Services on Demand
Journal
Article
Indicators
Related links
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.36 n.3 Texcoco Oct./Dec. 2018
https://doi.org/10.18781/r.mex.fit.1803-4
Scientific articles
Early morphological development of sclerotia of Sclerotinia sclerotiorum in the presence of potassium bicarbonate
1 Área de Microbiología, Posgrado de Edafología. Colegio de Postgraduados. Carretera México-Texcoco Km 36.5. Montecillo, Texcoco, Estado de México, CP. 56230 México
2 Centro de Investigación en Genética y Ambiente. Universidad Autónoma de Tlaxcala. Autopista Texmelucan-Tlaxcala Km 10.5. Ixtacuixtla CP. 90120, Tlaxcala, México
3 Instituto de Investigaciones en Ecosistemas y Sustentabilidad, Universidad Nacional Autónoma de México. Antigua Carretera a Pátzcuaro No.8701, Colonia Ex Hacienda de San José de la Huerta, Morelia, Michoacán, CP. 58190 México.
Sclerotinia sclerotiorum is a pathogen of great economic importance that causes significant losses in various crops. Control of the pathogen is difficult since this fungus forms resistant sclerotia that can survive in the soil for many years. This study evaluated the morphological development of S. sclerotiorum sclerotium primordia by using the Riddell technique, and different concentrations of potassium bicarbonate (KHCO3). The formation of primordia began from hyphae. However, as the concentration of KHCO3 increased, morphological changes were observed in the initiation phase of the sclerotia, as well as in the inhibition of their development when using a 50 mM concentration of KHCO3. This chemical compound modifies the morphology and inhibits the development of sclerotia in their initial stages; hence it may offer potential as an alternative to synthetic fungicides for the control of plant diseases caused by S. sclerotiorum.
Key words: antifungal agent; morphogenesis; inhibition; microscopy
Sclerotinia sclerotiorum es un patógeno de suma importancia económica que causa grandes pérdidas en varios cultivos. Controlar este patógeno es difícil porque forma estructuras de resistencia llamadas esclerocios que pueden mantenerse viables en el suelo por muchos años. Este estudio evaluó el desarrollo morfológico de los primordios de esclerocios de S. sclerotiorum utilizando la técnica de Riddell, y diferentes concentraciones de bicarbonato de potasio (KHCO3). La formación de los primordios de los esclerocios inició a partir de hifas; sin embargo, conforme las concentraciones de KHCO3 incrementaron, se observaron cambios morfológicos en la fase de iniciación de los esclerocios, así como en la inhibición de su desarrollo al utilizar una concentración de 50 mM de KHCO3. Este compuesto químico modifica la morfología e inhibe el desarrollo de esclerocios en su fase inicial y, por tanto, podría utilizarse como alternativa a los fungicidas sintéticos para controlar enfermedades de plantas causadas por S. sclerotiorum.
Palabras clave: agente antifúngico; morfogénesis; inhibición; microscopia
White mold is caused by Sclerotinia sclerotiorum, a fungus that belongs to the family Sclerotiniaceae. This is a destructive fungal pathogen for many agricultural crops such as sunflower, soybean, oilseed rape, bean, chickpea, canola, and onion (Hegedus and Rimmer, 2005: Bolton et al; 2006). It has little host specificity, thus being able to infect over 400 plant species, mainly dicotyledons (Fernando et al., 2004; Hegedus and Rimmer, 2005). The environmental conditions that promote the fungal infection are high humidity and temperatures between 15 and 25 °C (Saharan and Mehta, 2008). Secretion of fungal enzymes such as cellulases and pectinases, that soften and degrade plant tissues are involved in the plant infection process (Fernando et al., 2004; Bolton et al., 2006), as well as production of oxalic acid, which has toxic effects on the tissue of the host (Hegedus and Rimmer, 2005). One characteristic of this pathogen is the formation of sclerotia, fungal structures of resistance and dispersal, which under favorable conditions can remain viable for several years in soils (Bae and Knudsen, 2007; Calvo and Cary 2015; Smith et al., 2015).
