Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.37 n.1 Texcoco Jan. 2019 Epub Aug 21, 2020
https://doi.org/10.18781/r.mex.fit.1808-6
Review articles
A novel molecular approach in the study of parasite-host interaction
1 UAZ-Unidad Académica de Ciencias Biológicas;
2 CONACYT-INIFAP, Km. 24.5 Carretera Zacatecas-Fresnillo CP 98500. México;
3 INIFAP-Campo Experimental Zacatecas, km 24.5 Carretera Zacatecas-Fresnillo CP 98500. México.
Effectors have become the cornerstone of all investigations related to the interaction of parasites and their hosts, how they regulate the processes of infection at a molecular level, and how these molecules have evolved seem to be the most important issues that will have to be addressed in the following years. These new lines of research will be subject to the enormous progress that the next generation of sequencing technologies will have, and with them a paradigm shift in our vision of complex systems. However, there remain many questions to be elucidated about effectors, for example, how these proteins interact spatially and temporally in their hosts, possible co-operation between effectors, and the existence of protein complexes within host cells. This leads to the following questions: Do the effectors have the capacity for phenotypic expression beyond the genes that encode them? And above all, why are these proteins so widespread on a huge range of evolutionarily distant pathogens?
Key words: coevolution; virulence; parasitism
Los efectores, se han convertido en el eje fundamental de las investigaciones relacionadas a la interacción de los parásitos y sus hospederos, la manera en como regulan a nivel molecular los procesos de infección, y la forma en cómo estas moléculas han evolucionado, parecen ser las cuestiones más importantes que se tendrán que abordar en los siguientes años. Estas nuevas líneas de investigación, quedarán supeditadas al enorme progreso que tendrán la siguiente generación de tecnologías de secuenciación, y con ellas un cambio de paradigma en nuestra visión de los sistemas complejos. Sin embargo, aún quedan muchas cuestiones por dilucidar acerca de los efectores, por ejemplo, la manera en como estas proteínas interactúan de forma espacial y temporal en sus anfitriones, la posible cooperación entre los efectores, y la existencia de complejos proteicos dentro de las células huésped. De ello surgen las siguientes preguntas: ¿Los efectores tienen la capacidad de expresión fenotípica más allá de los genes que los codifican?, y sobre todo ¿Por qué estas proteínas se encuentran tan extendidas sobre una enorme gama de patógenos evolutivamente distantes?
Palabras clave: coevolución; virulencia; parasitismo
Parasites interact with their hosts through special proteins that have effects both on the cells they invade and on the host’s phenotype. Studies of the interaction between these proteins and the pathogenetic process are rapidly increasing within phytopathology in general and, therefore, we must keep our knowledge of this emerging approach up to date.
Parasite-host relationship. When referring to a host-parasite relationship, we mean a type of association between two players that perform active and essential roles. We know that a parasite depends metabolically and evolutionarily on its host, because they establish biological contact and molecular exchange to create a mutual relationship between the host’s defense and the parasite’s attack. In this arms race, parasites succeed because of the modulation produced by their “effectors” on the host’s defenses, potentially causing pathogenic actions or changes to the host’s homeostatic balance and adaptive response of its immune system.
Plant parasites and pathogens reprogram the host’s development and morphology (Le Fevre et al., 2015), and in this way the effector proteins of certain bacteria, such as phytoplasmas, modify the ecosystem architecture (Tomkins et al., 2018). Phytoplasmas cause phyllody in the host plants, supposedly to attract the insect vectors on which these bacteria’s transmission depends. Phytoplasma effectors in insects such as SAP54 improve their capacity to infect plants, and pathogens have a competitive advantage because their life cycle is extended (Sugio et al., 2011). Certain effectors, identified as PexRD54 for the first time in Phytophthora infestans, cause cell death in plant tissue of Nicotiana benthamiana (Białas et al., 2017) when the effectors are overexpressed as mature proteins. These are only some examples of the multiple studies that have revealed the molecular function of effectors as modulators of the host’s metabolism and gene expression. Another molecular system that has become a common target of parasites is the ubiquitination system, a post-translational gene regulation system which, in this case, is used by parasites to degrade the host’s immunity and alter its cell physiology, which benefits the invading parasite. Ubiquitin is a small protein that covalently binds to the lysine residues of other proteins, thereby “marking” them for degradation via the 26S proteasome. Certain pathogens have evolved to identify and exploit the weaknesses of this system and this has led to a greater pathogenic capacity to affect ubiquitin pathways in plants (Banfield, 2015).
Host resistance to diseases depends on the specific interaction between the resistance genes (R) and the corresponding avirulence genes (Avr). It has been suggested that the R genes encode for receptors that interact with ligands for the corresponding avirulence genes (De la Concepcion et al., 2018). Some host genes encode for effector protein recognition, such as the Solanum pimpinellifolium and Nicotiana paniculata MEcp2 gene that identifies the Ecp2 effector of parasitic fungi of the Capnodilaes class (Cladosporium fulvum) (Dagdas et al., 2016). It also seems that the phyllody (leaf-shaped flowers) produced by effector proteins (effector SAP54) on phytoplasmas is genetically related to a strong preference for insect-egg laying on plants infected by these bacteria. These facts lead us to believe that the changes in morphology are adaptive and that the parasite and host genomes will be jointly selected by evolution (Amselem et al., 2015). Other avirulence genes (such as AvrK1 and Avra10) encode for effector proteins in the fungus Blumeria graminis f.sp. hordei (Bgh), which increase their pathogenicity in barley plants (Di et al., 2017). Studies of rust fungi that affect many economically important plants such as coffee and soybean have served as excellent models for understanding the mechanisms that support pathogenesis, such as Melampsora lini, a rust pathogen, whose studies have shown how the sequences that encode for effectors are conserved in the genome of all these species and promote infection (Nemri et al., 2014), a fact that highlights the important role effectors play in the parasite-host interaction.
