Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.36 n.3 Texcoco Oct./Dec. 2018
https://doi.org/10.18781/r.mex.fit.1804-5
Phytopathological notes
Effect of Trichoderma spp. and phytopathogenic fungi on plant growth and tomato fruit quality
1 Centro de Investigación en Alimentación y Desarrollo- CIAD; AC., Cuauhtémoc, C.P. 31570, Chihuahua, México
2 Universidad de Ciencias y Artes de Chiapas- UNICACH, Villacorzo, C.P. 29000, Chiapas, México
3 Universidad Michoacana de San Nicolás de Hidalgo- UMSNH, C.P. 58880, Michoacán, México.
The objective of this study was to determine the effect of three Trichoderma species when applied alone and when are confronted with three phytopathogens on plant growth and tomato fruit quality. The substrate of the plants was inoculated with Trichoderma spp. alone and together in confrontation with Alternaria solani, Fusarium oxysporum y Phytophthora infestans. In plant, the height and weight, length and weight of roots, total chlorophyll content in leaves, and yield were determined. Fruits were characterized by color, weight, size, total soluble solids (TSS), titratable acidity, firmness and bromatological composition. Plants inoculated with Trichoderma showed significant increases in most of the variables evaluated, where T. longibrachiatum significantly promoted the growth plants (height ≥ 13%) and yield (14%), obtaining fruits with higher titratable acidity and lower TSS content. Pathogens decreased yield (23%). Diameter, firmness and protein content in fruits increased with the inoculation of Trichoderma. Color and fiber in fruits were not affected by treatments. Trichoderma strains showed potential to improve fruit quality.
Key words: antagonists; phytopathogens; TSS; firmness; bromatological analysis
El objetivo de este estudio fue determinar el efecto de tres especies de Trichoderma cuando se aplican solas y cuando se confrontan con tres fitopatógenos, sobre el crecimiento y calidad del fruto de jitomate. El sustrato de las plantas se inoculó con Trichoderma spp. solas y en confrontación con Alternaria solani, Fusarium oxysporum y Phytophthora infestans. En planta, se determinó la altura y peso, longitud y peso de raíces, contenido total de clorofila en hojas y rendimiento. Los frutos se caracterizaron por color, peso, tamaño, sólidos solubles totales (SST), acidez titulable, firmeza y composición bromatológica. Las plantas inoculadas con Trichoderma, mostraron incrementos significativos en la mayoría de las variables de respuesta evaluadas, donde T. longibrachiatum promovió significativamente el crecimiento de la planta (altura ≥13%) y el rendimiento (14%), obteniendo frutos con mayor acidez titulable y menor contenido de SST. Los patógenos redujeron el rendimiento (23%). El diámetro, firmeza y contenido de proteína en frutos, se incrementó con la inoculación de Trichoderma. El color y fibra en frutos no resultaron afectados por los tratamientos. Las cepas de Trichoderma, mostraron potencial para mejorar la calidad del fruto.
Palabras clave: antagonistas; fitopatógenos; SST; firmeza; análisis bromatológico
Tomato plants (Solanum lycopersicon L.) are susceptible to diverse pathogens, among which some fungi stand out for their ability to cause diseases that block vascular bundles of the plants, preventing the normal flow of water and nutrients, which is reflected in reduced growth, low fruit production, among other (Yadeta and Thomma, 2013; Shafique et al., 2016). Chemical fungicides are usually applied to control these pathogens with satisfactory results, with some limitations such as the development of pathogen-induced resistance, death of beneficial microorganisms, pollution of agricultural soils and other negative effects (Shafique et al., 2016). To address these limitations, the use of antagonistic fungi and bacteria has been proposed as an alternative biological control method. Within the group of fungi, Trichoderma genus stands out due to its broad ecological plasticity, versatile metabolism, easy reproduction and antagonistic capacity (Sharma and Gothalwal, 2017). Therefore, the use of Trichoderma strains could reduce the effects of diseases caused by multiple tomato phytopathogens when food sources are reduced by the competition for space and/or nutrients, production of antimicrobial compounds, direct parasitism and stimulation of plant defense mechanisms. Trichoderma strains also promote plants growth, improve fuit quality and crops yields through phytohormones production and promotion of the availability of phosphates and other minerals necessary for plants metabolism (Sharma and Gothalwal, 2017). This is the reason why some Trichoderma strains are used as biofertilizers and phytostimulants (Colla et al., 2015; Khan et al., 2017). Several studies have been conducted with excellent results to evaluate Trichoderma strains in vitro with excellent results regarding their capacity as antagonists and growth promoters of some agronomic variables such as root system, plant height, productivity, among other (Pelagio-Flores et al., 2017). However, only a few studies have analyzed the potential effect of these microorganisms on fruit quality or their ability to reduce the indirect effects of phytopathogenic fungi on fruits. Therefore, the objective of the study was to determine the effect of three Trichoderma species on tomato growth and quality, when applied alone or confronted with three phytopathogenic fungi.