During the formation of sclerotia, three stages or phases have been identified: 1) initiation: aggregation of hyphae, 2) development: hyphal growth for greater size, and 3) maturation: creation of surface boundaries, internal consolidation and melanization (Le Tourneau, 1979; Rollins and Dickman, 2001; Bolton et al., 2006; Saharan and Mehta, 2008). The initiation and maturation stages may be influenced by abiotic factors such as photoperiod, temperature, oxygen, and nutrient availability (e.g. carbon sources), and the morphogenesis and further development of sclerotia started between 12 and 24 h of fungal growth (Hansberg and Aguirre, 1990).
The sclerotium is composed by three layers: a thick and pigmented outer layer, an intermediate and thin layer, and an internal white layer called the inner medulla (Punja and Damiani, 1996; Bardin and Huang, 2001). Depending on environmental conditions, sclerotia grow belowground in one of two ways: 1) by forming mycelium that potentially infects roots and causes rot and wilting of plant tissues, or 2) by producing apothecia, in which ascospores are produced and released, then infecting aerial plant organs (Humpherson-Jones and Cooke, 1977; Mónaco et al., 1998; Bolton et al., 2006).
Bicarbonates possess antimicrobial properties of wide spectrum, and their efficiency has been proven for controlling many plant pathogenic fungi (Bombelli and Wright, 2006; Arslan, 2015). The Environmental Protection Agency (EPA) of the United States also recognizes bicarbonates as innocuous and safe compounds for both human health and environment (Palmer et al., 1997; Bombelli and Wright, 2006), since their use may decrease the utilization of pesticides. Some studies have shown that sodium, potassium and ammonium carbonates and bicarbonates inhibit the post-harvest growth of several fungal pathogens in fruits, vegetables and ornamental plants (Karabulut et al., 2003; Arslan et al., 2006; Jabnoun-Khiareddine et al., 2016). Bicarbonates alter the permeability of fungal membranes, inhibit the reactions of oxidative phosphorylation, and exert toxic effects on the structures of the pathogen (Avis, 2007). However, their efficacy depends on the concentration (0.2-3%) and on the susceptibility of each microorganism. For instance, treatments with sodium carbonate and bicarbonate improved the control of the green mold caused by Penicillium digitatum Sacc. (Trichocomaceae), in citrus fruits (Smilanick et al., 1999). Sodium and potassium bicarbonates also reduced powdery mildew caused by Leveillula taurica (Lév.) Arnaud (Erysiphaceae) in peppers (Fallik et al., 1997), and decreased the conidiogenesis by Helminthosporium solani Durieu y Mont. (Pleosporaceae) (Olivier et al, 1998). In addition, Bombelli and Wright (2006), and Türkkan et al. (2017) observed the growth inhibition of Botrytis cinerea Pers. Fr. (Sclerotiniaceae) when exposed to different bicarbonates with in vitro cultures. Plant disease control in carrot, cucumber and cantaloupe fruits has been also reported due to the application of bicarbonates (Aharoni et al., 1997; Bombelli and Wright, 2006).
Overall, the inhibitory effects of potassium bicarbonate (KHCO3) on the growth of S. sclerotiorum, as well as on the germination and formation of new sclerotia have been described (Ordóñez-Valencia et al., 2009), but the effects of this chemical compound during early stages of sclerotia morphological development are not well understood. Thus, the aim of this study was to evaluate the effects of different doses of potassium bicarbonate on the early stages and primordia development of sclerotia by S. sclerotiorum via microscopic observations.
MATERIALS AND METHODS
Sclerotinia sclerotiorum was obtained from the microbial collection of the Soil Microbiology Department, Colegio de Postgraduados. The fungus utilized in the present experiment was previously isolated from vegetative material of lettuce plantations at the Bajío region in Guanajuato, Mexico. In order to examine the effects of different concentrations of potassium bicarbonate (KHCO3) on the microscopic growth of S. sclerotiorum, the Riddell’s microculture technique was used (Riddell, 1950). This consisted in placing a V-shaped glass rod into glass Petri dishes which included microscopic slides and cover slips. Later, the Petri dishes were sterilized at 150 °C for 5 h. After sterilization, a 10 mm diameter-disk of Potato Dextrose Agar medium (PDA® Merck, Darmstat, Germany) enriched with each concentration of KHCO3 (Fermont®, Monterrey, Mexico; 0, 2, 4, 6, 8, 10, 25 and 50 mM), were placed on the microscopic slides. Then, S. sclerotiorum was inoculated with a sterile needle on one side of the PDA-disk, and later, the cover slip was placed on it. This procedure was repeated twice for each KHCO3 concentration, and a control without bicarbonate was also included.
