Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.37 n.2 Texcoco May. 2019 Epub Sep 30, 2020
https://doi.org/10.18781/r.mex.fit.1812-2
Scientific Articles
Chitosan and Pseudomonas fluorescens extracts for Alternaria alternata control in tomato (Solanum lycopersicum)
1 Laboratorio de Biotecnología Alimentaria, Unidad Profesional Interdisciplinaria de Biotecnología (UPIBI). Instituto Politécnico Nacional (IPN). Avenida Acueducto s/n, Barrio La Laguna Ticomán, Ciudad de México, CP 07340, México;
2 Departamento de Tecnología Postcosecha, Centro de Desarrollo de Productos Bióticos (CeProBi). IPN. Km 8.5 Carretera Yautepec-Jojutla, San Isidro, Yautepec CP 62731, Morelos, México.
Alternaria alternata is a fungus that causes damage to the tomato crop, it is characterized by producing black spots and wilting in plants and fruits; synthetic fungicides are the main tools to control this fungus. The objective of this study was to evaluate the effect of the application of mixtures of chitosan and extracts of Pseudomonas fluorescens in the control in vitro of A. alternata on mycelium and conidia, as well as the incidence and severity in greenhouse tomato plants. The use of the mixture of chitosan 1.5% (w/v) + extract of P. fluorescens 50% (v/v) resulted in 60 and 100% of in vitro inhibition of mycelial growth and conidia germination of A. alternata respectively. In greenhouse, the plants were inoculated with A. alternata, later they were sprayed with the mixture of chitosan 1.5% (w/v) + extract of P. fluorescens 50% (v/v) every 7 days until flowering. The incidence was 100%, while the severity was 51.8 and 38.9% for 7 days and 16.9 and 16.2% for 60 days, respectively. The mixture used is an option for the control of A. alternata.
Key words: antifungal activity; biological control; incidence; severity
Alternaria alternata es un hongo que ocasiona daños en el cultivo de jitomate, se caracteriza por producir manchas negras y marchitez en plantas y frutos; los fungicidas sintéticos son las principales herramientas para controlar este hongo. El objetivo del estudio fue evaluar el efecto de la aplicación de mezclas de quitosano y extractos de Pseudomonas fluorescens en el control de A. alternata bajo condiciones in vitro sobre micelio y conidios, así como la incidencia y severidad en plantas de jitomate en invernadero. El uso de la mezcla de quitosano 1.5% (p/v) + extracto de P. fluorescens 50% (v/v) resultó en 60 y 100% de inhibición in vitro de crecimiento micelial y germinación de conidios de A. alternata respectivamente. En invernadero, las plantas se inocularon con A. alternata, posteriormente se asperjaron con la mezcla de quitosano 1.5% (p/v) + extracto de P. fluorescens 50% (v/v) cada 7 días hasta la floración. La incidencia fue del 100 %, mientras que la severidad fue 51.8 y 38.9 para 7 días y 16.9 y 16.2% para 60 días, respectivamente. La mezcla utilizada es una opción para el control de A. alternata.
Palabras clave: actividad antifúngica; control biológico; incidencia; severidad
Mexico is one of the ten countries in the world with the highest production of tomato (Solanum lycopersicum). The crop satisfies the internal demand, and approximately 46% of the harvest is exported (FAO, 2016). Diverse phytopathogenic microorganisms may cause diseases in the tomato crop, and particularly fungi (Orberá et al., 2014). The genera which cause the greatest losses in pre- and postharvest are Alternaria, Botrytis, Penicillium, Colletotrichum and Rhizopus (Petriacq, 2018; Trigos et al., 2008). Their effects range from hardly visible symptoms to the devastation of complete plantations (Dean et al., 2012; Strange and Scott, 2005). Damages by Alternaria are common around the world and characteristically display black spots and wilting that affects leaves, stems, flowers and fruits (Agrios, 1997; Logrieco et al., 2009). Synthetic fungicides are the main tools for the control and management of plant diseases; however, excessive use produces important health and environmental problems, which has led to a demand for the management of agrochemical-free crops and foods by consumers (Sánchez-Bayo and Tennekes, 2015).
