Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.37 n.2 Texcoco May. 2019 Epub Sep 30, 2020
https://doi.org/10.18781/r.mex.fit.1808-7
Phytopathological notes
Reductive disinfestation, soil desiccation and Trichoderma harzianum to control Phymatotrichopsis omnivora in pecan tree nursery
1 Campo Experimental La Laguna INIFAP, Bulevar José Santos Valdez 1200 Pte., Colonia Centro Matamoros, Coahuila, México. C.P. 27440;
2 Universidad Autónoma Chapingo, Unidad Regional de Zonas Áridas. Carretera Gómez Palacio - Ciudad Juárez Km 40, Bermejillo, Durango. C.P. 35230;
3 Campo Experimental La Laguna INIFAP, Bulevar José Santos Valdez 1200 Poniente, Colonia Centro Matamoros, Coahuila, México. C. P. 27440.
In the laboratory, flooded soil with glucose, 0.5-4.0 mg g-1, the pH and potential oxidation-reduction (ORP) reached values of 6.2 and -250 mV, also, we observed up to 22 mmol L-1 of volatile fatty acids (VAFs). The supernatant of the soil was adjusted or not to pH ~ 4, then sclerotia of P. omnivora were submerged in it. The sclerotia died only in supernatants at pH ~ 4 that came from soil with 2.0 and 4.0 mg g-1 of glucose added. In the field, there were six treatments: a control or untreated soil (C); 55 t ha-1 of molasses was added and the ground was covered with plastic (M); inoculated soil with Trichoderma harzianum (T); dried soil (DS) and their combinations DS+M, DS+T and DS+M+ T. Where molasses was added, the pH and ORP reached 6.5 and -200 mV. The pH and ORP reached in soils with glucose and molasses are characteristic of soil reductive disinfestation (RSD). In the field, treatments were applied to the soil, with four plots per treatment and 12 pecan tree seeds were planted per plot. After three years, there was no difference in incidence and mortality of trees caused by P. omnivora, but the roots were invaded by Trichoderma sp.
Key words: soil reductive disinfestation; antagonism; biocontrol
En el laboratorio, suelo adicionado con glucosa 0.5-4.0 mg g-1 e inundado, el pH y el potencial oxidación-reducción (ORP) alcanzaron valores de 6.2 y -250 mV, también observamos hasta 22 mmol L-1 de ácidos grasos volátiles (VAFs). El sobrenadante del suelo se ajustó o no a pH ~ 4, luego los esclerocios de P. omnivora se sumergieron en él. Los esclerocios murieron solo en sobrenadantes a pH ~4 que provenían del suelo con 2.0 y 4.0 mg g-1 de glucosa añadida. En el campo, hubo seis tratamientos: un testigo o suelo no tratado (C); se agregaron 55 t ha-1 de melaza y el suelo se cubrió con plástico (M); suelo inoculado con Trichoderma harzianum (T); suelo desecado (DS) y sus combinaciones DS+M, DS+T y DS+M+T. Cuando se añadió melaza, el pH y el ORP alcanzaron 6.5 y -200 mV. El pH y el ORP alcanzados en suelos con glucosa y melaza son característicos de la desinfestación reductiva del suelo (RSD). En el campo, los tratamientos se aplicaron al suelo, con cuatro (repeticiones) parcelas por tratamiento y se sembraron 12 nueces por parcela. Después de tres años, no hubo diferencia en la incidencia y la mortandad de los árboles causada por P. omnivora, pero las raíces fueron invadidas por Trichoderma sp.
Palabras clave: desinfestación reductiva del suelo; antagonismo; control biológico
In Mexico, the pecan tree Carya illinoinensis (Wangenh.) Koch is a crop established in 112,000 ha, whose value in generating foreign currency was of 330 million dollars in 2015 (Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, 2018). The most important disease of the pecan tree roots is the cotton root rot root caused by the fungus Phymatotrichopsis omnivora (Duggar) Hennebert (Herrera-Pérez and Samaniego-Gaxiola, 2002). A recent method for the control of soil borne plant pathogens is the reductive soil disinfestation (RSD), where a source of carbon (C) such as molasses, wheat straw or compounds rich in carbohydrates are added to the soil, then it is saturated with water and covered with a plastic (Momma et al., 2006). In the RSD, the added residues are decomposed in an anaerobic condition, the pH of the soil solution becomes acidic, the oxidation-reduction potential or ORP (Eh) reaches negative (reductive) values and volatile fatty acids (VFAs), organic sulfur compounds and metal ions Mn2+ and Fe2+ are generated all toxic to fungi, bacteria and weeds (Hewavitharana et al., 2014; Momma, 2008; Momma et al., 2011). The VAFs are toxic to fungi, bacteria and nematodes (Shrestha et al., 2016) and the toxicity increases in acidified media (Samaniego-Gaxiola and Pedroza-Sandoval, 2013). The survival of sclerotia of P. omnivora was null after being immersed in VAFs solutions, as well as in flooded soil with glucose added (Samaniego-Gaxiola, 1994; Samaniego-Gaxiola and Balagurusamy, 2013). However, it has not been registered if soil infested with P. omnivora, flooded and added with glucose induce the changes of the RSD, if the VAFs in the solution of this soil alone or adjusting its pH can kill the sclerotia of the fungus. On the other hand, the sclerotia of P. omnivora are susceptible to die when they are dried or remain in contact with Trichoderma spp. (Samaniego-Gaxiola 2008; Samaniego-Gaxiola et al., 2010). Dried or inoculated soil with Trichoderma harzianum Rifai, could decrease the inoculum of P. omnivora. The RSD control methods, dried the soil and inoculating T. harzianum in soil, could be combined and give better results than individually, to protect the pecan tree from cotton root rot. Therefore, the objectives of this work were: i) to register the pH, ORP, production of VAFs in soil added with glucose and flooded permanently; ii) to evaluate the survival of sclerotia of P. omnivora after remaining in the supernatant coming from this soil, where the pH is adjusted or not to ~ 4; iii) to evaluate the incidence and death of the pecan tree sown in soils treated with RSD using molasses (M), inoculated with T. harzianum (T), dried soil (DS) and its combinations DS+T, DS+M and DS+T+M, and iv) record changes in pH and ORP in treated field soils.