In order to maintain the humidity in the Petri dishes, 10 mL of 10% glycerol were added. All Petri dishes were kept at room temperature conditions (~20 °C) and an approximate photoperiod of 12 h. Every day, the fungal growth was monitored under optical microscope (Leica CME, U.S.A.). Once the PDA-disk was fully covered with the fungal mycelium (approximately seven days of incubation), glycerol was replaced with a 10% formaldehyde solution, which was kept for 2 h for permanently fixing the fungal structures.
Later, the microscopic slide was removed from the Petri dish to prepare the fungal slides. The cover slip was carefully separated from the agar and placed on another clean slide on which a drop of the colorant cotton blue in lactophenol was added. The next step was to remove the PDA-disk from the original microscopic slide on which the colorant was also added, and a clean cover slip was immediately placed on it. In this way, four fungal preparations were obtained from each concentration of KHCO3, including the control without bicarbonate. Once the excess of colorant was removed, the stained fungal preparations were sealed with colorless nail polish, and evaluated under light microscope. The microscopic evaluations consisted on identifying the growth of the sclerotial primordia in each concentration of KHCO3. For this, an optical microscope (OLYMPUS BX51, Japan) was utilized for taking microphotographs of the fungal structures under phase contrast microscopy. Chi-square “goodness of fit” tests were performed in order to compare the effect of different concentrations of sodium bicarbonate on sclerotia formation for each one of the four structure phases. For it was used the VassarStats: Web Site for Statistical Computation (Lowry 2001-2018).
RESULTS AND DISCUSSION
The presented fungal structures are part of the process of sclerotia formation by S. sclerotiorum, and were microscopically observed before the initiation stage. The sclerotial formation in the control treatment (Figure 1, A-C) began with the proliferation of primary branching of main hyphae, thus, denoting the formation of the sclerotial primordia. As second structure, the hyphal branching became more profuse, and the presence of septa was observed in apical zones (Figure 1, D-F), while the third structure was characterized by presenting small hyphal clusters (Fig. 1, G-I). Finally, in the fourth structure, a massive cluster of hyphae was observed, in which some pigmented cells were visible (Figure 1, J-L). The initiation stage began with the union of several hyphal clusters, and this stage was macroscopically observed when a hyphal conglomerate starts growing on the surface of the culture medium.