An alternative for the reduction of agrochemicals is the use of biopolymers such as chitosan, which is an abundant compound in nature, and can be obtained by the partial deacetylation of chitin (main component of the exoskeleton of arthropods) through a thermal alkaline treatment; it has properties of biodegradability and inocuity, as well as antifungal activities, making it an ideal and easily manageable product (Waewthongrak et al., 2015). Diverse authors report that this biopolymer controls diseases caused by phytopathogenic fungi in papaya (Carica papaya), tomato (Physalis ixocarpa), banana (Musa × paradisiaca) and other crops (Bautista-Baños et al., 2003; De Oliveira et al., 2016; Liu et al., 2007; Maqbool et al., 2010). It also has the ability to produce films or coatings and it serves as a matrix to incorporate other additives or components that can add or strengthen some properties (Zargar et al., 2015).
In this sense, chitosan is an option for mixing with other antifungal agents, such as secondary metabolites like the ones found in the free extracts in Pseudomonas fluorescens cells. This bacteria displays control effects over phytopathogenic fungi in plantations of cabbage (Brassica oleracea), alfalfa (Medicago sativa), wheat (Triticum aestivum), broad bean (Vicia faba) and others (Alemu and Alemu, 2013; Mishra and Arora, 2012; Yanes et al., 2012;) by producing extracellular metabolites, which can be recovered from the culture medium and have an antifungal effect, without the need of having the bacteria present (Pal and McSpadden, 2006) and tackle the limitations of the use of biomass. The aim of this study was to evaluate the antifungal effect of the combination of chitosan and P. fluorescens extracts on the control in vitro of mycelia and A. alternata conidia, as well as the incidence and severity on tomato plants in greenhouse as an alternative possibility to reduce the use of agrochemicals.
Materials and methods
Biological material. The bacteria Pseudomonas fluorescens was isolated from the bacterial rhizosphere of strawberry stolons (Fragaria sp.) from the location of Ejido de la Finca, Villa Guerrero, State of Mexico (18° 53’ 07” N, 99° 37’ 36” W and an altitude of 1839 masl) during the 2012-2013 production cycle. The bacteria was purified and identified at the level of genus, following the methodology proposed by Schaad (1988). The molecular characterization was carried out by amplifying and sequencing the rRNA 16s and aligning with the bases of the National Center for Biotechnology Information (NCBI).
The phytopathogenic fungus Alternaria alternata was isolated from tomato fruits with typical symptoms of the disease, then identified and characterized molecularly in the Postharvest Physiology Lab in the Center for the Development of Biotic Products of the National Polytechnic Institute; it was then cultivated a Potato Dextrose Agar medium (PDA, BD Bioxon, Mexico) for 4-7 days and incubated at room temperature (25± 2 °C) for later trials.
Experiments performed in greenhouse were carried out using tomato plants of the Saladett (Eterno F1) variety in stages between vegetative (15 to 25 cm in height) to a flowering states, obtained from direct seeding in peatmoss pellets and grown in pots with an approximate capacity of 5 L, in a mixture of organic soil-peatmoss-agrolite in a proportion of 4:1:1, in greenhouse (32 °C, 75% RH, approximately) between February and June, 2017.
Preparation of chitosan and P. fluorescens extracts. The chitosan solution (Qs) (Sigma Aldrich, USA) was prepared at 3% (w/v) and obtained by dissolving 3.0 g of chitosan of low molecular molecular weight (50-190 kDa, 85% deacetylation) in 100 mL of distilled water, it was adding slowly glacial acetic acid (Fermont, Mexico) at 1% (v/v). The solution was stirred and warmed to 40 °C for 24 h, the pH was adjusted at 5.6 with sodium hydroxide 1 N and sterilized for 15 min at 15 psi. The P. fluorescens (EPf) extract was obtained by cultivating bacteria in a King´s B medium (25±2 °C, 72 h, 120 rpm), centrifuged for 15 min at 10015 xg, and the supernatant was filtered with 0.22 µm sterile membranes (Cole Palmer, USA) to retain the remaining cell biomass.