The extended phenotype. The concept of the extended phenotype (genes whose effects go beyond the cells in which they reside), introduced by Richard Dawkins in his classic book The Extended Phenotype (Dawkins, 2016), perfectly summarizes the idea that effectors act outside of parasites. Effectors are produced by genes residing in the pathogen’s genome, but they actually act in the interface with the host plant, or even inside the plant cells, providing an example of Dawkin’s extended genotype (Kamoun, 2007). Parasites can infect their hosts and cause severe changes in their appearance and performance, which are usually interpreted as being extended phenotypes that promote the parasite’s survival and ability (Le Fevre et al., 2015).
Some phytoplasmas that infect plants, such as Candidatus Phytoplasma trifolii, produce phyllody, supposedly to attract the insect vectors on which these bacteria depend for transmission (MacLean et al., 2014). However, the question remains as to whether plant morphological phenotypes, such as phyllody, directly benefit the vectors or whether they are secondary products of phytoparasitic infections (Hughes et al., 2012) (Figure 1).
MacLean et al. (2014) found that the SAP54 effector of phytoplasma induces phyllody in host cells, creating ecological niches to promote the vector’s colonization, and that these modifications in the host can be considered as being an extended phenotype caused by these proteins.
Incidence in the host cell. Many pathogen effectors are extraordinary examples of biological innovation and include some of the most important proteins known to function within plant cells, as shown in the diagram (Figure 2). Some of these effector proteins can even be specifically directed towards defense mechanisms that provide immunity against pathogen virulence genes, such as the Fusarium oxysporum Avr2 effector (Mccann, 2016). Plant bacteria, fungi, oomycetes and nematodes have developed the ability to manage effector proteins within host cells using different mechanisms (Hogenhout et al., 2009).
Biotrophic fungi and oomycetes have developed haustoria to manage effector proteins within the host cell (Whisson et al., 2007). Phytoparasitic nematodes use a specialized feeding organ known as a stylet to inject their effector proteins into a parasitized vascular cell (Davis et al., 2008).

Figure 1 (A) Healthy structures of Catharanthus roseus flowers, and (B) with phyllody and virescence caused by Candida tus Phytoplasma trifolii.
Some fungi proteins, particularly the selective toxin ToxA from the Pyrenophora tritici-repentis host, do not need the pathogen to move within plant cells (Sarma et al., 2005). ToxA moves within the host cells supposedly by designating a plant area receptor that binds to a protein motif formed by arginine-glycine-aspartate amino acids. Other effectors can suppress the host’s selective autophagy -plants use autophagy to protect themselves against pathogens- but how parasites are involved in these cell processes is still not known (Dagdas et al., 2016). One example of autophagy suppression is the PexRD54 effector produced by Phytophthora infestans, the causal agent of potato late blight (Washington et al., 2016).
Other effectors act in the apoplast. Some effectors act in the extracellular space of the plant-microbe interface, where they interfere with the plant’s apoplastic defenses and facilitate infection (Misas-Villamil and Van der Hoorn, 2008). These examples include the protein effectors secreted by Cladosporium fulvum (Cooke), which is an extracellular parasitic fungus found on tomato leaves that only grows in the apoplast and does not form haustorial structures (Thomma et al., 2005). All the known Cladosporium fulvum effectors (Avr2, Avr9, Avr4 and ECP2) are proteins rich in cysteine amino acid, which are believed to act exclusively in the apoplast (Thomma et al., 2005). Oomycetes such as Phytophthora infestans secrete apoplastic effectors as well as displacement effectors (cytoplasmic) in their hosts (Damasceno et al., 2008).
A common activity attributed to many Cladosporium fulvum apoplastic effects, as well as to other fungal pathogens and oomycetes, is the ability to protect themselves against plant hydrolytic enzymes such as proteases, glucanases and chitinases (Misas-Villamil and Van der Hoorn, 2008), which are the host plant’s defense mechanisms against exogenous agents such as parasites.
The C. fulvum Avr2 effector counteracts defense mechanisms because it is a cysteine-protease inhibitor directed at the Rcr3 and PIP1 tomato apoplastic enzymes, cysteines and proteases (van Esse et al., 2008). Phytophthora infestans also secretes protease-cysteine inhibitors, such as EPIC2B, which inhibits the PIP1 enzyme and other tomato apoplastic proteases (Tian et al., 2007). It also produces EPI1 and EPI10 effectors, which are serine-protease enzyme inhibitors that bind to and inhibit the P69B protein that is related to Phytophthora infestans pathogenesis. This serine-protease is similar to tomato subtilisin which is believed to act in defense mechanisms against pathogens (Tian et al., 2005). The genus Phytophthora spp. is also known to secrete glucanase inhibitors that inhibit the host’s apoplastic enzyme endo-β- 1,3 glucanase (Damasceno et al., 2008).
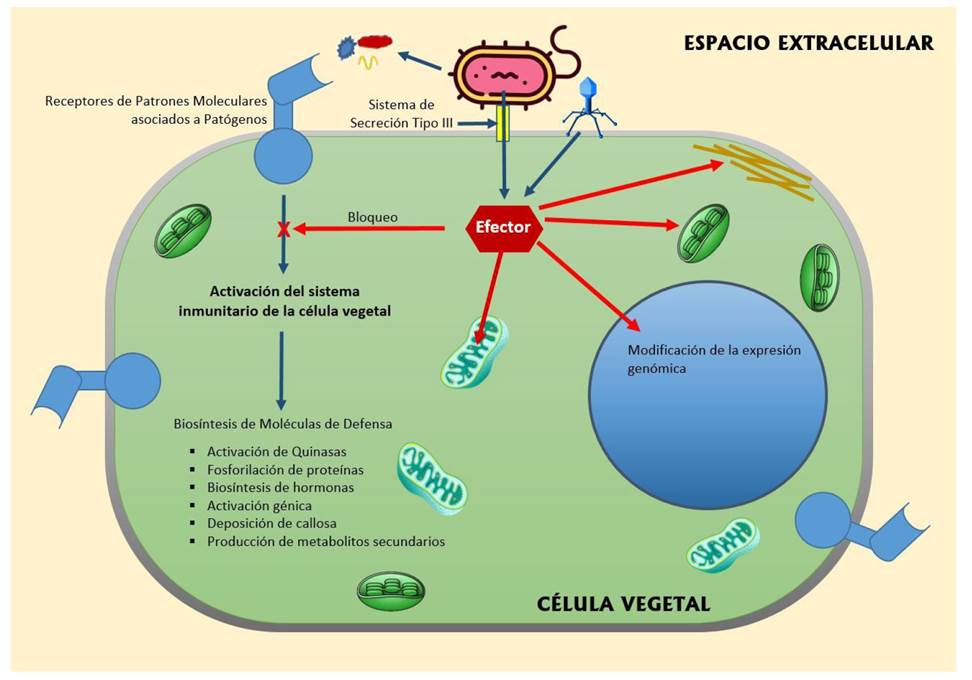
Figure 2 Pathways of effectors secreted by pathogens inside the cells of the host plant. Pathogens can inject their effectors into the plant cell cytoplasm through the type III secretion system. Depending on the type of effector, they can block the immune system of the plant cell, which is mediated by the receivers of molecular patterns associated with pathogens. Other types of effectors can alter the chloroplasts, mitochondria and cytoskeleton of the host cell to facilitate pathogen colonization. Effectors are also able to modify genomic expression to produce proteins that benefit pathogenicity.
