Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.37 n.1 Texcoco Jan. 2019 Epub Aug 21, 2020
https://doi.org/10.18781/r.mex.fit.1808-5
Phytopathological notes
Performance of native accessions of Carica papaya inoculated with PRSV-p using Aphis nerii
1 Programa de Agroecosistemas Tropicales, Campus Veracruz, Colegio de Postgraduados, Km 88.5 Carretera Federal Xalapa-Veracruz, Manlio F. Altamirano, Veracruz, CP 91690, México;
2 Programa de Fitosanidad-Fitopatología, Campus Montecillo, Colegio de Postgraduados, Km 36.5 Carretera México-Texcoco, Montecillo, Texcoco, Estado de México, CP. 56230, México;
3 Campo Experimental Cotaxtla, CIRGOC, Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, Km 34 Carretera Federal Veracruz-Córdoba, Municipio Medellín, Veracruz, C.P. 94274, México.
Tolerance to the papaya ring spot virus (PRSV-p) may exist in native Carica papaya plants. The response of 13 native papaya accessions (Acc) and the commercial variety Red Maradol to the incidence and severity of the PRSV-p, inoculated with the vector insect Aphis nerii, and their relation with the observed plant management during their collection were evaluated. Acc were collected in the wild, in backyards or in production fields. Three months old-plants were infected with the virus. Disease incidence and severity were measured nine times weekly. Acc 203 and 205 showed the lowest disease severity index (1.0), followed by 147a and 60 (both with 1.7), 94a (2.0) and 197a (2.2); Acc 64a (2.5) and 150 (2.8) reached intermediate severity, followed by 65 and 210b (with 3.3), and by 80, 169 and 188a (with 4.3). Red Maradol variety showed the highest severity index (4.8). In relation to the management in which plants were collected, mean severity in Acc coming from the wild was significantly lower (P=0.032) than in Red Maradol, although they were not different from Acc coming from cultivated orchards or from backyards. It is possible that PRSV-p tolerance genes might be found in wild Acc.
Key words: incidence; severity; tolerance; aphids
Es posible que exista tolerancia al virus de la mancha anular del papayo (PRSV-p) en plantas nativas de Carica papaya. Se evaluó la respuesta de 13 accesiones (Acc) de papayo nativo y la variedad Maradol roja respecto a la incidencia y severidad del PRSV-p, inoculado con el insecto vector Aphis nerii, y su relación con el manejo de la planta observado durante su colecta. Las Acc se colectaron en estado silvestre, en traspatio o cultivadas. A los tres meses de edad se inocularon las plantas con el virus. Se evaluó semanalmente la incidencia y severidad en nueve ocasiones. Las Acc 203 y 205 mostraron el menor índice de severidad (1.0), seguidas de 147a y 60 (con 1.7), 94a (2.0) y 197a (2.2); las Acc 64a (2.5) y 150 (2.8) alcanzaron una severidad intermedia, seguidas de la 65 y 210b (con 3.3), y de 80, 169 y 188a (con 4.3). La var. Maradol roja presentó el mayor índice de severidad (4.8). La severidad promedio en las Acc colectadas en manejo silvestre fue significativamente menor (P = 0.032) que en Maradol roja, aunque no fue diferente en las Acc que provenían de manejo de plantaciones cultivadas o de traspatio. Es posible que entre las Acc silvestres se encuentren genes de tolerancia al PRSV-p.