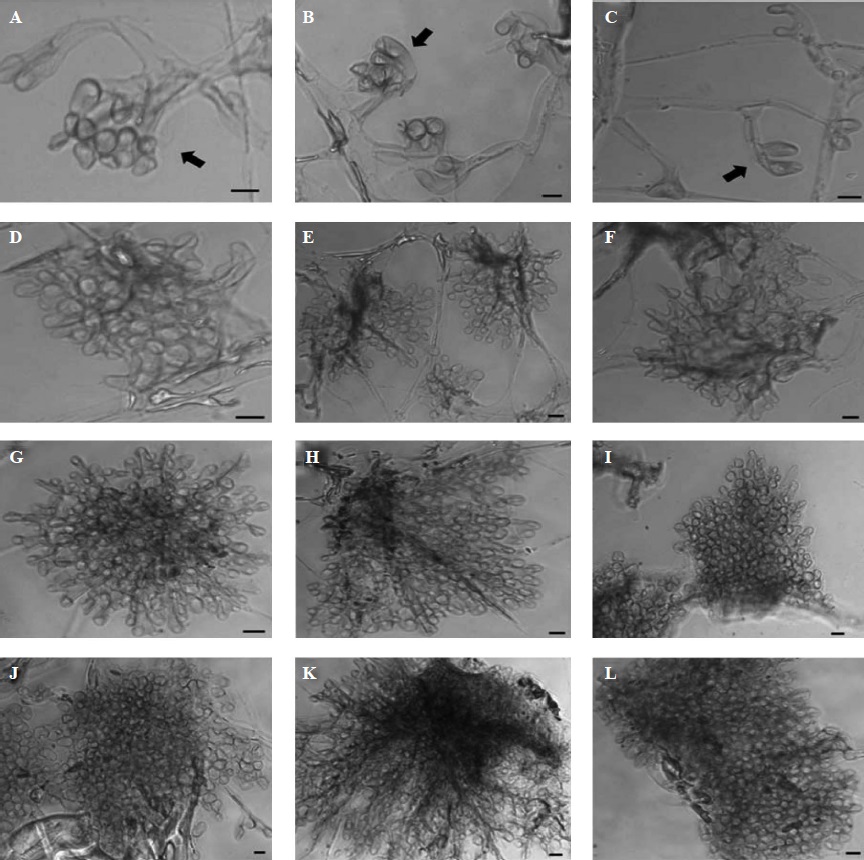
Figure 1 Microscopic developmental structures observed during the sclerotial formation of Sclerotinia sclerotiorum after seven days of fungal growth, without KHCO3 (Control). First structure: branching of hyphae, indicated by arrows (A-C); second structure: profuse branching of hyphae (D-F); third structure: clustering of hyphae (G-I); fourth structure: massive clustering of hyphae that build up the sclerotia in the initiation process (J-L). Microphotographs were taken in phase contrast microscopy at 40X magnification. Bars = 10 µm.
Results showed that the application of KHCO3 had inhibitory effects on the morphology of the sclerotial primordia (Table 1). In fact results from the 50 mM dose were not included on statistical analysis due the complete absence of mycelial growth. Unlikely the treatment without bicarbonate (control) and 2 mM bicarbonate, treatments with all the others concentrations of this chemical compound showed only three developmental structures, in which we noticed that the primordia were irregularly shaped with the formation of loose cells. As the concentration of KHCO3 increased, primordia became smaller and less compact. The increase in the concentration of bicarbonate resulted in growth inhibition of both hyphae and sclerotia (Figure 2 and 3). However, at 2 mM, 4 mM and 6 mM concentrations of KHCO3 only scarce morphological changes were observed.
Table 1 Frequencies of sclerotia on each structure phase. The value on last column indicates the probability of not effects due to sodium bicarbonate exposition for each structure phase (chi square “goodness fit”).
| Tratamientos Concentraciones de bicarbonato de sodio | ||||||||
| Fase de la estructura | Control | 2 mM | 4 mM | 6 mM | 8 mM | 10 mM | 25 mM | Probabilidad |
| I | 6 | 5 | 5 | 6 | 5 | 5 | 7 | 0.8606 |
| II | 5 | 5 | 5 | 5 | 5 | 5 | 0 | 0.2914 |
| III | 4 | 5 | 5 | 6 | 5 | 5 | 0 | <0.0001 |
| IV | 4 | 5 | 0 | 0 | 0 | 0 | 0 | <0.0001 |
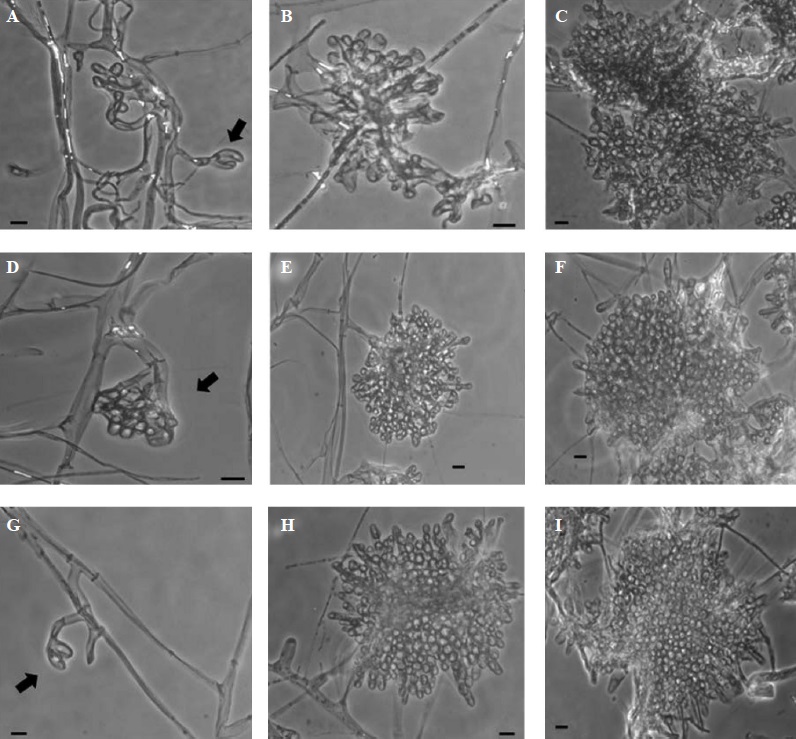
Figure 2 Microscopic developmental structures observed during the sclerotial formation of Sclerotinia sclerotiorum, after seven days of fungal growth. First (indicated by arrows), second, and third structure of sclerotial development in presence of KHCO3: (A-C) 2 mM, (D-F) 4 mM, and (G-I) 6 mM. Microphotographs were taken in phase contrast microscopy at 40X magnification. Bars = 10 µm.