Multiple targets in the cells of the host. Van der Hoorn and Kamoun defined operational targets as those host targets which, when manipulated by effectors, result in an altered defense or susceptible state. Therefore, it is important to distinguish operational targets from other types of host targets. These principles led to the idea that some decoy proteins of the host are not operational targets but that when disrupted by the effectors, they result in the host being recognized by the effector’s similar R proteins (van der Hoorn and Kamoun, 2008). The Pseudomonas syringae (Hall) AvrRpt2 protein is an effector of the T3SS type, which is a system that forms multiproteic complexes that prevent the presence of the effector in the extracellular medium; as a result, substrates are secreted by the plant cytoplasm into the extracellular medium with proteolytic activity against at least five Arabidopsis proteins, including the negative defense regulator RIN4 (Chisholm et al., 2006). AvrPto, another Pseudomonas syringae T3SS effector, is a kinase inhibitor that disables tomato Pto kinase (Xing et al., 2007). Other examples of multiple targets include Avr2 and EPIC2B protease inhibitors, which inhibit several tomato apoplastic proteases (van Esse et al., 2008).
Each interaction between an effector and a host protein can benefit the pathogen and have negative consequences or neutral effects on the pathogen-plant interaction (Table 1).
Molecular mimicry. Effectors produce analogous and imitators of plant hormones (Hogenhout et al., 2009). An example of this is coronatine, a toxin secreted by several repetitive genes of the Pseudomonas syringae genome, which is a structural and functional imitator of the jasmonoil-isoleucine (JA-ile) vegetal hormone (Bender et al., 1999). Coronatine has effects that improve plant bacterial colonization. Its effects include phytohormone saturation, which stops inducing the salicylic acid-mediated resistance response, and causes the plants’ stomata to open more, which in turn increases host infection. Other classic cases of hormone mimicry in plant pathogens include auxins and cytokinesins produced by different bacteria, such as Agrobacterium (Costacurta and Vanderleyden, 1995). There are also modified cytokinins produced by Rhodococcus fascians (Tilford) and Streptomyces turgidiscabies (Miyajima) fas operons (Hogenhout and Loria, 2008), and altered gibberellins produced by several fungi (Kawaide, 2006), such as Gibberella fujikuroi, a pathogen that affects rice seedlings (Tudzynski, 1999).
Besides hormone mimicry, effectors also include several surprising examples of molecular mimicry. The C-terminal region of the AvrPtoB effector of Pseudomonas syringae was found to be a structural and functional imitator of E3 ubiquitin-ligases in eukaryotes (Janjusevic et al., 2006). Degradation mediated by AvrPtoB of Fen kinase of the target host depends on the activity of the E3 ubiquitin ligase of AvrPto (Rosebrock et al., 2007).
Another example of molecular mimicry is the Xanthomonas vesicatoria Type III AvrBs3 effector which travels to the cell nucleus, where it acts as a transcriptional activator and binds to a conserved sequence promoter of the Xanthomonas vesicatoria genome, called upa box (Römer et al., 2007). Given that this box is also conserved in different genes of the pepper genome (Piper nigrum), it is believed that AvrBs3 imitates one of the host’s transcription factors (not yet discovered), which also points to this promotor sequence. Results of several studies have revealed that phytoparasitic nematodes secrete a series of proteins that imitate plant effectors, which allows the expression of plant genes that favor colonization by the pathogen (Cai et al., 2008).
Table 1 Effector proteins reported in previous studies of pathogen-host plant interaction.
