were suspended in sterile distilled water with 0.01% Tween 80. The final suspension was approximately 1.35’ 109 conidia/mL, this was then atomized onto the lower surfaces two d old detached and attached leaves (Taylor and Birdwell, 2000). In separate containers plants were also inoculated with sterile distilled water. The containers with inoculated seedlings were covered and maintained in a growth chamber (20 °C). One non-hosts species Pittosporum tobira was also inoculated in the same manner with the blastospores suspension. For microscopic analysis samples were taken at 24 h, 48 h, and 72 h post inoculation.
The specimens were analysed at 10 KV and 12 KV using scanning electron microscopy (SEM) with the JSM-35C® (JEOL LTD, Tokyo, Japan) in the Electronic Microscopy laboratory of the Department of Biology of the Autonomous University of Aguascalientes. Each specimen was fixed in 3% glutaraldehyde buffered with 0.1 M sodium phosphate (pH 7.2) for 1.5 h at room temperature, and then processed according to Dykstra and Reuss (2003). The dimensions (length and width) of the original blastospores as well as the secondary blastospores were analysed, the averages. To determine whether or not the dimensions of the two groups of blastospores were similar their averages were compared using the t-Student test. The budding rate of the blastospores after 24 h, 48 h and 72 h and the directional growth of the germination tubes were also analysed.
Scanning electron microscopy revealed that the blastospores on Quercus spp., leaves exhibited moderate budding 24 h post inoculation and increased significantly after 48 h and 72 h on leaves of Q. eduardii (Figures 1 A-B). and Q. potosina (Figures 1 C-D). There were no significant changes observed on the leaves of the non-host species even after 72 h (Figures 1 E-F) no budding was observed. After 48 h, multiple smaller blastospores (Figure 2 A-B) with dimensions 0.87 - 1.77 µm × 0.191 - 0.485 µm were observed on the leaves of Q. eduardii. A descriptive analysis of the measurements produced the following scores (n=30): length: medium = 1.41 µm, s=0.05, SD (standard deviation) = 0.27 µm for the width the values were: medium = 0.34 µm, s=0.01, SD=0.08 µm.
The dimensions of these smaller blastospores were compared to the blastopores which were originally inoculated. The dimensions of the original blastospores were 3.06 - 5.49 µm × 1.09 - 3.01 µm, and a descriptive analysis of the measurements (n=30) were as follows, length: medium = 4.12 µm, s=0.12, SD=0.68 µm for the width the values were: medium = 1.94 µm, s=0.09, SD = 0.50 µm To compare the difference between the two sets of blastospores a t-Student test was carried out. On comparing the width of the secondary blastospores (medium = 0.34 µm) and the width of the original blastospores (medium = 1.94 µm), the difference between the two means was significant, t-Student test = -17.69, p < .005. There was also a statistically significant differences between the length of the secondary blastospores (medium = 1.41 µm) and the original blastospores (medium= 4.12 µm), t-Student test = -23.05, p < .005.
Once formed the secondary blastospores produced germ tubes. The formation of germ tubes was recorded 48 h after inoculation in Q. eduardii. Although many germ tubes grew towards the stomata (Figure 2C) many were observed growing very randomly (Figure 2D). On average the germ tubes measured 1.69 µm after 48 h and 5.18 µm after 72 h.
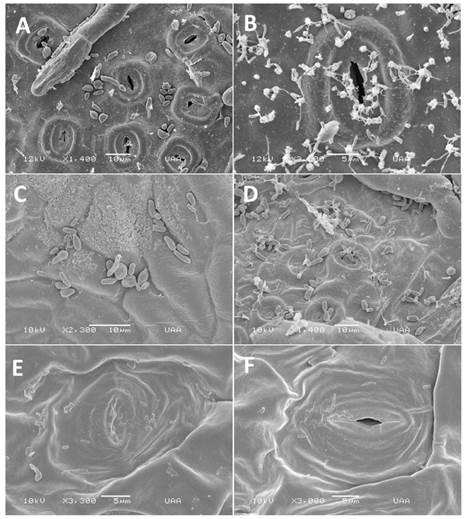
Figure 1 SEM of blastospores on inoculated leaves. A. Blastospores on Q. eduardii 24 h after inoculation. B. Blastospores on Q. eduardii 72 h after inoculation. C. Blastospore development on Q. potosina 24 h after inoculation. D. Blas tospore development on Q. potosina 72 h after inoculation. E. Blastospores on P. tobira 24 h after inoculation. F. Blastospores on P. tobira 72 h after inoculation.