Control tests in vitro of A. alternata: mycelial growth and conidia germination. For the inhibition trials, was carried out in a treatments design in a factorial arrengement, the factors and levels were Qs [0, 0.5, 1.0, and 1.5% (w/v)] and EPf [0, 15, 30 and 50% (w/v)] with six repetitions per treatment it was included Captan (0.25% w/v) as a positive control. The pH of all treatments was adjusted to 5.6. The experimental unit was a Petri dish (90 x 15 mm) with PDA culture medium; 0.5 mL of each of the treatments was evenly placed and distributed on the surface of the medium, and left to dry for 5 to 10 min. PDA discs (5 mm in diameter) with fungal mycelia were then placed in the center of the dishes and incubated at room temperature. The time limit for incubation was determined when the mycelial growth of the negative control (Qs 0% + Epf 0%) reached the edge of the dish. For each experimental unit was measured and averaged the diameter of culture in two directions. The percentage of mycelial inhibition was calculated following Korsten and Jager (1995) using the formula: Inhibition (%) = [(DC - DT) / DC) x 100], where DC is the diameter of the control culture, and DT is the diameter culture of the treatment.
For the conidia germination trials, it was used the mixture that displayed the most statistically significant mycelial inhibition in vitro (%)*=p≤0.05. Distilled water was included as a negative control, and Captan (0.25% w/v) as a positive control, with 6 repetitions for each treatment. It was placed 0.5 mL of each treatment on the surface of the Petri dishes with PDA, spread it out evenly, left it to dry for 5 to 10 min, inoculated using a conidial suspension (0.1 mL) (106 mL-1) and incubated at 25±2 °C for 4 days, and the percentage of conidia germination was determined.
A. alternata control tests in tomato plants in greenhouse conditions. For the trial in a greenhouse, it was used the mixture that presented the most the most in vitro mycelial inhibition (%) statistically significant. Distilled water was included as a negative control and Captan (0.25% w/v) as a positive control, with 15 repetitions per treatment. The plants were placed in wet chambers for 24 h before inoculation by spraying a suspension of conidia (106 mL-1). Inoculated plants were incubated for 72 h in wet chambers, and the treatments were later sprayed and taken to a greenhouse (32 °C, 75% HR, approximately). After the first application, the treatments were sprayed every 7 days until flowering. Incidence was evaluated as the fraction of experimental units per treatments with symptoms (leaf smut, chlorosis, defoliation or wilting) and the severity of the disease as the fraction of leaves damaged in regard to the total of leaves of each experimental unit per treatment (Terna et al., 2016). Evaluations were carried out 7, 30 and 60 days after inoculation (DAI).
Data analysis. For the treatments in vitro and in greenhouse, a completely random design was used, and they were analyzed using an ANOVA; the treatments were compared using Tukey’s Test (*=p≤0.05). Before the analysis, the severity data as percentages were transformed with a logarithm [log (x + 1)]. The analyses were carried out using SAS® 9.4.
Results and discussion
Biological material. The P. fluorescens bacteria isolated in strawberry stolons was Gram negative; in the specific yeast-dextrose-calcium carbonate (YDC) growth medium, the colonies displayed a creamy-white color after 48 h in incubation; in King B agar (KB), after 48 h of incubation, the colonies produced a yellow pigment that surrounded the colony, which was fluorescent greenish-yellow under UV light (Figure 1). The white colonies in YDC may belong to the genera Pseudomonas, Erwinia or Agrobacterium, while pigmentation and fluorescence in King´s B agar are typical of P. fluorescens (Schaad, 1988).