| Organismo | Efector (proteína) | Función en el hospedero (Objetivos operativos) | Referencia |
|---|---|---|---|
| Cladosporium fulvum | Ecp2 | Reconocimiento de secuencias especificas | (Dagdas et al., 2016) |
| (hongo) | |||
| Candidatus Phytoplasma trifolii | SAP54 | Cambios en la morfología de la planta, inducen fenotipos adaptativos para los vectores (filodia). | (Amselem et al., 2015) |
| (Bacteria) | |||
| Blumeria graminis f.sp (hongo) | AvrK1 y Avra10 | Codifican proteínas que aumentan su patogenicidad en plantas de cebada. | (Di et al., 2017) |
| (hongo) | |||
| Fusarium oxysporum | Avr2 | Interfieren de forma específica a mecanismos de defensa conservado para la inmunidad contra genes de virulencia de patógenos. | (Mccann, 2016) |
| Phytophthora infestans | PexRD54 | Suprime la capacidad de autofagia selectiva en el huésped, las plantas usan la autofagia para protegerse contra los patógenos. | (Washington et al., 2016) |
| Cladosporium fulvum (hongo) | Avr2, Avr9, Avr4 y ECP2 | Actúan en el espacio extracelular en la interface planta-microbio, donde interfieren con las defensas apoplásticas de la planta para infectarla. | (Thomma et al., 2005) |
| (hongo) | |||
| Pseudomonas syringae | AvrRpt2 | Actividad proteolítica contra al menos cinco proteínas de Arabidopsis, incluyendo el regulador de defensa negativo RIN4. | (Chisholm et al., 2006) |
| Xanthomonas vesicatoria | AvrBs3 | Actúa como un activador transcripcional, uniéndose a una secuencia promotora. | (Römer et al., 2007) |
| Pseudomonas syringae | HopAF1 | Suprime la inmunidad de la planta, este efector se encuentra en los genomas de muchas bacterias de este tipo y posiblemente esté relacionado con la proteína deamidasa. | (Hughes y Banfield, 2014) |
| Orden Tylenchida (nematodos) | Reprograman el desarrollo y la inmunidad en la planta. | (Lilley et al., 2018) | |
| (nematodos) | |||
| Phytophthora sojae | Avr1b | Suprime la muerte celular programada. | (Dou et al., 2008) |
| Xanthomonas vesicatoria | AvrBs3 | Hipertrofia celular. Se cree que estas lesiones cancerosas facilitan la liberación bacteriana del tejido infectado y aumentan su diseminación. | (Kay et al., 2007) |
Suppressing plant immunity. Suppression of a plant’s innate immunity has emerged as the primary function of effectors, particularly of T3SS effectors of plant pathogenic bacteria (Zhou and Chai, 2008). Some effectors, such as Pseudomonas syringae HopAF1, suppress a plant’s immunity; this effector is found in the genomes of many bacteria of this type and may be related to the deamidase protein, since deamidation is the irreversible substitution of an amide group by a carboxylate group (Hughes and Banfield, 2014). The Hyaloperonospora arabidopsidis (Hpa) pathogen translocates effector proteins to suppress host plant immunity (Wirthmueller et al., 2018). The way in which these effectors act to produce virulence is by suppressing the basal defense of the host plant’s immune system by not recognizing the molecular pattern associated with pathogens (known as PAMPs), which is one of the defense systems of plant cells (Kim et al., 2005). Some species of plant parasitic nematodes of the Tylenchida order secrete effector proteins into their hosts during the infection process in order to reprogram the plant’s development and immunity (Lilley et al., 2018).
Other T3SS effectors of phytopathogenic bacteria suppress hypersensitive cell death caused by several Avr proteins, a fact that in some cases explains previous observations of epistatic interactions among Avr genes (Abramovitch et al., 2006). The T3SS effectors are directed towards three plant processes that are essential for innate immunity, i.e., protein rotation, RNA homeostasis and phosphorylation pathways (Block et al., 2008).
Some fungi and oomycetes produce effectors that suppress cell death (Panstruga, 2003). This is based on histological observations of susceptible interactions and the prevalence of cell death suppressors among the T3SS bacterial effectors (Janjusevic et al., 2006). The Phytophthora infestans Avr3a effector suppresses hypersensitive cell death caused by another Phytophthora infestans protein (INF1 elicitin), which suggests a possible virulence function (Bos et al., 2006).
Another type of effectors is the RXLR type, which are characterized by having a domain with RXLR amino acids (Arginine-Leucine-Arginine) within their protein structure at the C-terminal end. The Phytophthora sojae effector of the RXLR type also suppresses programmed cell death caused by the BAX mouse protein in yeast and plants (Dou et al., 2008). Sohn (2007) demonstrated that administering Hyaloperonospora parasítica (Pers) ATR1 and ATR13 effectors increases Pseudomonas syringae virulence. ATR13 also suppresses the deposition of unchained calose by Pseudomonas syringae, which suggests that its action affects the basal defenses against pathogens (Sohn et al., 2007). These findings indicate that, like the bacterial T3SS effectors, the oomycetes RXLR effectors often act as plant immunity suppressors. However, the mechanisms through which the RXLR effectors interfere with immunity have not yet been correctly dilucidated (Hogenhout et al., 2009).
Effectors’ influence on plant development and performance. Some effectors affect the host plant’s performance and morphology (Hogenhout et al., 2009). Effectors of the AvrBs3 family, which are Xanthomonas transcriptional activators, cause cell division and colonization of susceptible hosts (Kay et al., 2007). Effectors can also activate a plant’s immune receptors, especially the nucleotide binding domain and proteins that contain repetitive regions rich in leucine (NLR), which enables plants to fight against invasive organisms; this interaction among effectors, their host targets and simultaneous immunity receptors is caused by complicated molecular mechanisms and an exceptionally dynamic coevolution (Białas et al., 2017). The presence of Xanthomonas citri in citrus cells is enough to cause macroscopic hyperplastic lesions similar to canker symptoms caused by the pathogen (Duan et al., 1999). Cankerous lesions cause bacteria to be released from infected tissue and favor their spread. The Xanthomonas vesicatoria AvrBs3 effector is also known to cause cell hypertrophy, although the impact of this symptom on bacterial ability is not very clear (Kay et al., 2007). Other organisms associated with plants alter their host’s morphology, which causes malformations that create a protective ecological niche or improve their spread. Classic examples include rhizobial nodules (Oldroyd and Downie, 2008), galls caused by Agrobacterium spp. (Chalupowicz et al., 2006) and witch’s broom caused by phytoplasmas (Hogenhout et al., 2009) (Figure 3).
Natural selection will favor effectors that have effects on the hosts’ phenotypes and will improve the pathogen’s ability (Hogenhout et al., 2009).
Effector genes evolve more rapidly than the nuclear genome. The rapid evolution of the gene is a hallmark of the pathogen’s adaptation (De la Concepcion et al., 2018). The biochemical adaptation of the effectors after they colonize the host is essential for the pathogen’s diversification and speciation (Dong et al., 2014). Genes that encode for effector proteins are direct targets of evolutionary forces that drive host and pathogen co-evolution (McCann and Guttman, 2008). Alleles of effectors that successfully increase the pathogen’s reproduction will be immediately favored by natural selection. Directional selection or positive selection is a type of natural selection that favors only one allele, and for this reason, the allelic frequency of a population continuously goes in one direction, given that this mechanism can also lead to adaptations (Futuyma, 2013).