Soil was extracted at a depth of 0-5 cm from the Hormiguero orchard located on the Torreón-San Pedro Km 16 road (25° 41’ 16.3” N 103° 19’ 52.4” W). To eight repetitions (glass flasks) of 1L were added 400 g of soil, then 80 ml of a glucose solution of 0.0, 0.5, 2.0 and 4.0 mg g-1, then 250 ml of water (flooded) and finally the flasks were incubated for five days at 28 °C. From the beginning of the incubation period, 20 ml of the supernatant of each flask were extracted daily. This sample was divided into two groups of vials (each with 10 ml), in both groups pH and ORP were measured, with a multi-parameter HI 9828 brand Hanna Instruments. A group of vials did not have an adjusted pH and the other were added H2SO4 to adjust the pH ~ 4; in the latter case, the amount of acid used to adjust the pH was quantified. In both groups of vials, 25 sclerotia of P. omnivora (per vial) were placed for one hour, and then the pH in the vials was measured. Sclerotia were extracted and germinated in sand. The sclerotia of P. omnivora were reproduced, harvested, managed and germinated as indicated by Samaniego-Gaxiola (2008).
From the second to the fifth day of incubation of the flasks, composite samples of the supernatant were taken, for each dose of glucose-soil 0.0, 0.5, 2.0 and 4.0 mg g-1. Each composite sample of 10 ml was analyzed for organic acids by high pressure liquid chromatography, on an Aminex HPX 87H column, 300 x 7.8 mm, with mobile phase: 5 mM H2SO4, flow conditions of 0.5 mL / min, 50 °C and an analysis of detection by arrangement of diodes at 210 nm. The quantification of the standard curve was obtained by reference standards. The profile of each acid was checked by absorption spectrum and corresponding retention time.
In the Hormiguero orchard, in March of 2012, one control and six randomized treatments were established, each treatment had four repetitions. The repetition was a micro-plot of 3x2 m, where 12 pecan-nut seeds were planted equidistantly. Between plots, towards both ends, a space of four meters of unseeded soil was left. The control treatment (C) consisted in the dry sowing of the walnut and then the application of a 15 cm irrigation sheet, similar irrigation sheet was applied for the rest of the treatments. The desiccated soil (DS) was a treatment where soil was extracted up to 60 cm and dried in the sun to reach a humidity of less than 5%, then the soil was returned to the well and the nuts were sown. The moisture that the soil reached in all treatments (soils with or without desiccation) was determined by taking two samples of 100 g for each depth and repetition; all the samples were weighed before and after drying 48 hours with dry air at 100 °C to determine their moisture content (W/W). The RSD was implemented using molasses (M) at a rate of 33 kg (55 t ha-1), molasses were dissolved in 800 L of irrigation water and poured into the plot, and then the surface of the plot was covered with black plastic polyethylene of 600 µm during 14 d. On the third day after applying the molasses, 100 L of a solution of H2SO4 (23 mmol L-1) was poured to the ground (removing the plastic). The plot was again covered with the plastic during 11 days, then it was uncovered by two weeks to allow aeration, and later the nuts were sown. An isolate of T. harzianum (T) obtained from pecan tree rhizosphere, was used to make an inoculum whose substrate was water, sand and wheat straw as described by Lewis and Papavizas (1984), the amount of inoculum was 3 kg (dry weight) per plot. Three other combined treatments were DS+M, DS+T and DS+M+T, depending on the case, first the soil was dried, then the molasses was added and, finally T. harzianum was applied.
In each plot, on the third day after applying the molasses, eight PVC tubes were vertically buried equidistantly to a depth of 60 cm. On the fourth and seventh days, half of the tubes were extracted, respectively, and cut into segments of 0-20, 20-40 and 40-60 cm, and frozen until the soil analysis. From each soil segment, a 2:1 water-soil extract was made and pH and ORP were measured.
During September to October of each the years 2013-2015, the incidence of cotton root rot and mortality of the pecan tree was evaluated. When the trees showed wilt leaves were considered sick; while dead tress had dried epidermis of branches and stem. Nevertheless, some diseased pecan trees withered and defoliated after October, but their wood was alive and rebounded the following year; at first, one of these trees was considered dead, but their roots were invaded at the same time by P. omnivora and Trichoderma sp. So, the rest of withered pecan trees with their alive epidermis, were not removed and their roots neither were inspected.