The result of the culture characterization was corroborated using molecular characterization (Table 1) with the analysis of the gene 16s and the alignment of the sequence of base pairs (bp) with those from the NCBI Gene Bank. The DNA had a molecular weight of 425 bp; the sequence was aligned in first, second and third places, respectively with Pseudomonas lutea (Access number NZ_JRMB01000004.1, NZ_JRMB01000003.1 and NZ_JRMB01000002.1, Kwak et al., 2016), with a similarity index of 99% and 467 bp for all sequences; the difference was two nucleotides (NZ_JRMB01000004.1) and three nucleotides (NZ_JRMB01000003.1, NZ_JRMB01000002.1) and maximum scores of 774, 769 and 769, respectively, out of a total score of 774. In seventh place, it aligned with P. fluorescens (access number NR113647, Redondo et al., 2012) with a value of 763 and an index of 99 %, with a difference of four nucleotides, both sequences with 425 pb. The differences between P. fluorescens nucleotides and the sequence under study are distributed at random throughout the sequence, unlike P. lutea, where the differences are found in the same codons for the three sequences; the difference of the nucleotides and the base pairs between the sequence under study and P. lutea indicates molecular differences that distinguish such species, whereas the differences between P. fluorescens and the sequence under study may be due to polymorphisms by substitution of base pairs, attributable to a possible mutation; based on the high molecular homology and culture characterization, the biocontrol agent was concluded to be P. fluorescens.
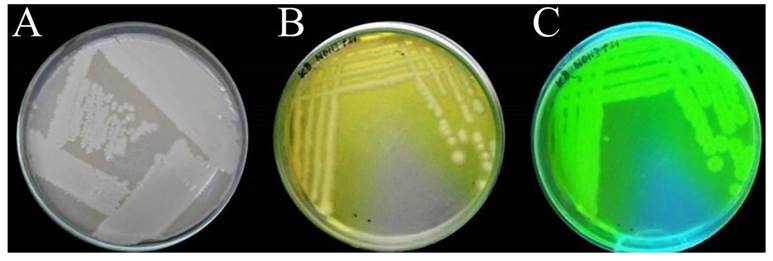
Figure 1 Cultural characteristics of P. fluorescens. Colonies in YDC medium YDC (A), yellow pigment in King´s B agar (B), and fluorescence under UV light (C).
In vitro A. alternata control tests: mycelial growth and conidia germination. The radial growth in the negative control A. alternata in PDA medium covered 100% of the Petri dish after 26 days of incubation. All treatments had a reduction mycelial growth statistically significant (*=p≤0.05) in regard to the negative control, as well as an inhibiting effect (Table 2, Figure 2).
In the treatments containing only Qs, the mycelial growth of A. alternata was inhibited between 9 and 33%, although in other studies the inhibiting effect was observed with concentrations starting at 0.5% of chitosan and higher than those reported in the present paper, with an inhibition of 54.6% for Fusarium solani f.sp. glycines (Prapagdee et al., 2007) and 100% for Botrytis cinerea (Liu et al., 2007) and Alternata chikunkiana (Meng et al., 2008).
Table 1 Sequence of base pairs of P. fluorescens isolated in Villa Guerrero, State of Mexico.
| CCGGGAACGTATTCACCGCGACATTCTGATTCGCGATTACTAGCGATTCCGACTTCACGCAGTCGAGTTGCAGACTGCGATCCGGACTACGATCGGTTTTATGGGATTAGCTCCACCTCGCGGCTTGGCAACCCTTTGTACCGACCATTGTAGCACGTGTGTAGCCCAGGCCGTAAGGGCCATGATGACTTGACGTCATCCCCACCTTCCTCCGGTTTGTCACCGGCAGTCTCCTTAGAGTGCCCACCATAACGTGCTGGTAACTAAGGACAAGGGTTGCGCTCGTTACGGGACTTAACCCAACATCTCACGACACGAGCTGACGACAGCCATGCAGCACCTGTCTCAATGTTCCCGAAGGCACCAATCCATCTCTGGAAAGTTCATTGGATGTCAAGGCCTGGTAAGGTTCTTCGCGTTGCTTC |
On the other hand, the individual antifungal effect of the EPfs were higher than for Qs in all cases, with a maximum inhibition of 47%; in comparison with other cell extracts, the extracts of Streptomyces griseus reduced the in vitro growth of Fusarium oxysporum f. sp. cubense (Zacky and Ting, 2013) by 33% and Bacillus subtilis extracts inhibited the in vitro mycelial development of Penicillium digitatum by 94% (Waewthongrak et al., 2015).