Many effector genes have evolved more quickly than the pathogen’s genome and often show extreme levels of positive selection with significantly higher rates of substitution of non-synonymous nucleotides for synonymous nucleotides (Ma and Guttman, 2008). In modular effector proteins, such as the bacterial T3SS effectors and oomycete RXLR effectors, their structural domains are under different selective pressure, depending on whether they function by secretion or conduct effector activity per se (Win, 2007). Therefore, terminal-N domains, such as the signal peptide, the RXLR domain and the T3SS directing sequence, typically show low levels of polymorphisms compared to those of the terminal-C effector region (Win et al., 2007).
Besides acting on nucleotide polymorphisms, natural selection acts on the polymorphisms of the number of copies of the effector genes (presence and lack of polymorphisms, and a varying number of gene copies). The effector genes of phytopathogenic fungi are located in loci with a high level of genomic plasticity, including regions rich in transposons and telomerase (Gout et al., 2006), which reduces the genetic recombination capacity and make the host more susceptible to phytopathogenic fungi. Recently, Yoshida et al. (2009) demonstrated that two loci of Magnaporthe oryzae (Herbert) effectors have a low diversity of nucleotides but a strong presence or lack of polymorphisms. This accelerated evolution of the parasites’ genomes will allow pathogens to successfully colonize their hosts and be better adapted to possible changes that in the future may occur in the genome (Jiang et al., 2006).
The association of effector genes with plastic genome loci could provide an adaptation mechanism to host resistance, thus increasing the genetic and epigenetic variation and enabling a rapid evolution (Hogenhout et al., 2009).

Figure 3 Plant morphology caused by pathogens, such as: A) modified sheaths in Sisymbrium irio plants; B) galls caused by Agrobacterium spp.; and C) witch’s broom caused by phytoplasmas in Capsicum annuum plants.
Evolution of effectors. Given that it is obvious that effectors increase the susceptibility to parasites, the host’s target alleles will evolve to avoid them. Recessive mutations in the xa13 rice gene make the promotor of this gene become insensitive to the effectors that activate Xanthomonas oryzae pv. oryzae transcription, which results in disease resistance (Sugio et al., 2007).
Another recessive gene of rice blight resistance (xa5) is caused by mutations in the IIIA transcription factor, which supposedly prevents actions performed by the related effector (Iyer-Pascuzzi and McCouch, 2007). Also, mutations in the elF4E elongation factor evade interactions with the VPg effector of potyvirus (Charron et al., 2008). An allele (Rcr3) of tomato cysteine-protease has also been identified as carrying a mutation that makes the protein insensitive to inhibition by the Cladosporium fulvum Avr2 effector (Shabab et al., 2008).
Many more examples are expected to be available in the future, since next-generation sequencing technologies are currently being explored which could systematically probe the variation in effector sequences as a mechanism to understand the selection evidence (Hogenhout et al., 2009). It is important to completely understand how the tripartite interaction among effectors, target effectors and R proteins (proteins that confer resistance to the host through direct or indirect recognition of a pathogen protein) evolves, given the conflict among the selective forces that occur between plants and pathogens in natural populations (van der Hoorn and Kamoun, 2008).
Perspectives
The study of effectors promises to be the new synthesis of multidisciplinary studies of parasite-host interactions, and this will mark the beginning of a new epistemological revolution in phytopathology. Effectors are a source of biological innovation whose results are just beginning to be elucidated and will undoubtedly be a rich source of scientific findings in the years to come. Effectors have proved to be some of the most important proteins involved in the eukaryotic cells of plants and animals. Studies of these proteins will provide important knowledge of insects’ immune system, bacterial virulence strategies, plant defense mechanisms against bacteria and herbivore insects, and reveal new pathways that affect plant vegetative growth and development. However, the biology of effectors is still in its infancy, and the available knowledge is limited to a few phytopathogen taxa and immunosuppression processes. But the gap could be filled soon by using new DNA sequencing technologies (next-generation sequencing) and the recently arrived “omics.” The sequences of pathogen and host genomes could provide a wider phylogenetic scope and thus a more comprehensive understanding of the pathosystem. All this information reinforces the importance of effectors as a fundamental cog in the wheel of this tripartite interaction. Also, the use of new tools and concepts for studying effectors will have important impacts on evolutionary biology and some of its concepts will be redefined, not to mention the new approaches that are emerging from the current study of “omics,” a new field of genetics that attempts to understand the molecular organization, evolution and architecture of the whole genome. This discipline has started to branch out into the study of proteomics (the study of all the proteins that are produced by an organism) and transcriptomics (the study of all cell RNAm), besides the latest computer system technology that can extrapolate infinite amounts of data, so the new effector biology is about to become the mother of all research about pathosystems.