A two-way analysis of variance (treatment effects) for repeated measurements or sampling time (rmANOVA) SAS (1999) was applied to the pH and ORP data of the laboratory experiment. In the field experiment, the pH and ORP analysis was an ANOVA for each sampling time, because it came from different samples. The incidence and mortality of pecan trees was analyzed each year separately by an ANOVA. When the statistical analyzes were significant (P<0.001), the mean comparison between treatments was performed with the multiple range test of Duncan means (P=0.05).
A pH of up to 6.2 was reached and maintained in soil amended with 4.0 mg g-1 of glucose, lower doses of glucose did not allow such a low pH or only remained until the second day; the soil without glucose maintained a pH around 8. The standard error of the pH values of the supernatant after placing sclerotia of P. omnivora increased with respect to the supernatant without the sclerotia (Figure 1).
The ORP descended only in soil with glucose as time passed, until reaching values of -50 to - 250 mV, and it remained around 50 mV in the soil without glucose (Figure 2).
The organic acids detected in the supernatants between days two to five from the soils amended with glucose are indicated in Table 1. The total of VAFs detected from the third day of incubation was almost twice (20 against 11 mmol L- 1) in 4.0 versus 2.0 mg g-1 of glucose added in soil; while only <0.2 mmol L-1 was detected from the other organic acids.
The amounts of sulfuric acid to adjust to ~ 4 the pH of the supernatant are shown in Figure 3; only the supernatants obtained from the soils with 2.0 and 4.0 mg g-1 of glucose added exceeded 60 μL and reached near of 180 μL of sulfuric acid.
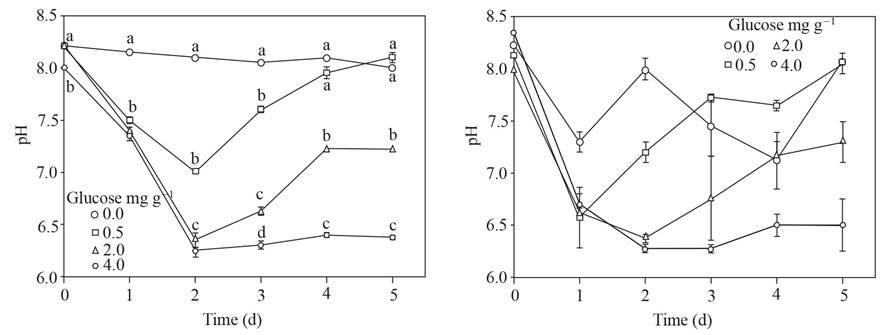
Figure 1 Left, pH of the supernatant obtained from soil with glucose added and flooded up to 5 d. Right, pH of the superna tant after placing 25 sclerotia of P. omnivora for one hour. Standard error bar n=8, the same letters above the bar are not significantly different according to the Duncan media separation test (P>0.05).
The pH values of the supernatants adjusted to pH ~ 4 and those reached in the supernatants where sclerotia of P. omnivora remained are shown in Figure 4; the pH of these last supernatants had values that ranged from about 4.5 to 2.5, in contrast with the pH of the supernatants without sclerotia with pH values of 4.2 to 3.9.
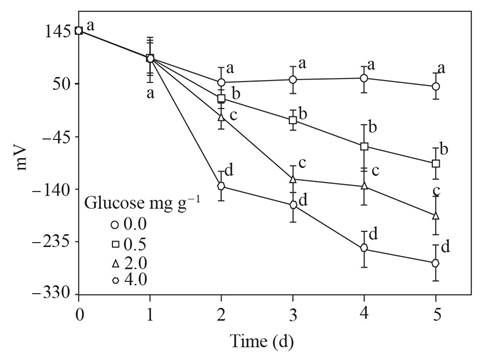
Figure 2 Oxidation-reduction potential (mV) of the supernatant obtained from soil with glucose added and flooded up to 5 d. Standard error bar n=8, the same letters above the bar are not significantly different according to the Duncan media separation test (P>0.05).
The sclerotia of P. omnivora survived between 90 to 100% in the supernatants where the pH was not adjusted, in contrast with a survival of 0 to 20% of the sclerotia that remained one hour in supernatants obtained from soil with 2.0 and 4.0 mg g-1 of glucose added, two days of flooding and which pH was adjusted ~ 4, (Figure 5). The percentage of moisture in the soils where treatments were applied showed that the humidity ranged from 6.8 to 9.6, from 9.6 to 11.1 and from 10.9 to 11.6% in the depths of 0-20, 20-40 and 40- 60 cm in non-dried soils, respectively; while in dried soils the humidity was 1.7 to 3.9%.
In the treatments where molasses was added to the soil, the average pH ranged from 7 to 6.5, but where the molasses was not added was from 7 to 7.5. Average ORP ranges from -20 to -60 mV were observed in soil treatments without molasses, in contrast in soils amended with molasses the ORP ranges were from -120 to -200 mV. The treatments (M) where molasses was added to the soils the minimum pH was 6.4, but where the molasses was not added (C) was 7.3; while in similar treatments, the minimum ORP of -60 mV and -200 mV, respectively (Figure 6).