Palabras clave: incidencia; severidad; tolerancia; áfidos
Papaya (Carica papaya L.) is originally from Central America and southern Mexico, although its area of domestication has not been entirely defined (Fuentes and Santamaría, 2014). Introduction of Maradol variety (var) in Mexico displaced and put at risk of extinction those native genotypes that lack adequate properties for the market, but displaying character diversity, including “cera”, “mamey” and “coco”, sold in local markets (Villanueva-Jiménez et al., 2015); in the wild is still possible to find “papaya de monte” or “pajarito” (Romero, 2013). Native papaya germplasm has not been evaluated in depth, especially their susceptibility to the papaya ring spot virus (PRSV-p), Potyvirus that reduces yield between 5 and 100%, hinders plant growth and drastically affects fruit size and quality. This virus is transmitted in a non-persistent manner by aphids (Hemiptera: Aphididae), being Myzus persicae, Aphis gossypii and A. nerii some of the most important (Villanueva-Jiménez et al., 2015; Hernández-Castro et al., 2015). The Papaya Network of the National System for Plant Genetic Resources for Food and Agriculture (SINAREFI, 2017) rescues native Mexican germplasm in which material tolerant to PRSV-p may exist. Therefore, the objective posed was to evaluate the response of 13 accessions (Acc) of native papaya trees and the red Maradol var regarding the incidence and severity of the PRSV-p, inoculated with the vector insect A. nerii, and its relation to plant management during its collection.
Material used. The original Acc were collected by Dr. Catarino Ávila Reséndiz (†), ex coordinator of the Papaya Network of SINAREFI (2017). Network´s South Southeast Orthodox Seed Conservation Center provided a limited number of seeds from these collections. Being recalcitrant, these seeds face germination problems after extended periods of conservation. We obtained a low number of individuals per accession, and therefore used the Acc that produced at least three plants. Table 1 presents the characteristics of each accession and the type of management in which they were collected. In addition, the var Red Maradol by Semillas del Caribe® was used, since it is the most widely grown in Mexico. Seeds were soaked for 48 h, replacing water every 8 h, and in gibberellic acid 0.1% for 12 h; they were placed on a folded sterilized moist cloth (15 x 20 cm) at 35 °C and 80 % relative humidity. Those that germinated were grown in trays with a mixture (1:1) of Peat Moss® and soil rich in organic matter. Seedlings were kept under a shade screen (75 %), irrigated and transplanted into pots (20 x 15 cm); after growing 8 to 10 true leaves, they were taken to a greenhouse at the Colegio de Postgraduados, Campus Veracruz. PRSV-p was obtained from papaya leaves gathered in a commercial orchard in Jamapa, Veracruz, Mexico. The presence of the pathogen was confirmed with RT-PCR, technique for the extraction of RNA from PRSV-p, with retrotranscription to generate complementary DNA and PCR with reverse transcriptase, performed using the Zymo Research® RNA extraction kit, as indicated by the manufacturer. RT-PCR was carried out in a Multigene and Labnet® thermocycler with the Promega RT-PCR System ®: PRSV-p CP region was amplified with 3F and 11R primers described by Noa-Carrazana et al. (2006). Aphids (A. nerii) from a virus-free culture were placed on leaves with PRSV-p for an acquisition period of 45 to 55 s (Gonsalves et al., 2010; Osorio-Acosta et al., 2010). Ten aphids were moved to each of 13 healthy Maradol papaya plants, kept there for 2 h, and then eliminated manually. Twenty days later, the transmission efficiency was confirmed via RT-PCR in leaves samples from each plant. Ten native plants PRSV-p positive were used as a source of inoculum.
Greenhouse test. It was established with three plants per Acc or var, which were inoculated three times with infective aphids with the methodology by Osorio-Acosta el al. (2010). 1st inoculation: 31/10/2017; 2nd 21/11/2017; 3rd 22/01/2018. A plant with infective aphids was confined 20 to 30 min in an entomological cage. Immediately afterwards, insects were removed.
Plants were kept at 24±3 °C in a greenhouse with an anti-aphid mesh for 63 days after inoculation. Weekly evaluations of PRSV-p incidence and severity of symptoms were carried out on leaves, stems and petioles (Table 2).