The mixtures of Qs and EPfs had a higher inhibition in comparison with the individual effects in all cases; the mixture with the highest concentration of both components (Qs 1.5% + EPf 50%) had the greatest effect of controlling the mycelial growth of A. alternata. The effect of the mixture of chitosan and other antifungal effects for the control of phytopathogenic fungi was reported earlier; the mixture of chitosan with beeswax and essential lemon oil inhibits the mycelial growth of Rhizopus stolonifer (Ramos-García et al., 2012) by 100%, the mixture of chitosan with ethanol (Qs 0.5% + ethanol 20%) reduced by up to 94% the deterioration caused by B. cinerea in grapes (Romanazzi et al., 2007), whereas the mixture of chitosan with papaya and custard apple leaf extracts reduced the mycelial growth of Colletotrichum gloeosporoides by up to 50% in comparison with the control (Bautista-Baños et al., 2003).
Table 2 In vitro mycelial growth and inhibition (%) of the mixtures of chitosan and extracts of P. fluore scens on A. alternata.
| Tratamiento (Qs % + EPf %) | Crecimiento de A. alternata (mm) | Inhibición (%) de A. alternata |
|---|---|---|
| Captan (0.25 % p/v) x | 24.8 h | 70.8 h |
| Qs 0.0 + EPf 0.0 y | 85.0 a | 0.0 a |
| Qs 0.5 + EPf 0.0 | 77.2 b | 9.2 b |
| Qs 1.0 + EPf 0.0 | 75.6 b | 11.0 b |
| Qs 1.5 + EPf 0.0 | 56.8 c | 33.2 c |
| Qs 0.0 + EPf 15 | 55.1 c | 35.2 c |
| Qs 0.0 + EPf 30 | 46.3 d | 45.5 d |
| Qs 0.0 + EPf 50 | 44.4 d e | 48.8d e |
| Qs 0.5 + EPf 15 | 52.7 c | 38.0 c |
| Qs 0.5 + EPf 30 | 43.5 d e | 48.8 d e |
| Qs 0.5 + EPf 50 | 39.6 e f | 54.9 e f |
| Qs 1.0 + EPf 15 | 43.8 d e | 48.5d e |
| Qs 1.0 + EPf 30 | 41.6 d e f | 51.0 d e f |
| Qs 1.0 + EPf 50 | 38.3 f g | 56.9 f g |
| Qs 1.5 + EPf 15 | 44.4 d | 47.8 d |
| Qs 1.5 + EPf 30 | 39.6 e f | 53.4 e f |
| Qs 1.5 + EPf 50 | 33.8 g | 60.2 g |
x Control positivo. y Control negativo. Medias con distinta letra son estadísticamente diferentes (Tukey *=p≤0.05) / x Positive control.
y Negative control. Means with different letters are statistically different (Tukey’s Test *=p≤0.05).
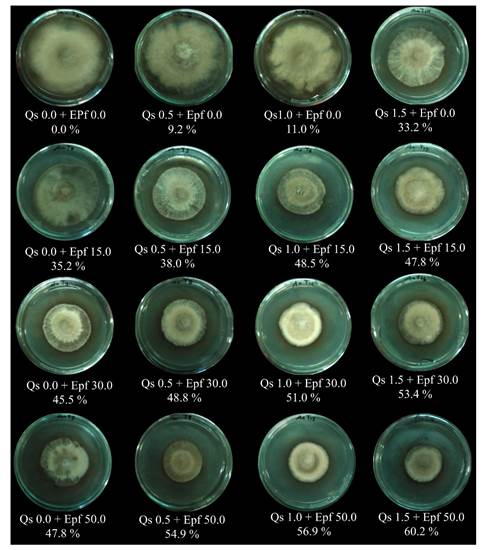
Figure 2 Figure 2. Effect of the control in vitro of the mixtures of chitosan and extracts of P. fluorescens (Qs % + Epf %) on A. alternata.