Literatura citada
Abramovitch RB, Anderson JC, and Martin GB. 2006. Bacterial elicitation and evasion of plant innate immunity. Nature Reviews Molecular Cell Biology 7: 601-611. http://dx.doi.org/10.1038/nrm1984. [ Links ]
Amselem J, Vigouroux M, Oberhaensli S, Brown JK, Bindschedler LV, Skamnioti P, Wicker T, Spanu PD, Quesneville H, and Sacristán S. 2015. Evolution of the EKA family of powdery mildew avirulence-effector genes from the ORF 1 of a LINE retrotransposon. BMC Genomics 16: 917. https://doi.org/10.1186/s12864-015-2185-x. [ Links ]
Banfield MJ. 2015. Perturbation of host ubiquitin systems by plant pathogen/pest effector proteins. Cellular microbiology 17: 18-25. https://doi.org/10.1111/cmi.12385 [ Links ]
Bender CL, Alarcón-Chaidez F, and Gross DC. 1999. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiology and molecular biology reviews 63: 266-292. Disponible en línea: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC98966/pdf/mr000266.pdf [ Links ]
Białas A, Zess EK, De la Concepcion JC, Franceschetti M, Pennington HG, Yoshida K, Upson JL, Chanclud E, Wu C-H, and Langner T. 2017. Lessons in effector and NLR biology of plant-microbe systems. Molecular Plant-Microbe Interactions 31: 34-45. https://doi.org/10.1094/MPMI-08-17-0196-FI. [ Links ]
Block A, Li G, Fu ZQ, and Alfano JR. 2008. Phytopathogen type III effector weaponry and their plant targets. Current opinion in plant biology 11: 396-403. http://dx.doi.org/10.1016/j.pbi.2008.06.007. [ Links ]
Bos JI, Kanneganti TD, Young C, Cakir C, Huitema E, Win J, Armstrong MR, Birch PR, and Kamoun S. 2006. The C‐terminal half of Phytophthora infestans RXLR effector AVR3a is sufficient to trigger R3a‐mediated hypersensitivity and suppress INF1‐induced cell death in Nicotiana benthamiana. The Plant Journal 48: 165-176. http://dx.doi.org/10.1111/j.1365-313X.2006.02866.x. [ Links ]
Cai H, Wei W, Davis RE, Chen H, and Zhao Y. 2008. Genetic diversity among phytoplasmas infecting Opuntia species: virtual RFLP analysis identifies new subgroups in the peanut witches’-broom phytoplasma group. Int J Syst Evol Microbiol 58: 1448-1457. http://doi.org/10.1099/ijs.0.65615-0. [ Links ]
Chalupowicz L, Barash I, Schwartz M, Aloni R, and Manulis S. 2006. Comparative anatomy of gall development on Gypsophila paniculata induced by bacteria with different mechanisms of pathogenicity. Planta 224: 429-437. https://doi.org/10.1007/s00425-006-0229-9. [ Links ]
Charron C, Nicolaï M, Gallois JL, Robaglia C, Moury B, Palloix A, and Caranta C. 2008. Natural variation and functional analyses provide evidence for co‐evolution between plant eIF4E and potyviral VPg. The Plant Journal 54: 56-68. http://dx.doi.org/10.1111/j.1365-313X.2008.03407.x. [ Links ]
Chisholm ST, Coaker G, Day B, and Staskawicz BJ. 2006. Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124: 803-814. http://dx.doi.org/10.1016/j.cell.2006.02.008. [ Links ]
Costacurta A, and Vanderleyden J. 1995. Synthesis of phytohormones by plant-associated bacteria. Critical reviews in microbiology 21: 1-18. http://dx.doi.org/10.3109/10408419509113531. [ Links ]
Dagdas YF, Belhaj K, Maqbool A, Chaparro-Garcia A, Pandey P, Petre B, Tabassum N, Cruz-Mireles N, Hughes RK, and Sklenar J. 2016. An effector of the Irish potato famine pathogen antagonizes a host autophagy cargo receptor. Elife 5: e10856. http://dx.doi.org/10.7554/eLife.10856.001. [ Links ]
Damasceno CM, Bishop JG, Ripoll DR, Win J, Kamoun S, and Rose JK. 2008. Structure of the glucanase inhibitor protein (GIP) family from Phytophthora species suggests coevolution with plant endo-β-1, 3-glucanases. Molecular Plant-Microbe Interactions 21: 820-830. http://dx.doi.org/10.1094/MPMI-21-6-0820. [ Links ]
Davis EL, Hussey RS, Mitchum MG, and Baum TJ. 2008. Parasitism proteins in nematode-plant interactions. Current opinion in plant biology 11: 360-366. https://doi.org/10.1016/j.pbi.2008.04.003. [ Links ]
De la Concepcion JC, Franceschetti M, Maqbool A, Saitoh H, Terauchi R, Kamoun S, and Banfield MJ. 2018. Polymorphic residues in rice NLRs expand binding and response to effectors of the blast pathogen. Nature plants 4: 576. Disponible en línea: [ Links ]
Di X, Cao L, Hughes RK, Tintor N, Banfield MJ, and Takken FL. 2017. Structure-function analysis of the Fusarium oxysporum Avr2 effector allows uncoupling of its immune‐suppressing activity from recognition. New Phytologist 216: 897-914. https://doi.org/10.1111/nph.14733 [ Links ]
Dong S, Stam R, Cano LM, Song J, Sklenar J, Yoshida K, Bozkurt TO, Oliva R, Liu Z, and Tian M. 2014. Effector specialization in a lineage of the Irish potato famine pathogen. Science 343: 552-555. http://doi.org/10.1126/science.1246300. [ Links ]
Dou D, Kale SD, Wang X, Chen Y, Wang Q, Wang X, Jiang RH, Arredondo FD, Anderson RG, and Thakur PB. 2008. Conserved C-terminal motifs required for avirulence and suppression of cell death by Phytophthora sojae effector Avr1b. The Plant Cell 20: 1118-1133. https://doi.org/10.1105/tpc.107.057067. [ Links ]
Duan Y, Castaneda A, Zhao G, Erdos G, and Gabriel D. 1999. Expression of a single, host-specific, bacterial pathogenicity gene in plant cells elicits division, enlargement, and cell death. Molecular Plant-Microbe Interactions 12: 556-560. https://doi.org/10.1094/MPMI.1999.12.6.556. [ Links ]