Incidence. The final incidence evaluated by symptoms was confirmed using the PRSV-p AGDIA® kit. Tissue was taken from 42 plants of the 13 Acc and the Red Maradol var; it was placed in bags and taken cold to the Virology Laboratory (Plant Health Program, Colegio de Postgraduados). Each sample was processed in a grinding mill (Dayton Electric®, Mod: 4Z522); the extract was gathered with 100 µl of the extraction buffer. Two replications were added per sample in a 96-well plate along with positive and negative controls. For the incubations, the enzyme-substrate mixture and the buffer washings were carried out following indications according to the manufacturer. Wells were read at 405 nm (Thermo Scientific®, Multiskan FC). A≥2.0 nm value was considered positive for PRSV-p. The percentage of incidence (I%) of the disease was determined using the formula: 1% = (n 1/N j )*100, where: n 1 = number of diseased plants per experimental plot during observation, and N j = total number of plants evaluated per experimental plot. Visual symptoms of the PRSV-p were displayed in 64.29% of inoculated plants from the Acc and Maradol var, confirmed by DAS-ELISA. Number of diseased plants in the 13 Acc and Red Maradol var increased with time, with final incidences of the PRSV-p inoculated with A. nerii between 33.3 and 100%. In other words, all Acc were susceptible to PRSV-p under greenhouse conditions, with clear differences in the proportion of infected plants (Table 3). Acc 65, 94a, 205 were the first to show symptoms of PRSV-p in a plant, on the 3rd week after inoculation (WAI), while red Maradol began with symptoms in two plants; Acc 80a, 210b, 147a, 60 and 203 displayed a plant with symptoms on the 4th WAI, and Acc 188a and 169a displayed two plants with symptoms; Acc 64, 150 and 197a were the last to show the first symptoms of PRSV-p, both in two plants up to the 5th WAI.
Table 1 Accession or variety, geographic location, type of fruit and degree of management of Carica papaya collected.
| Fecha | Accesión/Variedad | Localidad-Municipio, Estado | Latitud N Longitud O (°) | Altitud (m) | Tipo | Manejo | Peso fruto (g) | Color pulpa | °Brix | Grosor pulpa (cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 07/2010 | 60 | Ahuateno-Chicontepec, Veracruz | 20.97-98.17 | 642 | Mamey | Traspatio | 2020 | Amarilla-anaranjada | 11 | 3.7 |
| 08/2011 | 64a | Chilpancingo-Acapulco, Guerrero | 17.22-99.53 | 646 | Papaya | Traspatio | 650 | Amarilla-anaranjada | 9 | 3.1 |
| 08/2011 | 65 | Col. San José-Tierra Colorada, Guerrero | 17.16-99.53 | 273 | Criolla colorada | Traspatio | 800 | Amarilla-anaranjada | 8 | 3.0 |
| 09/2011 | 80 | El Pericón-Tecoanapa, Guerrero | 16.98-99.32 | 627 | Papayo | Traspatio | 78 | Amarilla | 13 | 2.1 |
| 09/2011 | 94a | Cariedad-San Marcos, Guerrero | 16.75-99.31 | 56 | Pico de pajarito | Silvestre | 7.1 | Amarilla | 21 | 0.6 |
| 07/2012 | 147a | Col. José María Pino Suarez-Huimanguillo, Tabasco | 17.78-93.64 | 25 | Papayitas | Silvestre | 8.5 | Amarilla-anaranjada | 18 | 0.9 |
| 07/2012 | 150 | Miguel Hidalgo-Huimanguillo, Tabasco | 17.67-93.74 | 24 | Papaya | Cultivada | 457 | Amarilla-anaranjada | 10 | 2.6 |
| 07/2012 | 169a | La Venta-Huimanguillo, Tabasco | 18.1-94.04 | 15 | Papaya | Cultivada | 834 | Amarilla | 11 | 3.4 |
| 07/2012 | 188a | San Carlos-Tenosique, Tabasco | 17.43-91.49 | 42 | P. Zapote | Cultivada | 2004 | Amarilla | 11 | 2.8 |
| 07/2012 | 197a | Tamulté de las Sabana-Centro, Tabasco | 18.16-92.79 | 8 | P. Zapote | Cultivada | 1980 | Amarilla | 9 | 2.9 |
| 11/2012 | 203 | Becan-Calakmul, Campeche | 18.51-89.46 | 277 | - | Silvestre | 7.2 | Amarilla | 17 | 0.6 |
| 11/2012 | 205 | El Plan de San Luis-Calakmul, Campeche | 18.53-89.56 | 255 | - | Silvestre | 11.5 | Amarilla | 14 | 1.3 |
| 12/2012 | 210b | Conguas-Calakmul, Campeche | 18.54-89.92 | 176 | - | Silvestre | 8.0 | Amarilla | 18 | 1.1 |
| 08/2017 | Maradol roja | Cuba | - | - | Variedad | Comercial | 1800 | Rojizo-anaranjada | 12 | 4.0 |
Table 2. Scale of severity and description of symptoms for the evaluation of papaya trees (C. papaya) infected by the PRSV-p virus. Adjusted fromRodríguez et al. (2013).