Table 1 Volatile fatty acids and other organic acids, detected in supernatants obtained from soil with 0.0, 2.0 and 4.0 mg g-1 glucose-soil doses added and flooded up to five days.
| Dose | Day | Acidsz in mmol L-1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Volatile fatty acids | Other organic acids | Total mmol L-1 | |||||||
| mg g-1 | AA | AB | AF | AC | AL | AS | Volatile | Other | |
| 0 | 5 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd |
| 2 | 2 | 4.85 | Nd | 0.93 | 0.01 | 0.02 | Nd | 5.78 | 0.03 |
| 3 | 9.71 | Nd | Nd | Nd | 0.01 | Nd | 9.82 | 0.01 | |
| 4 | 9.68 | 0.14 | Nd | 0.01 | Nd | Nd | 11.00 | Nd | |
| 5 | 11.15 | 0.08 | Nd | Nd | 0.01 | Nd | 11.23 | 0.01 | |
| 4 | 2 | 6.23 | 0.07 | 1.37 | 0.02 | 0.09 | 0.01 | 7.67 | 0.11 |
| 3 | 18.23 | Nd | 0.09 | Nd | Nd | 0.015 | 18.31 | 0.01 | |
| 4 | 20.06 | Nd | 0.07 | 0.01 | 0.04 | 0.051 | 20.12 | 0.10 | |
| 5 | 22.02 | Nd | 0.09 | 0.01 | 0.02 | 0.038 | 22.10 | 0.07 | |
z Ácidos, AA = acético; AB = butírico; AF = fórmico; AC = cítrico; AL = láctico; AS = succínico / z Acids, AA = acetic; AB = butyric; AF = formic; AC = citric; AL = lactic; AS = succinic.
Nd = No detectado / Nd = No detected.
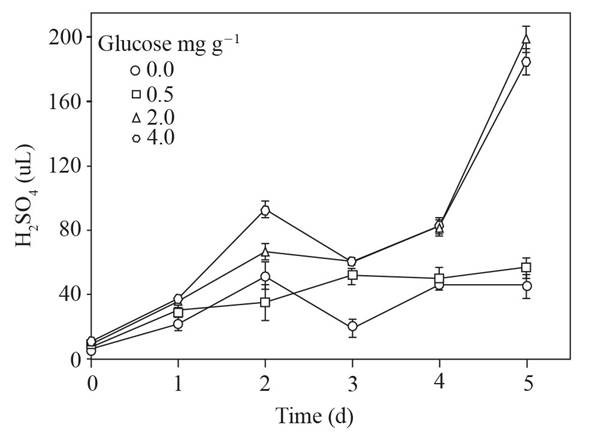
Figure 3 Sulfuric acid used to adjust the pH (~ 4) of the supernatant obtained from soil with glucose added and flooded up to 5 d. Standard error bar n=8.
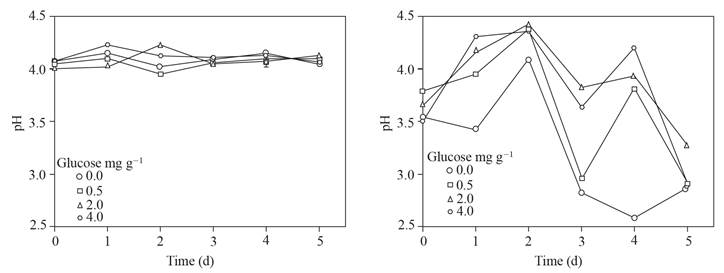
Figure 4 Left, pH adjusted to ~ 4 of the supernatant obtained from soil with glucose added and flooded up to 5 d. Right, pH of the supernatant after placing 25 sclerotia of P. omnivora for 1 h.
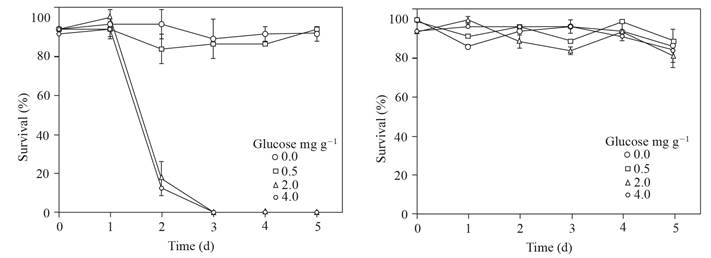
Figure 5 Survival of the sclerotia of P. omnivora in the supernatants, where the pH was not adjusted (right) or where the pH was adjusted to ~ 4 (left). Both supernatants were obtained from soil with glucose added and flooded up to 5 d. Standard error bar n=8.
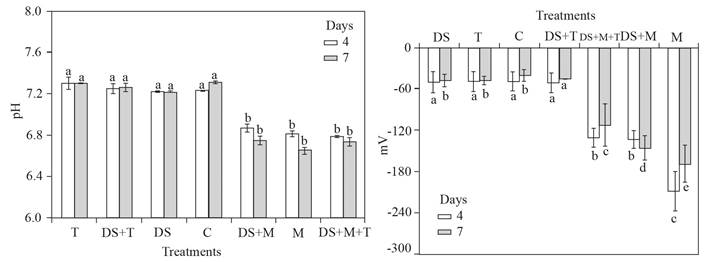
Figure 6 Left, changes in pH and right, ORP change (mV). Both changes after four or seven days for the treatments of with Molasses (M), with T. harzianum (T), dried soil (DS), no treatment or control (C) and combinations (DS+T, DS+M and DS+T+M). Standard error bar n = 4, the same letters above the bar are not significantly different according to the Duncan media separation test (P>0.05).