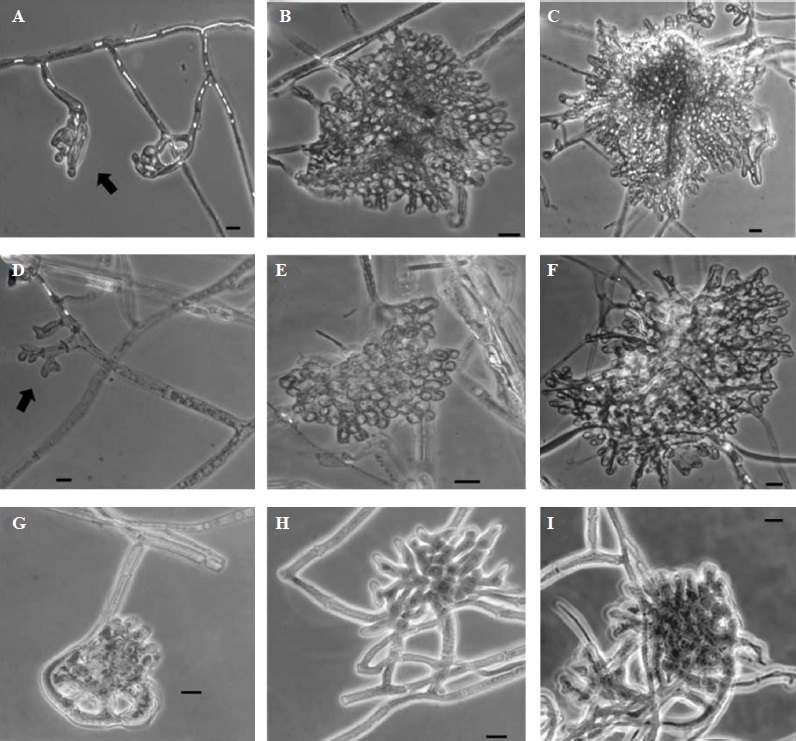
Figure 3 Microscopic developmental structures observed during the sclerotial formation of Sclerotinia sclerotiorum, after seven days of fungal growth. First (indicated by arrows), second and third structures of sclerotial development in presence of KHCO3: (A-C) 8 mM, and (D-F) 10 mM. For 25 mM a cellular disorganization of the primordium was observed (G-I), in which the second and third structures of sclerotial development were not observed. Microphotographs were taken in phase contrast microscopy at 40X magnification. Bars = 10 μm.
The most notorious morphological changes were evident after the fungus was exposed to concentrations greater than 8 mM of KHCO3. At 10 mM, the presence of primordia was noticed although not very well developed. Despite this, it was possible to observe the third structures of development (Figure 3, F), but it was not similar to the control by showing irregular formations of the sclerotium in which hyphal clusters were more loose (Figure 1, G-I). At 25 mM concentration, the formation of sclerotia primordia was scarce and disorganized (Figure 3, G-I), hence either the second or third structures of the sclerotium initiation could not be completed nor observed (Table 1). Finally, at the 50 mM concentration, no effects were noticed due to the absence of fungal growth in this treatment.