Three Trichoderma species were used: T. harzianum (Th) and T. asperellum (Ta) that were isolated from apple orchards in Chihuahua, Mexico, and T. longibrachiatum (Tl) and a strain insolated from soil in Brazilian soil, along with three phytopathogenic fungi: Alternaria solani (As), Fusarium oxysporum (Fo) and Phytophthora infestans (Pi) were isolated from tomato crops in Mexico. The pathogenicity of phytopathogenic fungi in planta and the antagonistic effect of Trichoderma species in vitro were confirmed by previous studies. Tomato seeds of cv. Merlice were purchased from DeRuiter™ (Monsanto Holland), and Bombus terrestris L. (Hymenoptera: Apidae) pollinator from Koppert Mexico, S.A. de C.V., Querétaro, Mexico. The experiment was conducted in a greenhouse in Cuauhtémoc, Chihuahua, Mexico. The seeds were germinated on 200-cavity trays and then transplanted to black plastic bags containing a mixture of autoclaved substrate prepared using loamy soil: vermiculite (Termolita, S.A. de C.V., Nuevo León, Mexico): peat moss (Lambert Peat Moss Inc., Quebec, Canada) at a 1:1:1 ratio. Fertilizer was applied every 30 days after transplanting (ddt) during the 2016 production cycle. Pollination was achieved by a Bombus terrestris beehive hive.
Fungi (antagonistic and phytopathogens) were grown for 5 d in a nutrient broth (BD Bioxon, Becton Dickinson de México, S.A. de C.V.), and Phytophthora infestans in V8 vegetable juice (V8 juice to which CaCO3 was added (3 g/L; Sigma-Aldrich). The cultures of the microorganisms were maintained at 28 °C for 5 d in constant shaking (180 rpm). The microorganisms were individually applied to substrate of 150 seedlings at 8 ddt using a completely randomized design with 10 replications (plants) per treatment. Conidia of the antagonist (3.9-7.8×107) and conidia of the phytopathogen (5.8-10.4×107) were inoculated in the substrate. Thirty plants were used to monitor the antagonists effects and 30 plants for the pathogens effects, (T2-T7; Table 1), 90 plants for confrontation tests between antagonists and pathogens (T8-T16; Table 1) and 10 plants without inoculum as a control (T1; Table 1). In the confrontation treatments, the corresponding pathogen was applied at 18 ddt (10 d after inoculating the first antagonists). The levels of antagonists in the substrate were maintained with three inoculum applications at 20-day intervals. The plants were evaluated from April to October in the 2016 production cycle.
Table 1 Treatments and concentration (conidia/mL) of microorganisms inoculated in substrate of tomato plants
| Tratamientox | conidias/mL de los microorganismos: | |||
| Código | Antagonistas | Fitopatógenos | Antagonistas | Fitopatógenos |
| T1 | Control | s/f | — | — |
| T2 | Trichoderma asperellum (Ta) | s/f | 2.9×106 | — |
| T3 | T. harzianum (Th) | s/f | 3.9×106 | — |
| T4 | T. longibrachiatum (Tl) | s/f | 5.2×106 | — |
| T5 | s/a | Fusarium oxysporum (Fo) | — | 1.3×106 |
| T6 | s/a | Alternaria solani (As) | — | 3.9×106 |
| T7 | s/a | Phytophthora infestans (Pi) | — | 2.6×106 |
| Juntos en confrontación (antagonistas vs patógenos) | ||||
| T8 | T. asperellum | F. oxysporum | 2.9×106 | 3.9×106 |
| T9 | T. asperellum | A. solani | 2.9×106 | 2.6×106 |
| T10 | T. asperellum | P. infestans | 2.9×106 | 1.3×106 |
| T11 | T. harzianum | F. oxysporum | 3.9×106 | 3.9×106 |
| T12 | T. harzianum | A. solani | 3.9×106 | 2.6×106 |
| T13 | T. harzianum | P. infestans | 3.9×106 | 1.3×106 |
| T14 | T. longibrachiatum | F. oxysporum | 5.2×106 | 3.9×106 |
| T15 | T. longibrachiatum | A. solani | 5.2×106 | 2.6×106 |
| T16 | T. longibrachiatum | P. infestans | 5.2×106 | 1.3×106 |
x s/a: without antagonists; s/f: without phytopathogens.