The greatest inhibition of mycelial growth in this study (60.2%) was lower than in previous studies, where antifungal agents were mixed with the substrate (PDA) as poisoned medium; for this investigation, it was applied as a cover on the surface of the culture medium to simulate the conditions of in vivo applications, where the biocontrol agents are sprayed on the surface of leaves and fruits (Feliziani et al., 2015; Saavedra et al., 2016). The mixture of Qs 1.5% + EPf 50% was used to evaluate the conidia germination, and had an inhibiting effect of 100%, equal to the effect of the commercial fungicide, in comparison with the negative control, 4 days after applying the treatments (Figure 3).
The control effect of the mixture Qs 1.5% + EPf 50% is related to the antifungal capacity of the components. The polycationic nature of the chitosan interferes with the negative charges of the cell membrane, modifies permeability and causes the leak of intracellular material, as well as the separation between cell membrane and wall of hyphae and conidia (Bautista-Baños et al., 2016; Sánchez-Domínguez et al., 2011). The slimming of hyphae and the loss of cytoplasmic content is due to the increase in membrane permeability, induced by the electrostatic interaction of the amino groups of the chitosan with the negative charges of the membrane (Palma et al., 2008).
The use of P. fluorescens extracts (Epf) is an interesting option for tackling the limitations of the use of cell biomass. P. fluorescens is commonly used as a biocontrol agent by the application of viable cells in the soil or plants, with variable results due to the lack of long term viability and inability to produce specialized resistance structures (endospores) like other biocontrol genera do (Narayasamy, 2003). In addition, inoculation is inconsistent between fields and between years, due to the variability in colonization, which results in a variable expression of the biocontrol mechanisms as antibiotics (Mark et al., 2006). Another limitation of the use of P. fluorescens is the environmental impact on the native saprophytic populations, with negative effects on the rhizosphere (Couillerot et al., 2008). In addition, there is the possible antibiotic transference (Nwosu, 2001) to other bacteria, it been documented the transference of plasmids due to the transference of plasmids between introduced and native strains of soil bacteria (Daane et al., 1996). The use of extracts produced by P. fluorescens (Epf) in this study show the in vitro antifungal effect as a viable alternative for the control of A. alternata.

Figure 3 Conidia germination of Alternaria alternata in PDA; negative control (A), mixture of Qs 1.5% + EPf 50% (B), Captan 0.25% w/v (C).
In the extracts of P. fluorescens in this study, the identities of the antifungal compounds were not determined, yet the biological control by P. fluorescens is attributed to the production of extracellular compounds such as phenazines, fluoroglucinols, pyrrolidine, pyrrolnitrine, cyclic lipopeptides, siderophores and hydrogen cyanide (Gerhardson, 2002; Hass and Defago, 2005). It has been suggested that these compounds act as enzyme inhibitors in the metabolism of glucose, which diffuse through the membrane, act as reduction agents, produce toxic compounds that affect the morphology of hyphae and conidia, and speed up the death process (Chin-A-Woeng et al., 2003; Premachandra et al., 2016).
A. alternata control trials in tomato in greenhouse. The incidence of the disease caused by A. alternata was 100 %. All plants treated showed the characteristic symptoms, such as leaves with dark brown to black circle-shaped spots and wilting. The severity of the disease showed variations throughout the application of the treatments; at the end of the applications we observed a behavior with no statistical difference between Qs 1.5 % + EPf 50 % and Captan (Table 3).
The success of a biological control product depends on the pathogen’s control ability, although reaching high effectiveness levels (95-98%) with the use of one single biocontrol agent is difficult (Guetsky et al., 2002). Nowadays there is a search for the mixtures of biological agents or additives to overcome the variable performance of the biocontrol agents and increase the effectiveness of the control (Droby, 2006). The use of mixtures of biocontrol agents that display various or different action mechanisms will give rise to synergistic, and in some cases, antagonistic effects (Guetsky et al., 2001).