| Severidad | Descripción de síntomas | Rango promedio para calcular severidad |
|---|---|---|
| 0 | Ausencia de síntomas (0%) | 0 |
| 1 | Síntoma inicial del virus, aún sin estar bien definido (1-9%) | 5 |
| 2 | Síntoma evidente, pero puntual en un órgano (10-24%) | 17 |
| 3 | Síntoma que no se encuentra generalizado en todos los órganos y que abarca el 25-50% del órgano afectado | 38 |
| 4 | Síntoma severo y generalizado en el órgano, aunque sólo en 51-75% del órgano | 63 |
| 5 | Síntoma severo y generalizado en todos los órganos (76-100%). | 88 |
The proportion of plants affected in red Maradol and in Acc 188a and 80 increased gradually until they reached a final incidence of 100 %, while in Acc 169, 65, 210b, 94a, 64a, 150 and 197a, incidence increased slowly until it reached 67 %. Acc 147a, 60, 203 and 205 maintained the same proportion of affected plants during the entire period (33%), and they were less affected by the virus. This might be due to a lower susceptibility to transmission by A. nerii on these accessions. Selecting Acc with transmission indices of less than 100% might guarantee the production of fruit in the orchard; this factor, although it is not the strongest, might provide tolerance to the virus. Rodríguez et al. (2013) also mentioned that all Acc of papaya plants evaluated in the field were sensitive to PRSV-p, with differences in time of infection between them. It is therefore likely that the differences in tolerance of the Acc to the PRSV-p have a genetic origin. Gonsalves et al. (2010) indicate that no natural resistance to this virus has been found in C. papaya, although tolerant lines have been found in the Philippines, Taiwan, Thailand, and Florida, USA, which, once are diseased, do not produce adequate fruits for the market, either. However, delaying the beginning of the infection allows for a production of quality fruits (Hernández-Castro et al., 2015), which is why it is crucial to recognize the Acc with a long incubation period to help obtain the first healthy fruits. In the fields of Taiwan, the incidence of the disease in non-transgenic papaya lines appeared 29 days after transplanting, while transgenic lines acquired the disease five months later (Bau et al., 2004).
Table 3. Temporary increase of the incidence of PRSV-p in 13 accessions of native papaya and the var red Maradol under greenhouse conditions.