Chlorophyll was estimated in 10 leaves per plant, four times every 20 ddt, with a Spad 502DL Plus chlorophyll meter (Konica Minolta Brand, USA). At the end of the production cycle, plant height and weight (fresh and dry), root length and weight (fresh and dry) and stem diameter were evaluated. The number of fruits per plant was also recorded. Tomatoes were harvested at the red ripening stage of physiological maturity and stored at 4 °C for 3 d until they were analyzed. Fruits were evaluated for weight, diameter (polar and equatorial), color, total soluble solids (TSS; °Brix), tritratable acidity and firmness. Color (L*, a* and b*) was measured using a Minolta CR300 colorimeter. The °Brix values were determined using a PAL-1 digital refractometer (Atago Co. Ltd., Tokyo, Japan). The titratable acidity was expresed as the % of citric acid (NMX-F-102-S-1978) by taking 50 mL of tomato pulp diluted 25 times and tritrated with 0.1 N of NaOH, using 0.5% phenolphthalein as a color indicator. Firmness was evaluated using 50 tomatoes of each treatment, recording the maximum force (N), a puncture test was conducted using a Ta-XTPlus texture analyzer (Surrey, UK), starting at 30 cm/min and 15 cm distance. For the bromatological analysis, mashed tomato subsamples (from 1 to 10 g, depending on the parameter measured) were prepared using 20 tomatoes randomly taken from each treatment in order to measure moisture, ashes, protein (N × 6.25), fat and total fiber, according to the official methods of analysis (925.10, 923.03, 991.20 and 920.35) of AOAC (2002).
The experiment included 16 treatments (Table 1) with 10 tomato plants each, arranged in a completely randomized design. Data were analized using the SAS version 9.0 program and then subjected to an analysis of variance (ANOVA) and media separation using Tukey’s test (p= 0.05). Fruit measurements were taken by triplicate, and the obtained data were subjected to an ANOVA and media separation using Tukey’s test (p=0.05). Both were individually calculated.
The three Trichoderma isolates promoted plant growth in at least one of the evaluated response variables (Figures 1 and 2) and increased root fresh and dry weight (210.9 to 264.5, and 47.5 to 52.4 g, respectively) and root length (Figure 1). Trichoderma longibrachiatum showed the highest values for these variables and a significant increase in height (1.56 m, Figure 1) of 20 cm more than that of the controls. These results were in agreement with the existing literature (Pelagio-Flores et al., 2017). These positive effects confirm Trichoderma’s capacity as a plant growth and root biomass promoter, which could be due to its capacity to solubilize phosphates, micronutrients and mineral cations important for plants metabolism (Tucci et al., 2011). In contrast, when inoculated with F. oxysporum, P. infestans and A. solani, most of the phytopathogens exhibited less response variability (Figure 1), especially in plant height, since the plants were 14, 27 and 33 cm shorter than the control plants and also had shorter roots (28, 27 and 29 cm, respectively; Figure 1), while Trichoderma strains produced a positive effect on the stem diameter and enhanced chlorophyll accumulation in leaves. Like the rest of the treatments, except for those where As and TlvsPi were used, Trichoderma strains significantly increased yield (Figure 2). Trichoderma longibrachitum produced the highest yield with 240 g more per plant than the control plants. This could be due to the capacity of the genus to produce phytohormones such as auxins, cytokinins and gibberellins (Domínguez et al., 2016), specially indoleacetic acid (IAA) that stimulates plant growth and increases root growth (Tucci et al., 2011). Molla et al. (2012) demonstrated that when Trichoderma was inoculated in tomato plants, fruit yield increased 3-11%. These results are similar to the results of our study (8 to 27%) and are in agreement with those reported by Tucci et al. (2011) and Domínguez et al. (2016). In the field, crop productivity increases up to 300% have been documented when using Trichoderma (Khan et al., 2017). The yield of plants treated with F. oxysporum and ) alone was significantly lower compared with that of the controls, except for P. infestans, and ranged between 33,000 and 39,000 kg Ha-1, where plants treated with F. oxysporum had the lowest yields (200 g less than the control; Figure 2). For the rest of treatments (confrontations), the yields were lower than those of the control plants, except for TavsFo, ThvsPi and TlvsPi treatments, but varied among them (Figure 2). Fruits from plants treated with Trichoderma strains were larger than those from the plants of rest of the treatments (Figure 2). Plants inoculated with T. asperellum produced larger fruits. However, in some confrontation treatments no difference in size with respect to the control was observed. In contrast, plants inoculated with the pathogens alone and together in confrontation with Trichoderma strains produced small fruits. This could be due to a poor assimilation of nutrients as a result of the disease induced by the pathogens. On the other hand, the low density of the pathogen used in our study may have reduced the protective effect against pathogens on the tomato plants (Sharma and Gothalwal, 2017).