In this study, we observed the formation process of sclerotia during their initiation phase in which the four stages of development were identified (Bolton et al., 2006; Saharan and Mehta, 2008). However, the sclerotial formation showed variations depending on the concentration of KHCO3. The inhibitory effect of bicarbonates on the growth of several species of phytopathogenic fungi, especially during postharvest, has been recorded before (Aharoni et al., 1997; Palmer et al., 1997; Bombelli and Wright, 2006; Jabnoun-Khiareddine et al., 2016), and Ordóñez-Valencia et al. (2009) demonstrated that KHCO3 inhibited the growth of S. sclerotiorum in in vitro cultures. The inhibitory effect of bicarbonate salts on fungi was probably due to reduced fungal cell turgor pressure, which resulted in collapse and shrinkage of hyphae (Türkkan et al., 2017).
The formation of sclerotial primordia by S. sclerotiorum was initiated by branching and clustering of hyphae (Figure 1), resulting in a mass of cells that eventually originate mature sclerotia. Similar effects were observed by Smits and Noguera (1988) in the formation of sclerotia of Macrophomina phaseolina, which began from hyphal branching and entwinements, besides the increase in size of the associate cells and the reduction in size of the sclerotial mass. Townsend and Willets (1954) observed different development patterns (thickening, branching, and septation of main hyphae and their entwinement) in Rhizoctonia solani, Botrytis allii, B. cinerea, and Sclerotium cepivorum.
In the present study, KHCO3 resulted in microscopic morphological changes during early phases of sclerotia development. The increase of bicarbonate concentrations resulted in less profuse and loose hyphal branching, leading to the decrement and consequent absence of well-formed sclerotia (Table 1). Igwegbe et al. (1977) reported that the addition of 50 µg mL-1 of 6-metilpurine caused significant reduction in the sclerotia formation by S. rolfsii.
The addition of KHCO3 caused an increase in pH (from 6.5 to 8.0) in the culture medium (Ordóñez-Valencia et al., 2009), which resulted in reductions of fungal growth. In this regard, Alexander (1977) mentioned that many fungi grow better under acidic conditions than alkaline, because an acidic environment is not conducive to the existence of either bacteria or actinomycetes, resulting in the monopoly of fungi for utilization of organic substrates (Giri et al., 2005). On the other hand, it has been observed that both growth and development of sclerotia of S. sclerotiorum depend on the pH and the production of oxalic acid (Rollins and Dickman, 2001; Chen et al., 2004). Neutral or alkaline pH values inhibit the formation of sclerotia, and the production of oxalic acid helps reducing the alkaline pH of the medium, creating more favorable conditions for the development of sclerotia (Rollins and Dickman, 2001).
Although some reports have described the negative effects of bicarbonate on certain plant pathogenic fungi (Bombelli and Wright, 2006; Ordóñez-Valencia et al., 2009), yet the present study is one of the first reports describing inhibitory effects of KHCO3 on S. sclerotiorum during the initial phases of the sclerotia formation as well as on the morphology of sclerotial primordia.
The inhibitory effects of KHCO3 on fungal growth and development may in part be explained by affecting vital biochemical processes such as the biogenesis of either the fungal cell wall and/or the apical wall (Sentandreu et al., 1994; Sideri and Georgiou, 2000). Certain antimicrobial compounds cause oxidative stress in fungi which may show morphological changes, impaired growth rate, and low content of proteins and ATP (Harel et al., 2005; Marcet-Houben and Gabaldon, 2011). In this regard, the application of KHCO3 may account on the production of reactive oxygen species (ROS) as a response of the stress generated by this salt, then, causing alterations on the morphology and development of S. sclerotiorum.
Bicarbonate ions cause alterations in oxidation and nitration reactions in biological systems, regulate pH, and stimulate the production of either reactive nitrogen species such as peroxynitrite (ONOO-) or superoxide (O2-) (Knorev et al., 2000; Arai et al., 2005; Lushchak et al., 2009). As a result of oxidative stress in combination with abiotic factors also have negative effects on the sclerotia formation in filamentous fungi (Georgiou et al., 2006). Moreover, Sideri and Georgiou (2000) demonstrated that the production of hydrogen peroxide (H2O2) in S. rolfsii (Typhulaceae) exposed to different light and iron conditions; the highest production of H2O2 was recorded during early stages of fungal growth. However, as sclerotia become mature the hydrogen peroxide production decreased. Nevertheless, further research is needed to elucidate the effects of KHCO3 on either physiological, biochemical or molecular processes during fungal morphogenesis.