| Accesiones | Inicio de la incidencia (Semana) | Incidencia inicial (%) | Incidencia final (%) | Área bajo la curva de incidencia vs. severidad |
|---|---|---|---|---|
| Maradol | 3 | 67 | 100 | 242 |
| 188a | 4 | 67 | 100 | 201 |
| 80 | 4 | 33 | 100 | 139 |
| 169 | 4 | 67 | 67 | 155 |
| 65 | 3 | 33 | 67 | 155 |
| 210b | 4 | 33 | 67 | 132 |
| 64a | 5 | 67 | 67 | 107 |
| 94a | 3 | 33 | 67 | 101 |
| 150 | 5 | 67 | 67 | 92 |
| 197a | 5 | 67 | 67 | 57 |
| 147a | 4 | 33 | 33 | 77 |
| 60 | 4 | 33 | 33 | 57 |
| 203 | 4 | 33 | 33 | 41 |
| 205 | 3 | 33 | 33 | 39 |
Severity. The visual estimation of the severity value per plant was transformed into the mean percentage rank indicated in the description of symptoms (Table 2); this constituted the level of damage during the evaluation (X ki ), which was used to calculate the mean severity index (IS) per accession, using the formula , where, in addition, N ki = number of plants with the level of damage at the moment of evaluation; and N j = total number of plants evaluated. All Acc and the var red Maradol displayed an increase in the proportion of symptoms evaluated in the nine weeks after inoculation (WAI), although there were differences between them. The highest PRSV-p severity was observed in the Red Maradol var (with a mean severity index of 4.8), followed by Acc 88, 169 and 188a (4.3), Acc 210b and 65 (3.3), Acc 150 (2.8) and 64a (2.5), and were therefore considered the most susceptible to the disease (Figure 1, Right). The high susceptibility of red Maradol to the PRSV-p is probably caused by not been selected for tolerance to the virus. From those Acc that presented the latest incidence, 205 and 203 displayed the lowest mean severity index (1.0) on week nine. Acc 60 and 147a displayed a mean severity index of 1.7, followed by 94a with a value of 2.0, and by 197a with 2.2 (Figure 1, Left). These genotypes have the potential of being used as a source of tolerance in genetic breeding programs for papaya. In Cuba, Rodríguez et al. (2013) evaluated four promising accessions for seven months under field conditions; in three of them they obtained a low mean final severity of the symptoms reported for stem, petiole, foliage and fruit: “Sapote de Pilón en Cuba” (2.3), “Amarilla de Duaba” (2.4) and “Amarilla de Nava” (2.5); the Acc “Tallo Morado de Nava” was the most effective in the trial (3.1), yet in none of the four was the final severity in fruit above 2.5; the authors considered these materials as tolerant and with potential to be used as base material in genetic breeding programs for papaya in that country. The level of tolerance by severity in this investigation is comparable with that of 10 transgenic lines that Bau et al. (2003) classified as resistant, since they kept symptoms moderate five WAI. Because the present test was carried out during nine WAI, it is possible for the severity to increase during the lifetime of the plants. However, a delay in the infection of at least 5 weeks at the beginning of the development of the plant may produce differences in yield of over 50%, even in susceptible materials such as Red Maradol (Hernández-Castro et al., 2010). On the other hand, advanced intergenetic crosses (F6) of C. papaya (var Arka Surya) with Vasconcellea cauliflora carried out by Yanthan et al. (2017) produced seven progenies tolerant to PRSV-p under field conditions. In previous years, the group of Gonsalves et al. (2010) introduced the capside of the PRSV-p strain HA 5-1 to transgenic varieties of the Solo group and developed PRSV-p resistance. However, Mexico has still not allowed the production of transgenic varieties of papaya.
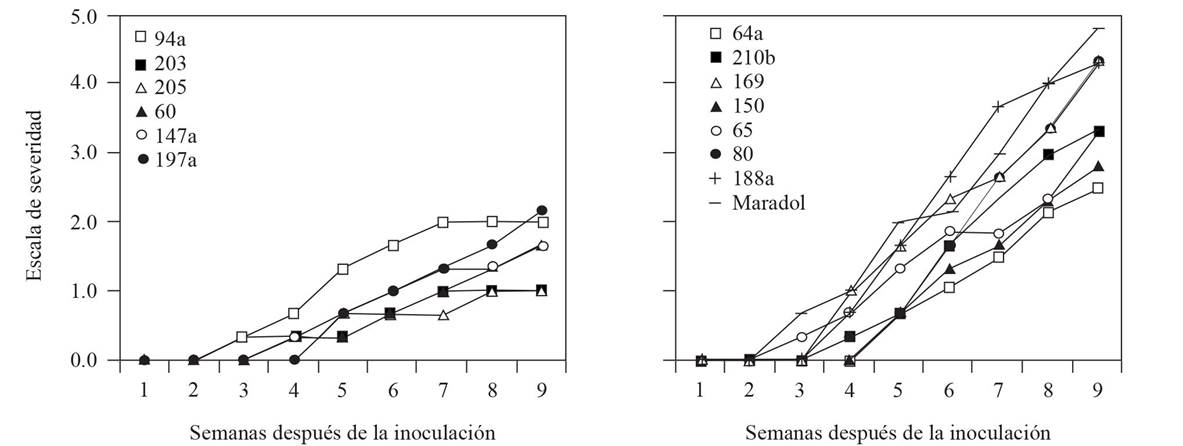
Figure 1 Dynamics of the mean severity index PRSV-p inoculated with A. nerii for nine weeks after inoculation. Left: six accessions with the lowest severity index. Right: Red Maradol variety and seven accessions with the highest severity index.