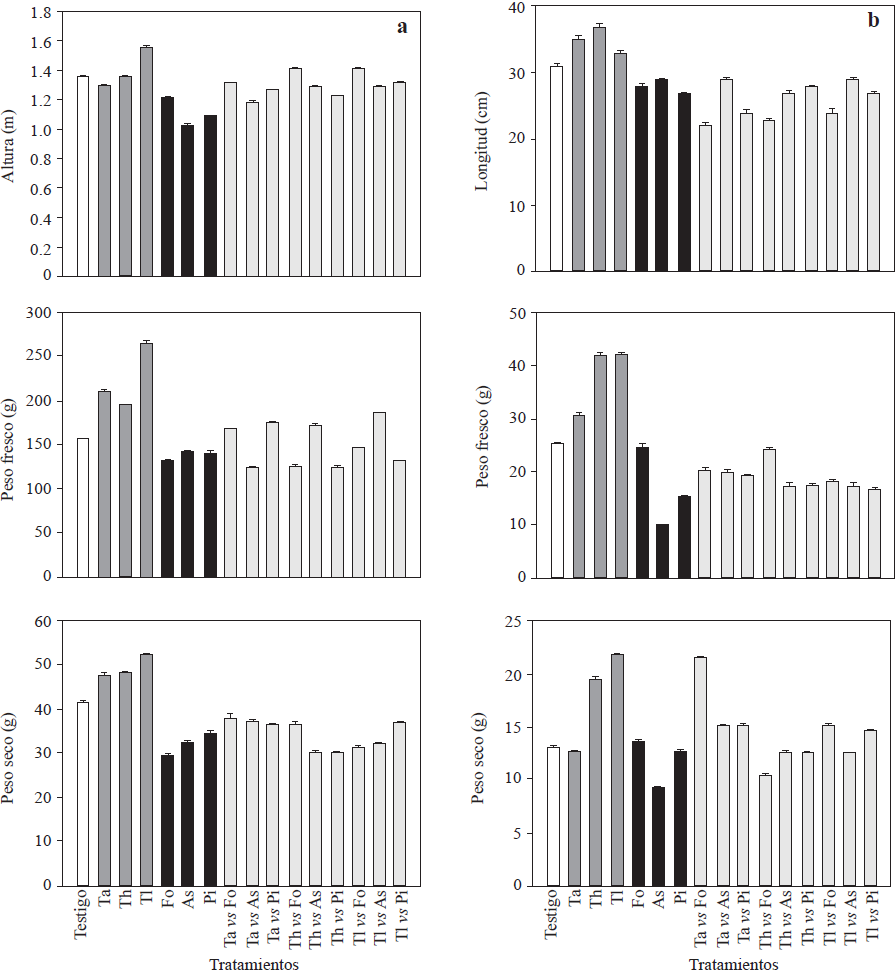
Figure 1 Growth variables (height, length, fresh and dry weight) estimated in cv. Merlice tomato plants influenced by inoculation of Trichoderma species and phytopathogenic microorganisms, alone and together in confrontation: a) plants; b) roots. Ta: T. asperellum; Th: T. harzianum, Tl: T. longibrachiatum, Fo: F. oxysporum, Pi: P. infestans, As: A. solani. Data are shown as means ± standard error.