There are diverse reports about the management of diseases caused by fungi in crops of nutritional interest with the application of mixtures of chitosan with extracts of biocontrol microorganisms or their viable cells, yet results are variable. Contrary to this study, Postma et al., (2009) observed that a mixture of extract of Lysobacter enzymogenes and chitosan (1.0%) did not inhibit the disease caused by Pythium aphanidermatum in cucumber plants, but the mixture with viable biomass reduced the incidence of the disease by 74 %. On the other hand, concentrations starting at 0.01 % of chitosan reduce the number of viable cells of the biocontrol agent Cryptococcus laurentii (Ting et al., 2012), therefore a mixture of both agents is not viable.
The combination of chitosan with the extracts of P. fluorecens (Qs + Epf) proved to be effective for the control of A. alternata. The mode of action of the chitosan can be attributed to a direct antimicrobial effect on the pathogen or the induction of resistance of the plant (Bakeer et al., 2016). On the other hand, the main mechanism of action attributable to P. fluorecens is the production of different types of antibiotics (Walsh et al., 2001). In this study, the reduction of the severity of A. alternata may be due to the combination of the different action mechanisms of both agents.
Table 3 Effect of the application of the mixture Qs 1.5% + EPf 50% on the severity in tomato plants in the greenhouse.
| Tratamiento | Severidad (%) | ||
|---|---|---|---|
| 7 DDIx | 30 DDIx | 60 DDIx | |
| Control negativo (agua) | 57.4 a | 36.0 a | 21.3 a |
| Control positivo (Captan) | 38.9 b | 21.0 b | 16.9 b |
| Qs1.5 % + EPf50 % | 51.8 a | 29.8 ab | 16.2 b |
Medias con distinta letra en cada columna son estadísticamente diferentes (Tukey *=p≤0.05) / Means with different letters in each column are statistical different (Tukey´s Test *=p≤0.05).
x DDI (días después de la inoculación) / x DAI (days after inoculation).
The best results of disease inhibition are obtained with chitosan in concentrations above 0.5% (Miranda, 2016), although the action mechanisms exerted on the phytopathogenic fungi could also inhibit the growth of some biocontrol microorganisms (Xing et al., 2015). Therefore, the antifungal mixture of this study is an alternative to this behavior, since there is no antagonistic effect of chitosan on the inoculum or on the cells of P. fluorescens, and the effect of both antifungal agents is maintained.
Conclusions
The P. fluorescens extracts, chitosan and the mixture of both agents inhibited mycelial growth and the conidia germination of A. alternata in trials in vitro. In the trials in greenhouse, all plants presented the typical symptoms of the A. alternata fungus. The antifungal effect of the mixtures of chitosan and extracts of P. fluorescens was greater than the control agents used individually. The trials in the greenhouse show that there is no significant difference between the use of the mixture of chitosan and extracts of P. fluorescens and the commercial agrochemical, hence the mixture could be a strategy for the control of phytopathogenic fungi in fruit and vegetable crops.