Futuyma D. 2013. Evolution. 3rd edn. Sunderland, MA. Sinauer Associates, Inc. [ Links ]
Gout L, Fudal I, Kuhn ML, Blaise F, Eckert M, Cattolico L, Balesdent MH, and Rouxel T. 2006. Lost in the middle of nowhere: the AvrLm1 avirulence gene of the Dothideomycete Leptosphaeria maculans. Mol Microbiol 60: 67-80. http://dx.doi.org/10.1111/j.1365-2958.2006.05076.x. [ Links ]
Hogenhout SA, and Loria R. 2008. Virulence mechanisms of Gram-positive plant pathogenic bacteria. Current opinion in plant biology 11: 449-456. http://dx.doi.org/10.1016/j.pbi.2008.05.007. [ Links ]
Hogenhout SA, Van der Hoorn RA, Terauchi R, and Kamoun S. 2009. Emerging concepts in effector biology of plant-associated organisms. Molecular Plant-Microbe Interactions 22: 115-122. http://dx.doi.org/10.1094/MPMI-22-2-0115. [ Links ]
Hughes DP, Brodeur J, and Thomas F. 2012. Host manipulation by parasites. Oxford University Press. [ Links ]
Hughes R, and Banfield M. 2014. Production of RXLR effector proteins for structural analysis by X-ray crystallography. Methods in molecular biology (Clifton, NJ) 1127: 231-253. Disponible en línea: [ Links ]
Iyer-Pascuzzi AS, and McCouch SR. 2007. Recessive resistance genes and the Oryza sativa-Xanthomonas oryzae pv. oryzae pathosystem. Molecular Plant-Microbe Interactions 20: 731-739. http://dx.doi.org/10.1094/MPMI-20-7-0731. [ Links ]
Janjusevic R, Abramovitch RB, Martin GB, and Stebbins CE. 2006. A bacterial inhibitor of host programmed cell death defenses is an E3 ubiquitin ligase. Science 311: 222-226. http://dx.doi.org/10.1126/science.1120131. [ Links ]
Jiang RH, Weide R, van de Vondervoort PJ, and Govers F. 2006. Amplification generates modular diversity at an avirulence locus in the pathogen Phytophthora. Genome research 16: 827-840. https://doi.org/10.1101/gr.5193806. [ Links ]
Kamoun S. 2007. Groovy times: filamentous pathogen effectors revealed. Current opinion in plant biology 10: 358-365. https://doi.org/10.1016/j.pbi.2007.04.017. [ Links ]
Kawaide H. 2006. Biochemical and molecular analyses of gibberellin biosynthesis in fungi. Bioscience, biotechnology, and biochemistry 70: 583-590. https://doi.org/10.1271/bbb.70.583. [ Links ]
Kay S, Hahn S, Marois E, Hause G, and Bonas U. 2007. A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science 318: 648-651. http://dx.doi.org/10.1126/science.1144956. [ Links ]
Kim MG, Da Cunha L, McFall AJ, Belkhadir Y, DebRoy S, Dangl JL, and Mackey D. 2005. Two Pseudomonas syringae type III effectors inhibit RIN4-regulated basal defense in Arabidopsis. Cell 121: 749-759. http://dx.doi.org/10.1016/j.cell.2005.03.025. [ Links ]
Le Fevre R, Evangelisti E, Rey T, and Schornack S. 2015. Modulation of host cell biology by plant pathogenic microbes. Annual review of cell and developmental biology 31: 201-229. http://dx.doi.org/10.1146/annurev-cellbio-102314-112502. [ Links ]
Lilley CJ, Maqbool A, Wu D, Yusup HB, Jones LM, Birch PR, Banfield MJ, Urwin PE, and Eves-van den Akker S. 2018. Effector gene birth in plant parasitic nematodes: Neofunctionalization of a housekeeping glutathione synthetase gene. PLoS genetics 14: e1007310. https://doi.org/10.1371/journal.pgen.1007310. [ Links ]
Ma W, and Guttman DS. 2008. Evolution of prokaryotic and eukaryotic virulence effectors. Current opinion in plant biology 11: 412-419. https://doi.org/10.1016/j.pbi.2008.05.001. [ Links ]
MacLean AM, Orlovskis Z, Kowitwanich K, Zdziarska AM, Angenent GC, Immink RG, and Hogenhout SA. 2014. Phytoplasma effector SAP54 hijacks plant reproduction by degrading MADS-box proteins and promotes insect colonization in a RAD23-dependent manner. PLoS biology 12: e1001835. http://dx.doi.org/10.1371/journal.pbio.1001835. [ Links ]
McCann HC, and Guttman DS. 2008. Evolution of the type III secretion system and its effectors in plant-microbe interactions. New Phytologist 177: 33-47. http://dx.doi.org/10.1111/j.1469-8137.2007.02293.x. [ Links ]
Mccann L. 2016. Characterisation of the Cf-Ecp2 gene encoding for recognition of the conserved fungal effector Ecp2 in Solanum pimpinellifolium and Nicotiana paniculata. University of East Anglia. [ Links ]
Misas-Villamil JC, and Van der Hoorn RA. 2008. Enzyme-inhibitor interactions at the plant-pathogen interface. Current opinion in plant biology 11: 380-388. https://doi.org/10.1016/j.pbi.2008.04.007. [ Links ]
Nemri A, Saunders DG, Anderson C, Upadhyaya NM, Win J, Lawrence G, Jones D, Kamoun S, Ellis J, and Dodds P. 2014. The genome sequence and effector complement of the flax rust pathogen Melampsora lini. Frontiers in plant science 5: 98. https://doi.org/10.3389/fpls.2014.00098. [ Links ]
Oldroyd GE, and Downie JA. 2008. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol 59: 519-546. http://dx.doi.org/10.1146/annurev.arplant.59.032607.092839. [ Links ]
Panstruga R. 2003. Establishing compatibility between plants and obligate biotrophic pathogens. Current opinion in plant biology 6: 320-326. https://doi.org/10.1016/S1369-5266(03)00043-8. [ Links ]
Römer P, Hahn S, Jordan T, Strauß T, Bonas U, and Lahaye T. 2007. Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science 318: 645-648. http://dx.doi.org/10.1126/science.1144958. [ Links ]