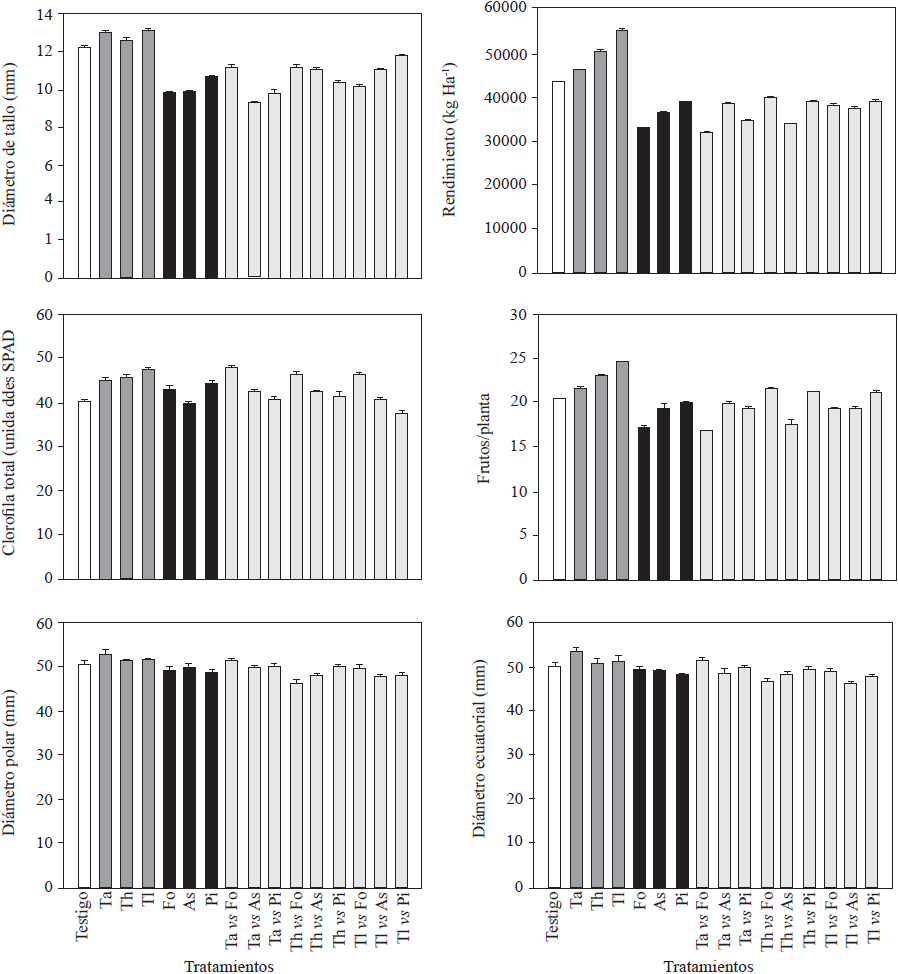
Figure 2 Agronomic variables (stem diameter, total chlorophyll, yield, number of tomatoes) and diameter (polar and equatorial) of cv. Merlice tomato fruits influenced by inoculation of Trichoderma species and phytopathogenic microorganisms, alone and together in confrontation. Ta: T. asperellum; Th: T. harzianum, Tl: T. longibrachiatum, Fo: F. oxysporum, Pi: P. infestans, As: A. solani. Data are shown as means ± standard error.
Overall, the TSS did not show a clear tendency, where the fruits harvested from plants inoculated with Trichoderma strains had the lowest °Brix content (3.5-4.0). By contrast, fruits of plants inoculated with A. solani had a higher TSS content (Table 2), possibly due to a faster rate of carbohydrate hydrolysis, a fact that may have implications for shelf life of tomatoes in the market (Tigist et al., 2013), while the increase in titratable acidity of fruits from plants treated with Trichoderma may be due to maturity and weight variability, because fruits larger in size tend to become more acid (Tigist et al., 2013). Fruit firmness ranged from 20.9 to 51.6 N. The application of T. harzianum increased fruit firmness 1.2 times compared with that of the control plants, which can be attributed to the induction of phytohormones biosynthesis when the plant’s defense mechanisms were activated, mainly ethylene, a chemical compound responsible for the expression of plants maturity genes (Shafique et al., 2016; Table 2). According to Colla et al. (2015), firm fruits are more resistant to microorganisms’ attacks. In our study, the evaluated microorganisms did not modify the color of the fruit (Table 2).
Table 2 Total soluble solids (ºBrix), titratable acidity (citric acid %), firmness and fruit color indicated by the CIELAB System parameters, L, a and b of fruits of cv. Merlice tomato plants, influenced by inoculation of Trichoderma species and phytopathogenic microorganisms, alone and together in confrontation.