For each year, there was no significantly difference for incidence and death of pecan trees. In 2013, neither pecan trees were detected with symptoms of cotton root rot, nor any died. For 2014, the incidence of the disease was 2 to 48% and for 2015 was 27 to 69%. The mortality of pecan trees ranged from 2 to 29 and 27 to 54%, in the years 2014 and 2015, respectively. The DS+T+M treatment had the lowest incidence and mortality of pecan trees, with 0, 2, and 27% for the years 2013, 2014, and 2015, respectively.
The RSD can be induced with crop residues, fertilizers or organic materials that provide a source or input of organic C for soil microorganisms; some substrates are as diverse as molasses, glucose, ethanol, bran and cereal straws, canola residues (Brassica napus), grape marc, alfalfa, mustard seeds (Brassica juncea), grass residues, liquid pig manure, among others (Conn et al., 2005; Hewavitharana and Mazzola, 2016; Shrestha et al., 2016).
When C sources are crop residues or green manure, the amounts that are usually used for RSD range from 20 to 40 t ha-1, which allows the control of fungi, bacteria, nematodes and weeds (Katase et al., 2009; Shrestha et al., 2016; Shennan et al., 2014).
Two indicators of the RSD process are changes in pH towards acidity (<7) and ORP towards reductive (-200 mV) (Momma, 2008); these changes were observed in this work, both in soil added with glucose and with molasses. In this work, the amount of C added as glucose (2.0-4.0 mg g-1) necessary to induce the death of the sclerotia of P. omnivora was consistent with previous works (Liu et al., 2016; Samaniego-Gaxiola, 1994) and according to the amount of C (4 mg C g-1) calculated for the control of other plant pathogens in soil (Shennan et al., 2014). The acetic acid in solution necessary to kill more than 50% of sclerotia of P. omnivora in a period of time between 5 and 1 h was from 17 to 33 mmol L-1, respectively (Samaniego-Gaxiola and Balagurusamy, 2013); in contrast, in this study, we observed that 80% of the sclerotia died in 1 h after remaining in the supernatants at pH ~ 4 that contained 5.8 to 22 mmol L-1 of VAFs (Table 1 and Figure 5). This suggests that the supernatants may contain other toxic compounds to sclerotia and that the pH needs to be adjusted so that these compounds increase their toxicity. Other organic compounds such as allyl isothiocyanate, sulfides, disulfides and trisulfides are produced with the RSD using mustard seeds, which have been shown to be antifungal and nematicidal (Hewavitharana et al., 2014); as well as the Fe2+ and Mn2+ ions are equally toxic under RSD conditions for Fusarium oxysporum f. sp. lycopersici (Momma et al., 2011). In concordance with Samaniego-Gaxiola and Pedroza-Sandoval (2013); Tenuta et al. (2002); Tenuta and Lazarovits (2002) the Henderson-Hasselbalch equation predicts the toxicity and chemical forms of the VAFs, nitrous acid and ammonia as a function of pH. Additionally, in Lazarovits et al. (2005) the RSD allowed the control of phytopathogenic fungi if the VAFs, nitrous acid and the ammonia were in a pH ≤ 5 and pH ≥ 8, respectively. The latter, is also in concordance with our results, where the sclerotia only died in the supernatants where the pH was adjusted ~ 4.
Three times more sulfuric acid was needed to adjust to the pH ~ 4 of the supernatants with 0.0 and 0.5 than with 2 and 4 mg g-1 of glucose added in soil (Figure 3) which points to the formation of compounds with the ability to buffer pH, in addition to the VAFs. Likewise, the changes in pH observed in the solutions where the sclerotia remained (Figure 4) suggest that they respond to the modification of the pH from their environment, which has been previously reported (Samaniego-Gaxiola, 2013).
In the field, soils with or without molasses showed differences in pH and the ORP (Figure 6). Although, during the first year there was no symptom of the disease in the pecan trees, in the following years we observed the opposite, possibly due to the colonization of the roots by P. omnivora outside the treated soils and the restoration of a favorable condition for it. In the pecan trees from the orchard Hormiguero, during the three years of our field work none of the adult pecan trees died, however, in a nursery around our workplace, 179 (11% of 1504) pecan trees died by the cotton root rot (Samaniego-Gaxiola et al., 2014). Which suggests that the Phymatotrichopsis is widely distributed in the soil of Hormiguero orchard, but the adult pecan tree tolerates its attack and survives and the young tree does not. This would explain why the adult trees from the Hormiguero orchard did not die during the three years of this study. Although, this work did not have the scope to assess the role of Trichoderma spp. in orchard soils, the colonization of Trichoderma over P. omnivora strands, and tree roots was evident, in contrast, the pecan tree death of pecan trees for the third year was 27-54% (Figure 7) well above the 11% indicated, which suggests that none of our treatments had effect in protecting the pecan tree from P. omnivora.