Rosebrock TR, Zeng L, Brady JJ, Abramovitch RB, Xiao F, and Martin GB. 2007. A bacterial E3 ubiquitin ligase targets a host protein kinase to disrupt plant immunity. Nature 448: 370-374. http://doi.org/10.1038/nature05966. [ Links ]
Sarma GN, Manning VA, Ciuffetti LM, and Karplus PA. 2005. Structure of Ptr ToxA: An RGD-containing host-selective toxin from Pyrenophora tritici-repentis. The Plant Cell 17: 3190-3202. https://doi.org/10.1105/tpc.105.034918. [ Links ]
Shabab M, Shindo T, Gu C, Kaschani F, Pansuriya T, Chintha R, Harzen A, Colby T, Kamoun S, and van der Hoorn RA. 2008. Fungal effector protein AVR2 targets diversifying defense-related cys proteases of tomato. The Plant Cell 20: 1169-1183. https://doi.org/10.1105/tpc.107.056325. [ Links ]
Sohn KH, Lei R, Nemri A, and Jones JD. 2007. The downy mildew effector proteins ATR1 and ATR13 promote disease susceptibility in Arabidopsis thaliana. The Plant Cell 19: 4077-4090. https://doi.org/10.1105/tpc.107.054262. [ Links ]
Sugio A, Yang B, Zhu T, and White FF. 2007. Two type III effector genes of Xanthomonas oryzae pv. oryzae control the induction of the host genes OsTFIIAγ1 and OsTFX1 during bacterial blight of rice. Proceedings of the National Academy of Sciences 104: 10720-10725. http://dx.doi.org/10.1073/pnas.0701742104 [ Links ]
Sugio A, MacLean AM, Grieve VM, and Hogenhout SA. 2011. Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis. Proceedings of the National Academy of Sciences 108: E1254-E1263. Disponible en línea: [ Links ]
Thomma BP, van Esse HP, Crous PW, and de Wit PJ. 2005. Cladosporium fulvum (syn. Passalora fulva), a highly specialized plant pathogen as a model for functional studies on plant pathogenic Mycosphaerellaceae. Molecular plant pathology 6: 379-393. http://dx.doi.org/10.1111/j.1364-3703.2005.00292.x. [ Links ]
Tian M, Benedetti B, and Kamoun S. 2005. A second Kazal-like protease inhibitor from Phytophthora infestans inhibits and interacts with the apoplastic pathogenesis-related protease P69B of tomato. Plant Physiol 138: 1785-1793. https://doi.org/10.1104/pp.105.061226. [ Links ]
Tian M, Win J, Song J, van der Hoorn R, van der Knaap E, and Kamoun S. 2007. A Phytophthora infestans cystatin-like protein targets a novel tomato papain-like apoplastic protease. Plant Physiol 143: 364-377. https://doi.org/10.1104/pp.106.090050. [ Links ]
Tomkins M, Kliot A, Marée AF, and Hogenhout SA. 2018. A multi-layered mechanistic modelling approach to understand how effector genes extend beyond phytoplasma to modulate plant hosts, insect vectors and the environment. Current opinion in plant biology 44: 39-48. https://doi.org/10.1016/j.pbi.2018.02.002. [ Links ]
Tudzynski B. 1999. Biosynthesis of gibberellins in Gibberella fujikuroi: biomolecular aspects. Applied microbiology and biotechnology 52: 298-310. https://doi.org/10.1007/s002530051. [ Links ]
van der Hoorn RA, and Kamoun S. 2008. From guard to decoy: a new model for perception of plant pathogen effectors. The Plant Cell 20: 2009-2017. https://doi.org/10.1105/tpc.108.060194. [ Links ]
van Esse HP, van’t Klooster JW, Bolton MD, Yadeta KA, Van Baarlen P, Boeren S, Vervoort J, de Wit PJ, and Thomma BP. 2008. The Cladosporium fulvum virulence protein Avr2 inhibits host proteases required for basal defense. The Plant Cell 20: 1948-1963. https://doi.org/10.1105/tpc.108.059394. [ Links ]
Washington E, Mukhtar M, Finkel O, Wan L, Banfield M, Kieber J, and Dangl J. 2016. Pseudomonas syringae type III effector HopAF1 suppresses plant immunity by targeting methionine recycling to block ethylene induction. Proc Natl Acad Sci U S A 113: E3577-3586. Disponible en línea: [ Links ]
Whisson SC, Boevink PC, Moleleki L, Avrova AO, Morales JG, Gilroy EM, Armstrong MR, Grouffaud S, Van West P, and Chapman S. 2007. A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature 450: 115-118. http://dx.doi.org/10.1038/nature06203. [ Links ]
Win J, Morgan W, Bos J, Krasileva KV, Cano LM, Chaparro-Garcia A, Ammar R, Staskawicz BJ, and Kamoun S. 2007. Adaptive evolution has targeted the C-terminal domain of the RXLR effectors of plant pathogenic oomycetes. The Plant Cell 19: 2349-2369. https://doi.org/10.1105/tpc.107.051037. [ Links ]
Wirthmueller L, Asai S, Rallapalli G, Sklenar J, Fabro G, Kim DS, Lintermann R, Jaspers P, Wrzaczek M, and Kangasjärvi J. 2018. Arabidopsis downy mildew effector HaRxL106 suppresses plant immunity by binding to RADICAL‐INDUCED CELL DEATH1. New Phytologist 220: 232-248. Disponible en línea: [ Links ]
Xing W, Zou Y, Liu Q, Liu J, Luo X, Huang Q, Chen S, Zhu L, Bi R, and Hao Q. 2007. The structural basis for activation of plant immunity by bacterial effector protein AvrPto. Nature 449: 243-247. http://dx.doi.org/10.1038/nature06109. [ Links ]
Yoshida K, Saitoh H, Fujisawa S, Kanzaki H, Matsumura H, Yoshida K, Tosa Y, Chuma I, Takano Y, Win J et al. 2009. Association Genetics Reveals Three Novel Avirulence Genes from the Rice Blast Fungal Pathogen Magnaporthe oryzae. The Plant Cell 21: 1573-1591. https://doi.org/10.1105/tpc.109.066324. [ Links ]
Zhou J-M, and Chai J. 2008. Plant pathogenic bacterial type III effectors subdue host responses. Curr Opin Microbiol 11: 179-185. https://doi.org/10.1016/j.mib.2008.02.004. [ Links ]
Received: September 04, 2018; Accepted: October 26, 2018











 text in
text in 