| Código | Tratamiento | xSST (°Brix) | Acidez Titulable | Firmeza (N) | L | a | b |
| T1 | Testigo | 4.4 ± 0.1abc | 0.34 ± 0.02ab | 37.4 ± 0.4abcde | 49.2 ± 1.2a | 14.4 ± 0.9ab | 19.2 ± 0.9ab |
| T2 | T. asperellum | 3.5 ± 0.2c | 0.41 ± 0.02a | 28.2 ± 0.3bcde | 48.8 ± 0.5a | 15.2 ± 0.4a | 18.8 ± 1.1ab |
| T3 | T. harzianum | 3.7 ± 0.2bc | 0.41 ± 0.01a | 45.2 ± 0.4ab | 49.4 ± 0.3a | 14.5 ± 0.7ab | 21.4 ± 0.8a |
| T4 | T. longibrachiatum | 4.0 ± 0.2abc | 0.40 ± 0.02a | 20.9 ± 0.4e | 50.4 ± 0.9a | 14.7 ± 0.5ab | 21.3 ± 1.3a |
| T5 | F. oxysporum | 4.3 ± 0.3abc | 0.24 ± 0.002b | 23.3 ± 0.2cde | 48.9 ± 0.8a | 14.6 ± 0.7ab | 20.2 ± 1.3a |
| T6 | A. solani | 4.8 ± 0.3a | 0.39 ± 0.02a | 36.2 ± 0.3abcde | 50.7 ± 0.7a | 15.0 ± 0.9ab | 22.5 ± 1.0a |
| T7 | P. infestans | 4.5 ± 0.3ab | 0.32 ± 0.04ab | 51.6 ± 1.4a | 49.1 ± 0.2a | 14.5 ± 0.5ab | 21.1 ± 1.5a |
| Juntos en confrontación (antagonistas vs patógenos) | |||||||
| T8 | T. asperellum vs F. oxysporum | 4.1 ± 0.3abc | 0.32 ± 0.04ab | 50.4 ± 1.2a | 49.8 ± 0.9a | 13.5 ± 1.4ab | 19.0 ± 0.9ab |
| T9 | T. asperellum vs A. solani | 4.5 ± 0.4ab | 0.34 ± 0.04ab | 44.7 ± 0.3ab | 50.0 ± 1.0a | 14.2 ± 0.6ab | 21.0 ± 0.7a |
| T10 | T. asperellum vs P. infestans | 4.2 ± 0.3abc | 0.31 ± 0.01ab | 39.2 ± 0.2abcd | 49.3 ± 1.1a | 14.5 ± 1.2ab | 19.8 ± 1.4ab |
| T11 | T. harzianum vs F. oxysporum | 4.2 ± 0.3abc | 0.31 ± 0.04ab | 20.4 ± 0.1e | 49.5 ± 1.1a | 14.1 ± 1.0ab | 21.2 ± 1.2a |
| T12 | T. harzianum vs A. solani | 4.4 ± 0.4abc | 0.39 ± 0.02a | 37.0 ± 0.3abcde | 46.9 ± 1.0ab | 15.8 ± 0.5a | 18.9 ± 1.8ab |
| T13 | T. harzianum vs P. infestans | 4.1 ± 0.5abc | 0.34 ± 0.03ab | 29.1 ± 0.4bcde | 50.1 ± 1.2a | 15.6 ± 1.6a | 21.4 ± 1.2a |
| T14 | T. longibrachiatum vs F. oxysporum | 4.5 ± 0.3ab | 0.31 ± 0.03ab | 21.6 ± 0.1cde | 49.8 ± 1.0a | 13.2 ± 0.6bc | 21.6 ± 1.1a |
| T15 | T. longibrachiatum vs A. solani | 3.9 ± 0.2abc | 0.25 ± 0.02b | 40.5 ± 0.7abc | 40.8 ± 1.2b | 11.4 ± 1.0c | 17.8 ± 1.5b |
| T16 | T. longibrachiatum vs P. infestans | 4.4 ± 0.1abc | 0.39 ± 0.008a | 32.5 ± 0.3bcde | 50.6 ± 1.8a | 14.7 ± 1.4ab | 21.8 ± 0.7a |
xMeans with the same literal between columns are statistically equal, according to Tukey’s test (p=0.05). ± standard error.
The bromatological composition of the studied fruits was within the parameters reported in the literature (Pinela et al., 2012). Fruits moisture content ranged from 96.3 to 98.9 %. Moisture of tomatoes from plants treated with Trichoderma was similar to that of the control. However, fruits from plants inoculated with the pathogens alone and in confrontation showed an increase in moisture content (Table 3). The high level of moisture content in plants inoculated with the phytopathogens alone and in confrontation may be due to water stress induced by the pathogens, which may have inhibited both plants and fruits normal transpiration (Yadeta and Thomma, 2013). The protein content ranged from 0.33 to 0.72 % (Table 3). Tomatoes from plants treated with Trichoderma and the pathogens alone had higher protein content than those of the control plants. The tomato fat content ranged from 0.03 to 0.15 % (Table 3). This variable was lower in fruits from plants treated with Trichoderma and higher in fruits from plants treated with F. oxysporum. Regarding raw fiber, fruits from all the treatments had a similar content (1.2 a 1.8), with no significant differences (Table 3). Pinela et al. (2012) reported an ash content higher than the content found in our study, where 0.38 was the highest value, and a lower fat content ranging from 0.03 to 0.17 %, values that are similar to those of our study. The decrease of agronomic variables in plants and fruit quality parameters caused by the phytopathogens may be largely attributed to the defficient assimilation of nutrients by the diseased plants (Pusztahelyi et al., 2015).