Literatura citada
Agrios, GN. 1997. Plant Pathology. 4th Edition. Academic Press. San Diego, California, USA. 300-303. https://doi.org/10.1017/S0014479700015507 [ Links ]
Alemu F, and Alemu T. 2013. Antifungal activity of secondary metabolites of Pseudomonas fluorescens isolates as a biocontrol agent of chocolate spot disease (Botrytis fabae) of faba bean in Ethiopia. African Journal of Microbiology Research. 7: 5364-5373. https://doi.org/10.5897/AJMR2013.5899 [ Links ]
Bakeer AR, El-Mohamedy RSR, Saied NM, Abd-El-Kareem. 2016. Field suppression of Fusarium soil borne diseases of tomato plants by the combined applications of bio agents and chitosan. 3: 1-10. https://doi.org/10.9734/bbj/2016/24985 [ Links ]
Bautista-Baños S, Hernández-López M, Bosquez-Molina E, and Wilson CL. 2003. Effects of chitosan and plant extracts on growth of Colletotrichum gloeosporioides, anthracnose levels and quality of papaya fruit. Crop Protection 22: 1087-1092. https://doi.org/10.1016/S0261-2194(03)00117-0 [ Links ]
Bautista-Baños S, Barrera NL, Hernández-López M, and Rodríguez-González F. 2016. Morphological and ultrastructural modifications of chitosan-treated fungal phytopathogens. (251-275). In: Bautista-Baños S, Romanazzi G. and Jiménez-Aparicio A. (Eds.). Chitosan in the preservation of agricultural commodities. Academic Press/Elsevier USA 394p. https://doi.org/10.1016/c2014-0-03033-x [ Links ]
Chin A Woeng TFC, Bloemberg GV and Lugtenberg BJJ. 2003. Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytologist. 157: 503-523. https://doi.org/10.1046/j.1469-8137.2003.00686.x [ Links ]
Couillerot O, Prigent-Combaret C, Caballero-Mellado J and Moënne-Loccoz. 2008. Pseudomonas fluorescens and closely-related fluorescent pseudomonads as biocontrol agents of soil-borne phytopathogens. Letters in Applied Microbiology. 48: 505-512. https://doi.org/10.1111/j.1472-765x.2009.02566.x [ Links ]
Daane LL, Molina JA, Berry EC and Sadowsky MJ. 1996. Influence of earthworm activity on gene transfer from Pseudomonas fluorescens to indigenous soil bacteria. Applied Environmental Microbiology. 62: 515-521. https://www.ncbi.nlm.nih.gov/pubmed/8593052. [ Links ]
Dean R, Van Kan JAL, Petrorius ZA, Hammond KE, Di Pietro AD, Spanu PD, Rudd JJ, Dickman M. 2012. The top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology. 13: 414-430. https://doi.org/10.1111/j.1364-3703.2011.00783.x [ Links ]
De Oliveira ENJ. 2016. Fungal growth control by chitosan and derivates (62-76). In: Sultan S (Eds). Fungal Pathogenicity. IntechOpen. USA. https://doi.org/10.5772/63308 [ Links ]
Droby, S. 2006. Improving quality and safety of fresh fruit and vegetables after harvest by the use of biocontrol agents and natural materials. Acta Horticulturae. 709:45-51. https://doi.org/10.17660/actahortic.2006.709.5 [ Links ]
FAO (Food and Agriculture Organization of the United Nations). 2016. FAOSTAT Statistics Database. http://www.fao.org/faostat/ (consulta noviembre 2018). [ Links ]
Feliziani E, Landi L, and Romanazzi G. 2015. Preharvest treatments with chitosan and other alternatives to conventional fungicides to control postharvest decay of strawberry. Carbohydrate Polymers. 132: 111-117. https://doi.org/10.1016/j.carbpol.2015.05.078 [ Links ]
Gerhardson B. 2002. Biological substitutes for pesticides. Trends in Biotechnology 20: 338-343. https://doi.org/10.1016/s0167-7799(02)02021-8 [ Links ]
Guetsky R, Shtienberg D, Elad Y, Fischer E, Dinoor A. 2001. Combining biocontrol agents to reduce the variability of biological control. The American Phytopathological Society. 91: 1024:1031. https://doi.org/10.1094/phyto.2001.91.7.621 [ Links ]
Guetsky R, Shtienberg D, Elad Y, Fischer E, Dinoor A. 2002. Improving biological control by combining biocontrol agents each with several mechanisms of disease suppression. Biological Control. 92: 976:985. https://doi.org/10.1094/phyto.2002.92.9.976 [ Links ]
Hass D and Defago G. 2005. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nature Reviews Microbiology. 3: 307-319. https://doi.org/10.1038/nrmicro1129. [ Links ]
Korsten, L, and Jager EE. 1995. Mode of action of Bacillus subtilis for control of avocado postharvest pathogens. SAAGA Yearbook 18: 124-130. http://agris.fao.org/agris-search/search.do?recordID=ZA9600511 [ Links ]
Received: December 19, 2018; Accepted: February 10, 2019











 text in
text in 