Management of accessions during collections. IS data were analyzed by Kruskal and Wallis to separate the groups of Acc regarding the management observed during the collection of the material. The analysis showed differences (P=0.032) between the percentage of symptoms of the Acc related to management. Acc found in the wild with no management presented the lowest severity, in comparison with the commercial var Red Maradol, while Acc with backyard management and planted in orchards did not present different percentage of severity between them (Figure 2). This confirms that it is in wild genotypes where the largest gene bank may lie for tolerance to the infection of the virus, since these are genotypes that have not yet been selected by man. In this regard, d’Eeckenbrugge et al. (2014) mention that within Caricaceae there are 21 wild species of the genus Vasconcellea, which have a potential to resist the virus PRSV-p, and could be used to generate resistance in commercial populations. In the Philippines, Alviar et al. (2012) indicate that Sinta is a papaya hybrid with a parent line of the genus Cariflora that inherits to the hybrid a high PRSV-p tolerance, therefore infected plants do not reduce their yields. This highlights the importance of evaluating a var or accession regarding the infection of the virus as a strategy for the development of resistant varieties.
Despite its preliminary connotation, we suggest to replicate this investigation with more accessions, evaluating them until fruit set. Due to the limitations for the use of transgenics, Mexican efforts to maintain accession banks of wild papaya material should take advantage of the papaya complete genome availability to identify genetic markers and identify the tolerance or resistance genes in outstanding C. papaya materials (Porter et al. 2013), so the advancement of development programs for resistance genotypes to this and other viruses can be systematized in a quick and efficient manner.
Literatura citada
Alviar NA, Sta Cruz FC and Hautea DM. 2012. Assessing the responses of tolerant papaya (Carica papaya L.) varieties to papaya ringspot virus (PRSV) infection and establishment of symptom severity rating scale for resistance screening. Philippine Journal of Crop Science 37:20-28. http://www.cssp.org.ph/pjcs/abstracts/volume-37-issue-no-2-august-2012/ [ Links ]
Bau HJ, Ying-Huey C, Tesong-Ann Y, Jiu-Sherng Y and Shyi-Dong Y. 2003. Broad-spectrum resistance to different geographic strains of papaya ringspot virus in coat protein gene transgenic papaya. Phytopathology 93:112-120. https://apsjournals.apsnet.org/doi/10.1094/PHYTO.2003.93.1.112 [ Links ]
Bau HJ, Ying-Huey C, Tesong-Ann Y, Jiu-Sherng Y, Chi-Hsiung H, Chien-Yih L and Shyi-Dong Y. 2004. Field evaluation of transgenic papaya lines carrying the coat protein gene of papaya ringspot virus in Taiwan. Plant Disease 88:594-599. https://doi.org/10.1094/PDIS.2004.88.6.594 [ Links ]
d’Eeckenbrugge G, Drew R, Kyndt T and Scheldeman X. 2014. Vasconcellea for papaya improvement. In: Ming R and Moore PH. (eds.) Genetics and Genomics of Papaya. Springer, New York. pp. 47-79. https://link.springer.com/chapter/10.1007/978-1-4614-8087-7_4 [ Links ]