Table 3 Bromatological analysis of cv. Merlice tomatoes influenced by inoculation of Trichoderma species and phytopathogenic microorganisms, alone and together in confrontation.
| Código | Tratamiento | Composición bromatológica (g/100 g pf)x | ||||
| Humedad (%) |
Ceniza (%) |
Proteína (%) |
Grasa (Lípidos) (%) |
Fibra (%) |
||
| T1 | Testigo | 96.8 ± 0.1ab | 0.09 ± 0.01b | 0.35 ± 0.09d | 0.08 ± 0.001bcd | 1.2 ± 0.01a |
| T2 | T. asperellum | 96.7 ± 0.1ab | 0.03 ± 0.004c | 0.43 ± 0.05cd | 0.06 ± 0.002bcd | 1.6 ± 0.08a |
| T3 | T. harzianum | 96.3 ± 0.2b | 0.02 ± 0.004c | 0.60 ± 0.1abc | 0.05 ± 0.008bcd | 1.3 ± 0.06a |
| T4 | T. longibrachiatum | 96.8 ± 0.1ab | 0.03 ± 0.003c | 0.44 ± 0.07cd | 0.03 ± 0.005d | 1.6 ± 0.08a |
| T5 | F. oxysporum | 97.1 ± 0.3ab | 0.38 ± 0.06a | 0.70 ± 0.15ab | 0.15 ± 0.002a | 1.6 ± 0.05a |
| T6 | A. solani | 97.7 ± 0.1ab | 0.08 ± 0.01bc | 0.33 ± 0.02d | 0.04 ± 0.001cd | 1.8 ± 0.02a |
| T7 | P. infestans | 97.4 ± 0.2ab | 0.10 ± 0.03b | 0.72 ± 0.06a | 0.06 ± 0.001bcd | 1.3 ± 0.04a |
| Juntos en confrontación (antagonistas vs patógenos) | ||||||
| T8 | T. asperellum vs F. oxysporum | 98.5 ± 0.1a | 0.03 ± 0.002c | 0.41 ± 0.08cd | 0.05 ± 0.003bcd | 1.5 ± 0.09a |
| T9 | T. asperellum vs A. solani | 98.5 ± 0.1a | 0.03 ± 0.003c | 0.34 ± 0.08d | 0.09 ± 0.009b | 1.3 ± 0.03a |
| T10 | T. asperellum vs P. infestans | 98.6 ± 0.5a | 0.04 ± 0.006c | 0.38 ± 0.1cd | 0.04 ± 0.002cd | 1.4 ± 0.08a |
| T11 | T. harzianum vs F. oxysporum | 98.4 ± 0.2a | 0.03 ± 0.004c | 0.43 ± 0.02cd | 0.07 ± 0.001bcd | 1.7 ± 0.09a |
| T12 | T. harzianum vs A. solani | 98.6 ± 0.8a | 0.03 ± 0.003c | 0.42 ± 0.12cd | 0.06 ± 0.002bcd | 1.8 ± 0.04a |
| T13 | T. harzianum vs P. infestans | 98.7 ± 0.1a | 0.03 ± 0.005c | 0.49 ± 0.08bbcd | 0.05 ± 0.002bcd | 1.6 ± 0.06a |
| T14 | T. longibrachiatum vs F. oxysporum | 98.9 ± 0.0a | 0.02 ± 0.008c | 0.39 ± 0.06cd | 0.08 ± 0.002bc | 1.7 ± 0.03a |
| T15 | T. longibrachiatum vs A. solani | 98.7 ± 0.0a | 0.04 ± 0.001c | 0.42 ± 0.02cd | 0.06 ± 0.001bcd | 1.4 ± 0.03a |
| T16 | T. longibrachiatum vs P. infestans | 98.2 ± 0.3a | 0.03 ± 0.004c | 0.55 ± 0.12abcd | 0.04 ± 0.001cd | 1.3 ± 0.08a |
xpf: Fresh weight. Means with the same letter between columns are statistically equal, according to Tukey’s test (p=0.05). ± Standard error.