Figura 2 SEM of developing secondary blastospores. A-B Multiple secondary blastospores forming on the surface of the original inoculum (arrows showing secondary blastospores). C. Germination tubes formed on the apical end of the secondary blastospores growing towards the stomata. D. Germination tubes growing in a randomized pattern (arrows showing germination tubes).
Although budding was observed on the surface of Q. potosina after 48 h post inoculation no secondary blastospores were observed there was also no formation of germ tubes. Disease symptoms developed only on the leaves on Q. eduardii in 30% of the plant that were inoculated after one month (Figures 3 A-D), these results are similar to Taylor and Birdwell (2000).
Since the 2000 study of Taylor and Birdwell there has been no other comprehensive study into the interaction between Quercus spp. and T. caerulescens. One of the major differences between the present and the study of Taylor and Birdwell is that, in this present study an isolate of T. caerulescens obtained from Quercus eduardii (white oak) which is endemic to the Sierra Fria, Aguascalientes was used, whereas, the isolate used by Taylor and Birdwell in their experiments was derived from Quercus nigra (red oak) native to the eastern and south-central United States and can be found growing in all the coastal states. Taylor and Birdwell (2000) concluded that after 48 h germ tubes formed, this research confirms their observations that germ tubes formed after 48 h of contact with a suitable host. Many germ tubes grew towards the stomata; however, they were also observed growing arbitrarily (growing over and away from opened stomatal pores). This growth pattern was also described by Taylor and Birdwell (2000), they claimed that germ tubes frequently grew to extensive lengths and that the longer germ tubes growth appeared random rather than directional. The growth pattern exhibited by the germ tubes has led to the hypothesis that the orientation of the germ tubes is being affected by other factors in addition to chemotactic gradients emanating from the stomata (carbon dioxide concentration) however, because of the limited scope of this investigation it was not possible to determine the other factors involved.
Taylor and Birdwell (2000) stated the germ tubes formed from the apical ends of the original cells (conidia/blastospores) that were used to inoculate the leaves. This present study provides a stark contrast to their findings. The isolate from the Sierra Fria behaved in a very distinctive manner, the original conidia first produced a number of significantly smaller cells which were dubbed secondary blastospores. These secondary blastospores were the only cells capable of forming germ tubes, no germ tubes were ever observed originating from the larger blastospores. This investigation presents the first findings of these secondary blastospores.

Figure 3 Formation of leaf blisters on a leaves of Q. eduardii 40 days after being inoculated with T. caerulescens.
Another finding of interest is that although the isolate used was obtained from Q. eduardii, it was capable of multiplying on the surface of Q. potosina, thereby weakening the argument that the T. caerulescens (at least this isolate) is ultra-specific and not capable of growth on another Quercus spp apart from the species that it was isolated from (Taylor and Birdwell, 2000). Researches attributed this new finding to the fact that in Mexico, there is a high degree of hybridization among the endemic species of Quercus spp. (Valencia, 2004) and it is possible that because of this similitude of the genetic information among these species this isolate can grow and possibly infect multiple Quercus spp.
In this study, it was determined that the blastospores of this isolate of T. caerulescens found infecting Q. eduardii of the Sierra Fria of Aguascalientes, were not capable of directly forming germination tubes, instead, the original inoculum formed numerous smaller blastospores which formed germination tubes. This research is the first time that these secondary blastospores have been observed in Taphrina spp. The study also showed that although the blastospores were capable of multiplying on Q. potosina no germination tubes were formed.
Literatura citada
Arriaga L, Espinoza JM, Aguilar C, Martínez E, Gómez L and Loa E. 2000. Regiones Terrestres Prioritarias de México. México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). 609 p. http://bioteca.biodiversidad.gob.mx/janium/Documentos/1036.pdf [ Links ]
Dykstra MJ, and Reuss LE. 1993. Biological electron microscopy: theory, techniques, and troubleshooting. 1st edition. Boston, MA. Springer. USA. 1-73. https://doi.org/10.1007/978-1-4684-0010-6. [ Links ]
Fonseca A and Rodrigues MG. 2011 Taphrina Fries (1832). Pp. 1291-1298. In: Kurtzman CP, Fell JW, Boekhout T (eds.) The Yeasts A Taxonomy Study. Elsevier. USA. 2354 p. [ Links ]
Horst RK. 2008. Westcott’s Plant Disease Handbook. 7th edn. Springer. New York, USA. 295-297. https://doi.org/10.1108/09504120910925832. [ Links ]
Lindsey BE, Rivero L, Calhoun CS, Grotewold E and Brkljacic J. 2017. Standardized Method for High-throughput Sterilization of Arabidopsis Seeds. Journal of Visualized Experiments 128: 1-7. https://doi.org/10.3791/56587. [ Links ]
Moreno-Rico O, Marmolejo-Monsivais JG, Moreno-Manzano CE and Pérez-Hernández K. 2015. Primer reporte de Taphrina caerulescens en encinos (Quercus sp.) de la Sierra Fría de Aguascalientes. Congreso Nacional XVI e Internacional de Fitopatología. México. p. 4. [ Links ]
Nagao H and Katumoto K. 1998. Leaf blister of Quercus phillyraeoides caused by Taphrina caerulescens. Mycoscience 39: 173-178. https://doi.org/10.1007/bf02464056. [ Links ]
Rodrigues MG and Fonseca A. 2003. Molecular systematic of the dimorphic ascomycete genus Taphrina. International Journal of Systematic and Evolutionary Microbiology 53:607-616. https://doi.org/10.1099/ijs.0.02437-0 [ Links ]
Rossi V, Bolognesi M, Languasco L and Giosuè S. 2006. Influence of environmental conditions on infection of peach shoots by Taphrina deformans. Phytopathology 9: 155-163. https://doi.org/10.1094/phyto-96-0155 [ Links ]
Rossi V and Languasco L. 2007. Influence of environmental conditions on spore production and budding in Taphrina deformans, the causal agent of peach leaf curl. Phytopathology 97: 359-365. https://doi.org/10.1094/phyto-97-3-0 [ Links ]
Taylor J and Birdwell DO. 2000. A scanning electron microscopic study of the Infection of Water Oak (Quercus nigra) by Taphrina caerulescens. Mycologia 92: 309-311. https://doi.org/10.2307/3761 [ Links ]
Taylor J and Birdwell DO. 2000. A scanning electron microscopic study of the Infection of Water Oak (Quercus nigra) by Taphrina caerulescens. Mycologia 92: 309-311. https://doi.org/10.2307/3761566. [ Links ]
Valencia S. 2004. Diversidad del género Quercus (Fagaceae) en México. Boletín de La Sociedad Botánica de México 45: 33-53. https://doi.org/10.17129/botsci.1692. [ Links ]
Received: February 28, 2019; Accepted: April 12, 2019











 text in
text in 