Fuentes G and Santamaría JM. 2014. Papaya (Carica papaya L.): Origin, domestication, and production. In: R Ming and Moore PH (eds.). Genetics and Genomics of Papaya. Springer, New York. pp. 3-15. https://doi.org/10.1007/978-1-4614-8087-7_1 [ Links ]
Gonsalves D, Tripathi S, Carr JB and Suzuki JY. 2010. Papaya ringspot virus. The Plant Health Instructor. https://doi.org/10.1094/PHI-I-2010-1004-01 [ Links ]
Hernández-Castro E, Villanueva-Jiménez JA, Mora-Aguilera JA y Nava-Díaz C. 2010. Barreras de maíz en una estrategia de manejo integral para controlar epidemias del virus mancha anular del papayo (PRSV-P). Agrociencia 44:339-349. http://www.redalyc.org/pdf/302/30215552008.pdf [ Links ]
Hernández-Castro E, Nava DA, Mora AJA, Villanueva-Jiménez JA, Vargas AD and Palemón FA. 2015. Incidence of the papaya ringspot virus (PRSV-p) and management in the state of Guerrero, Mexico. In: Dimitrov TS and Vitanova II. Tropical Fruits. Nova Science Publishers Inc. pp. 119-127. https://www.novapublishers.com/catalog/product_info.php?products_id=54114 [ Links ]
Noa-Carrazana J, González LD, Ruiz-Castro B, Piñero D and Silva-Rosales L. 2006. Distribution of papaya ringspot virus and papaya mosaic virus in papaya plants (Carica papaya L.) in Mexico. Plant Disease 90:1004-1011. https://doi.org/10.1094/PD-90-1004 [ Links ]
Osorio-Acosta F, Villanueva-Jiménez JA, Celis-León B, Morales-Rodríguez A y José-Pablo R. 2016. Insecticidas en la transmisión del virus de la mancha anular de Carica papaya L., mediante Aphis nerii (Boyer de Fonscolombe). Agroproductividad 9(10):68-74 http://www.revista-agroproductividad.org/index.php/agroproductividad/article/view/834 [ Links ]
Porter BW, Christopher DA and Zhu YJ. 2013. The phylogeny of Caricaceae. In: Ming R and Moore PH (eds.). Genetics and Genomics of Papaya. Springer, New York. pp. 277-307. https://doi.org/10.1007/978-1-4614-8087-7_15 [ Links ]
Rodríguez D, Alonso M, Tornet Y, Valero L, Lorenzetti, ER and Pérez R. 2013. Assessment of Cuban papaya (Carica papaya L.) accessions against ringspot. Summa Phytopathologica 39:24-27. https://doi.org/10.1590/S0100-54052013000100004 [ Links ]
Romero RJA. 2013. Manejo y conservación de germoplasma de la familia Caricaceae. Tesis de Doctorado. Montecillo, Texcoco, Edo. de México . 145 p. http://www.secheresse.info/spip.php?article35498 [ Links ]
SINAREFI, Sistema Nacional de Recursos Fitogenéticos para la Alimentación y la Agricultura. 2017. MacroRed Frutales. SNICS, SAGARPA, México. 62 p. https://www.gob.mx/snics/acciones-y-programas/macro-red-frutales (consulta: mayo 2018). [ Links ]
Villanueva-Jiménez JA, Abato-Zárate M y Reyes-Pérez N. 2015. Plagas del papayo en México. In: Otero-Colina G, Abato-Zárate M y Villanueva-Jiménez JA. (eds.). Ácaros Asociados al Cultivo de Papayo en México. Editorial Colegio de Postgraduados. México. pp. 29-40. https://www.researchgate.net/publication/294893439 [ Links ]
Yanthan LJ, Vasugi C, Dinesh MR, Reddy K and Das R. 2017. Evaluation of F6 intergeneric population of papaya (Carica papaya L.) for resistance to papaya ring spot virus (PRSV). International Journal of Current Microbiology and Applied Sciences 6:289-298. https://doi.org/10.20546/ijcmas.2017.605.033 [ Links ]
Received: August 23, 2018; Accepted: December 14, 2018











 text in
text in 