The Trichoderma species used in this study had a positive effect on tomato plants because they improved height, biomass, chlorophyll, yield and fruit quality variables under greenhouse conditions. Based on these results, the evaluated Trichoderma strains would be a good alternative to promote plant growth and improve fruit quality attributes in horticultural crops.
Acknowledgments
The author María Fernanda Ruiz Cisneros thanks the Consejo Nacional de Ciencia y Tecnología (CONACyT) for the scholarship awarded for her Doctorate in Science studies.
REFERENCES
AOAC. 2002. Official methods of analysis. Association of Official Agricultural Chemists. 17a Ed. Washington, D.C., USA. [ Links ]
Colla G, Rouphael Y, Di Mattia E, El-Nakhel C and Cardarelli M. 2015. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. Journal of the Science of Food and Agriculture 95:1706-1715. https://dx.doi.org/10.1002/jsfa.6875 [ Links ]
Domínguez S, Rubio MB, Cardoza RE, Gutiérrez S, Nicolás C, Bettiol W, Hermosa R and Monte E. 2016. Nitrogen Metabolism and growth enhancement in tomato plants challenged with Trichoderma harzianum expressing the Aspergillus nidulans acetamidase amdS gene. Frontiers in Microbiology 7:1182. https://dx.doi.org/10.3389/fmicb.2016.01182 [ Links ]
Khan MY, Haque MM, Molla AH, Rahman MM and Alam MZ. 2017. Antioxidant compounds and minerals in tomatoes by Trichoderma-enriched biofertilizer and their relationship with the soil environments. Journal of Integrative Agriculture 16:691-703. https://dx.doi.org/10.1016/S2095-3119(16)61350-3 [ Links ]
Molla AH , Haque MM , Haque MA and Ilias GNM. 2012. Trichoderma-enriched biofertilizer enhances production and nutritional quality of tomato (Lycopersicon esculentum Mill.) and minimizes NPK fertilizer use. Agricultural Research 1:265-272. https://dx.doi.org/10.1007/s40003-012-0025-7 [ Links ]
NMX-F-102-S-1978. Determinación de la acidez titulable en productos elaborados a partir de frutas y hortalizas. Norma mexicana. Dirección general de normas. Disponible en línea: https://www.colpos.mx/bancodenormas/nmexicanas/NMX-F-102-S-1978.PDF [ Links ]
Pelagio-Flores R, Esparza-Reynoso S, Garnica-Vergara A, López-Bucio J and Herrera-Estrella A. 2017. Trichoderma-Induced acidification is an early trigger for changes in Arabidopsis root growth and determines fungal phytostimulation. Frontiers in Plant Science 8:822. https://dx.doi.org/10.3389/fpls.2017.00822 [ Links ]
Pinela J, Barros L, Carvalho AM and Ferreira ICFR. 2012. Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal homegardens. Food and Chemical Toxicology 50:829-834. https://dx.doi.org/10.1016/j.fct.2011.11.045 [ Links ]
Pusztahelyi T, Holb IJ and Pócsi I. 2015. Secondary metabolites in fungus-plant interactions. Frontiers in Plant Science 6:573. https://dx.doi.org/10.3389/fpls.2015.00573 [ Links ]
Shafique HA, Sultana V, Ehteshamul-Haque S and Athar M. 2016. Management of soil-borne diseases of organic vegetables. Journal of Plant Protection Research 56:221-230. https://dx.doi.org/10.1515/jppr-2016-0043 [ Links ]
Sharma PK and Gothalwal R. 2017. Trichoderma: A potent fungus as biological control agent. Pp:113-125. In: Singh J., Seneviratne G. (eds) Agro-Environmental Sustainability. Springer, Cham. https://dx.doi.org/10.1007/978-3-319-49724-2_6 [ Links ]
Tigist M, Workneh TS and Woldetsadik K. 2013. Effects of variety on the quality of tomato stored under ambient conditions. Journal of Food Science and Technology 50:477-486. https://dx.doi.org/10.1007/s13197-011-0378-0 [ Links ]
Tucci M, Ruocco M, de Masi L, de Palma M and Lorito M. 2011. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Molecular Plant Pathology 12:341-354. https://dx.doi.org/10.1111/j.1364-3703.2010.00674.x [ Links ]
Yadeta KA and Thomma BP. 2013. The xylem as battleground for plant hosts and vascular wilt pathogens. Frontiers in Plant Science 4:1-12. https://dx.doi.org/10.3389/fpls.2013.00097 [ Links ]
Received: April 16, 2018; Accepted: August 16, 2018











 text in
text in 