In La Laguna, Mexico, in soil of pecan tree orchards, it is common to find species of Trichoderma (Samaniego-Gaxiola y Chew-Madinaveitia, 2007) and even on the roots or in the rhizosphere (unpublished). Although, there was no significantly difference in any year for incidence and death of pecan trees, Trichoderma treatments had the lowest incidence of the disease (Figure 7). It is possible that T. harzianum used in this work or other native Trichoderma, become antagonists of P. omnivora, permanently colonize the soil and roots or pecan tree acquire resistance to the attack of the phytopathogen; although these relationships were as beyond the scope of this work and it is still under investigation, it was evident the presence of T. harzianum in the soil and on the roots of the pecan tree, which was initially considered as dead (Figure 8).
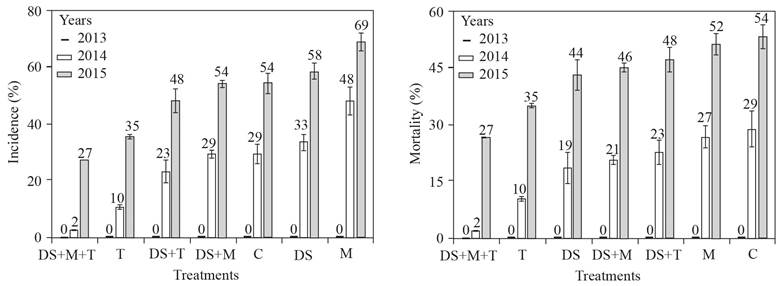
Figure 7 Figure 7. Incidence of cotton root rot (left) and tree mortality (right) in the treatments applied to the Molasses soil (M), with T. harzianum (T), dried soil (DS), no treatment or control (C) and the combinations (DS+T, DS+M and DS+M+T). Standard error bar n=4, number above the bar indicates the percentage of incidence and death of pecan trees.
Shrestha et al. (2013) found that after the RSD, sclerotia of Sclerotium rolfsii Sacc. were colonized between 93-94% by Trichoderma spp. natives of the soil. Consequently, fungi and antagonistic bacteria of phytopathogenic fungi in the soil could be established after the RSD, which is a potential benefit of this method. This idea is supported by studies, where RSD eliminated pathogens and modified soil biota by favoring antagonists of the phytopathogens and the beneficial nutrient cycle for crop growth (Huang et al., 2015). Although, the input of C used in the RSD and subsequent establishment of crops, will determine the type of dominant microbiota in the soil (Huang et al., 2016); that is, the addition of sources of C in the RSD, induces a change of microbiota in the soil, but this also depends on both the C source and the native microbiota of the crop that will be established. An additional modality of the RSD is to use several substrates as a source of C for the control of a single pathogen (Phytophthora nicotianae) (Serrano-Pérez et al., 2017).
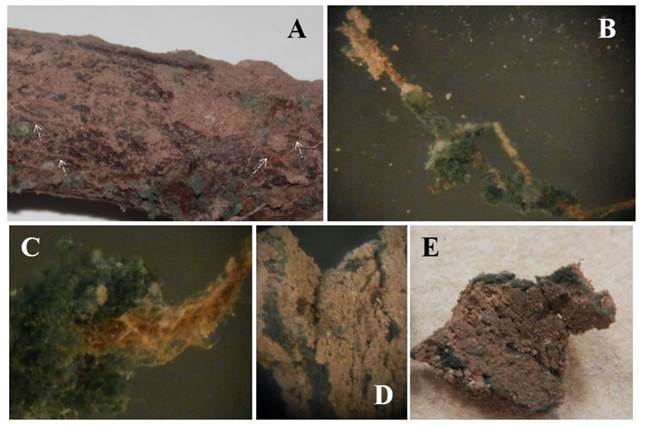
Figure 8 Trichoderma sp. on pecan tree root, strands of P. omnivora and in soil. A. Strands on roots (white arrows), green ar eas are Trichoderma sp. over strands and roots. B and C. Trichoderma sp. wrapping the strands. D and E. Strands wrapped by Trichoderma sp. inside the cracks of the soil.
According to Liu et al. (2018) after RSD it was possible to reduce the populations of Fusarium oxysporum f. sp. niveum in soil cultivated with watermelon (Citrullus lanatus (Thunb.) Matsum and Nakai) also, the wilt caused by F. oxysporum was significantly reduced; but the populations of the pathogen were restored when watermelon was planted in a second cycle, the disease was reestablished and the increase of other crop pathogens such as Monosporascus was favored. In our case, P. omnivora possibly recolonizes the soil from the second year, where the strands were evident on the roots of the pecan tree.
Acknowledgment
To the fund SAGARPA-CONACYT by financing through the key project 2011-13-175247. Also, to Daniela Samaniego Castruita for revising the manuscript and its version in English.
REFERENCES
Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (Sistema de Centros Públicos de Investigación CONACYT). 2018. La nuez pecanera en México. Disponible en línea: https://centrosconacyt.mx/objeto/nuez-pecanera/ [ Links ]
Conn KL, Tenuta M and Lazarovits G. 2005. Liquid swine manure can kill Verticillium dahliae microsclerotia in soil by volatile fatty acid, nitrous acid, and ammonia toxicity. Phytopathology 95(1):28-35. http://dx.doi.org/10.1094/PHYTO-95-0028 [ Links ]
Herrera-Pérez T y Samaniego-Gaxiola JA. 2002. Enfermedades del nogal. Pp. 177-206. En: Arreola, AA y Reyes JI (eds). Tecnología de Producción del Nogal Pecanero. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP), Campo Experimental La Laguna. Matamoros, Coahuila, México. 220 p. http://biblioteca.inifap.gob.mx:8080/jspui/bitstream/handle/123456789/1967/Tecnologia%20de%20produccion%20de%20nogal%20pecanero.pdf?sequence=1 [ Links ]
Hewavitharana SS, and Mazzola M. 2016. Carbon source-dependent effects of anaerobic soil disinfestation on soil microbiome and suppression of Rhizoctonia solani AG-5 and Pratylenchus penetrans. Phytopathology 106(9):1015-1028. http://dx.doi.org/10.1094/PHYTO-12-15-0329-R [ Links ]
Hewavitharana SS, Ruddell D, and Mazzola M. 2014. Carbon source-dependent antifungal and nematicidal volatiles derived during anaerobic soil disinfestation. European Journal of Plant Pathology 140(1):39-52. http://dx.doi.org/10.1007/s10658-014-0442-5 [ Links ]
Huang X, Liu L, Wen T, Zhu R, Zhang J and Cai Z. 2015. Illumina MiSeq investigations on the changes of microbial community in the Fusarium oxysporum f. sp. cubense infected soil during and after reductive soil disinfestation. Microbiological research 181:33-42. https://doi.org/10.1016/j.micres.2015.08.004 [ Links ]
Huang X, Liu L, Wen T, Zhang J, Wang F and Cai Z. 2016. Changes in the soil microbial community after reductive soil disinfestation and cucumber seedling cultivation. Applied microbiology and biotechnology 100(12):5581-5593. DOI 10.1007/s00253-016-7362-6 [ Links ]
Katase M, Kubo C, Ushio S, Ootsuka E, Takeuchi T and Mizukubo T. 2009. Nematicidal activity of volatile fatty acids generated from wheat bran in reductive soil disinfestation. Nematological Research 39(2):53-62. https://swfrec.ifas.ufl.edu/docs/pdf/veg-hort/asd/Nematicidal_activity_volatile_fatty_acids_SD.pdf [ Links ]
Lazarovits G, Conn KL, Abbasi PA and Tenuta M. 2005. Understanding the mode of action of organic soil amendments provides the way for improved management of soilborne plant pathogens. Acta Horticulturae 698:215. https://doi.org/10.17660/ActaHortic.2005.698.29 [ Links ]
Lewis JA and Papavizas GC. 1984. A new approach to stimulate population proliferation of Trichoderma species and other potential biocontrol fungi introduced into natural soils. Phytopathology 74(10):1240-1243. https://www.apsnet.org/publications/phytopathology/backissues/Documents/1984Articles/Phyto74n10_1240.pdf [ Links ]
Liu L, Chen S, Zhao J, Zhou X, Wang B, Li Y, Zheng G, Zhang J, Cai Z and Huang X. 2018. Watermelon planting is capable to restructure the soil microbiome that regulated by reductive soil disinfestation. Applied Soil Ecology 129:52-60. https://www.researchgate.net/profile/Jun_Zhao33/publication/325028296_Watermelon_planting_is_capable_to_restructure_the_soil_microbiome_that_regulated_by_reductive_soil_disinfestation/links/5b2c7922a6fdcc8506bc861e/Watermelon-planting-is-capable-to-restructure-the-soil-microbiome-that-regulated-by-reductive-soil-disinfestation.pdf [ Links ]
Liu L, Kong J, Cu, H, Zhang J, Wang F, Cai Z and Huang X. 2016. Relationships of decomposability and C/N ratio in different types of organic matter with suppression of Fusarium oxysporum and microbial communities during reductive soil disinfestation. Biological Control 101:103-113. http://dx.doi.org/10.1016/j.biocontrol.2016.06.011 [ Links ]
Momma, N. 2008. Biological soil disinfestation (BSD) of soilborne pathogens and its possible mechanisms. Japan Agricultural Research Quarterly 42(1):7-12. https://www.jstage.jst.go.jp/article/jarq/42/1/42_7/_pdf [ Links ]
Momma N, Kobara Y and Momma M. 2011. Fe2+ and Mn2+, potential agents to induce suppression of Fusarium oxysporum for biological soil disinfestation. Journal of General Plant Pathology 77(6):331-335. http://dx.doi.org/10.1007/s10327-011-0336-8 [ Links ]
Momma N, Yamamoto K, Simandi P and Shishido M. 2006. Role of organic acids in the mechanisms of biological soil disinfestation (BSD). Journal of General Plant Pathology 72(4):247-252. http://dx.doi.org/10.1007/s10327-006-0274-z [ Links ]
Samaniego-Gaxiola JA. 1994. Viabilidad de los esclerocios de Phymatotrichum omnivorum (Shear) Dugg. en suelos inundados y complementados con glucosa. Revista Mexicana de Fitopatología 12(1):125-133. [ Links ]
Samaniego-Gaxiola, JA. 2008. Efecto del pH en la sobrevivencia de esclerocios de Phymatotrichopsis omnivora Dugg Hennebert II expuestos a Tilt y Trichoderma sp. Revista Mexicana de Fitopatología 26(1):32-39. http://www.redalyc.org/html/612/61226106/ [ Links ]
Samaniego GJA. 2013. Supervivencia de los esclerocios de Phymatotrichopsis omnivora en función del pH in vitro. Revista Mexicana de Ciencias Agrícolas 4(3):337-351. http://www.redalyc.org/service/redalyc/downloadPdf/2631/263127575001/1 [ Links ]
Samaniego-Gaxiola, JA and Balagurusamy N. 2013. Survival of soil-borne fungus Phymatotrichopsis omnivora after exposure to volatile fatty acids. Journal of General Plant Pathology 79(2):105-109. https://link.springer.com/article/10.1007/s10327-013-0436-8 [ Links ]
Samaniego-Gaxiola JA y Chew-Madinaveitia Y. 2007. Diversidad de géneros de hongos del suelo en tres campos con diferente condición agrícola en La Laguna, México. Revista Mexicana de Biodiversidad 78(2):383-390. http://revista.ib.unam.mx/index.php/bio/article/view/407/377 [ Links ]
Samaniego-Gaxiola JA, Fontes-Puebla AA, Tarango-Rivero SH y Pedroza-Sandoval A. 2014. Comportamiento de la Pudrición Texana (Phymatotrichopsis omnivora) en Vivero de Nogales. Revista mexicana de fitopatología 32(1):26-37. http://rmf.smf.org.mx/Vol3212014/Articulos/ComportamientodelaPudricionTexana.pdf [ Links ]
Samaniego GJA, Ordóñez MHJ, Pedroza SA y Cueto-Wong C. 2010. Relationship between the drying of the sclerotia of Phymatotrichopsis omnivora and its survival. Revista Mexicana de Micología 32(1):49-58. http://www.redalyc.org/pdf/883/88319899006.pdf [ Links ]
Samaniego-Gaxiola JA y Pedroza-Sandoval A. 2013. Usos potenciales de los ácidos grasos volátiles en suelo, agua y aire. Terra Latinoamericana 31(2):155-163. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0187-57792013000300155 [ Links ]
Serrano-Pérez P, Rosskopf E, De Santiago A and Rodríguez-Molina MC. 2017. Anaerobic soil disinfestation reduces survival and infectivity of Phytophthora nicotianae chlamydospores in pepper. Scientia horticulturae 215:38-48. http://dx.doi.org/10.1016/j.scienta.2016.12.003 [ Links ]
Shennan C, Muramoto J, Lamers J, Mazzola M, Rosskopf E N, Kokalis-Burelle N, Momma N, Butler DM and Kobara Y. 2014. Anaerobic soil disinfestation for soil borne disease control in strawberry and vegetable systems: current knowledge and future directions. In VIII International Symposium on Chemical and Non-Chemical Soil and Substrate Disinfestation 1044:165-175. https://doi.org/10.17660/ActaHortic.2014.1044.20 [ Links ]
Shrestha U, Ownley BH, Rosskopf E N, Dee ME and Butler DM. 2013. Optimization of amendment C: N ratio in anaerobic soil disinfestation for control of Sclerotium rolfsii. In Proceedings of Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, SanDiego, CA. 14-1. https://www.researchgate.net/profile/Utsala_Shrestha/publication/309479087_OPTIMIZATION_OF_AMENDMENT_CN_RATIO_IN_ANAEROBIC_SOIL_DISINFESTATION_FOR_CONTROL_OF_SCLEROTIUM_ROLFSII/links/581248b108ae1f5510c2a2cd/OPTIMIZATION-OF-AMENDMENT-CN-RATIO-IN-ANAEROBIC-SOIL-DISINFESTATION-FOR-CONTROL-OF-SCLEROTIUM-ROLFSII.pdf [ Links ]
Shrestha U, Augé RM and Butler DM. 2016. A meta-analysis of the impact of anaerobic soil disinfestation on pest suppression and yield of horticultural crops. Frontiers in Plant Science 7 article 1254:1-20. http://dx.doi.org/10.3389/fpls.2016.01254 [ Links ]
Statistical Analysis System (SAS Institute). 1999. SAS. [ Links ]
Tenuta M, Conn KL and Lazarovits G. 2002. Volatile fatty acids in liquid swine manure can kill microsclerotia of Verticillium dahliae. Phytopathology 92(5):548-552. https://apsjournals.apsnet.org/doi/pdf/10.1094/PHYTO.2002.92.5.548 [ Links ]
Tenuta M and Lazarovits G. 2002. Ammonia and nitrous acid from nitrogenous amendments kill the microsclerotia of Verticillium dahliae. Phytopathology 92(3):255-264. https://apsjournals.apsnet.org/doi/pdfplus/10.1094/PHYTO.2002.92.3.255 [ Links ]
Received: August 28, 2018; Accepted: March 28, 2019











 text in
text in 