CONCLUSIONS
The present results clearly demonstrate that KHCO3 is effective in controlling both growth and development of S. sclerotiorum under in vitro conditions, which would help reducing the formation of sclerotia, as well as being a valid alternative for replacing or reducing the use ofiguraf synthetic fungicides. However, this is a preliminary study; thus, the efficiency of KHCO3 must also be studied in a soil including plant-pathogen interactions. Moreover, S. sclerotiorum can spend 90% of its life cycle as sclerotia, thus it is important to direct more research efforts to know in detail the processes involved in either early initiation or further development of this persistent fungal structures.
Acknowledgements
Authors thanks the National Council of Science and Technology (CONACYT, Mexico) for financial support to Claudia Ordóñez-Valencia (MSc. studies), and to the grant SEP-CONACyT 58594. Authors also thanks the comments and suggestions of two anonymous reviewers.
REFERENCES
Aharoni Y, Fallik E, Copel A, Gil M, Grinberg S, and Klein JD. 1997. Sodium bicarbonate reduces postharvest decay development on melons. Postharvest Biology and Technology 10:201-206. https://doi.org/10.1016/S0925-5214(97)01412-9 [ Links ]
Alexander M. 1977. Introduction to soil microbiology, 2nd edition. New York, John Wiley and Sons. [ Links ]
Arai H, Berlett BS, Chock PB, and Stadtman ER. 2005. Effect of bicarbonate on iron-mediated oxidation of low-density lipoprotein. Proceedings of the National Academy of Sciences 102:10472-10477. https://doi.org/10.1073/pnas.0504685102 [ Links ]
Arslan U. 2015. Evaluation of antifungal activity of mono and dipotassium phosphates against phytopathogenic fungi. Fresenius Environmental Bulletin 24:810-816. Disponible en línea: https://www.researchgate.net/publication/281927544_Evaluation_of_antifungal_activity_of_mono_and_dipotassium_phosphates_against_phytopathogenic_fungi [ Links ]
Arslan K, Ilhan U and Karabulut OA. 2006. Evaluation of food additives and low-toxicity compounds for the control of bean rust and wheat leaf rust. Journal of Phytopathology 154:534-541.https://doi.org/10.1111/j.1439-0434.2006.01144.x [ Links ]
Avis TJ. 2007. Antifungal compounds that target fungal membranes: applications in plant disease control. Canadian Journal of Plant Pathology 29:323-329. https://doi.org/10.1080/07060660709507478 [ Links ]
Bae YS and Knudsen GR. 2007. Effect of sclerotial distribution pattern of Sclerotinia sclerotiorum on biocontrol efficacy of Trichoderma harzianum. Applied Soil Ecology 35:21-24. https://doi.org/10.1016/j.apsoil.2006.05.014 [ Links ]
Bardin SD and Huang HC. 2001. Research on biology and control of Sclerotinia diseases in Canada. Canadian Journal of Plant Pathology 23:88-98. https://doi.org/10.1080/07060660109506914 [ Links ]
Bolton MD, Thomma BPHJ and Nelson BD. 2006. Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Molecular Plant Pathology 7:1-16. https://doi.org/10.1111/j.1364-3703.2005.00316.x [ Links ]
Bombelli EC y Wright ER. 2006. Efecto del bicarbonato de potasio sobre la calidad del tomate y acción sobre Botrytis cinerea en postcosecha. Ciencia e Investigación Agraria 33:197-203. Disponible en línea: https://www.researchgate.net/publication/28138683_Efecto_del_bicarbonato_de_potasio_sobre_la_calidad_del_tomate_y_accion_sobre_Botrytis_cinerea_en_poscosecha [ Links ]
Calvo AM and Cary JW. 2015. Association of fungal secondary metabolism and sclerotial biology. Frontiers in Microbiology 6:1-16. https://doi.org/10.3389/fmicb.2015.00062 [ Links ]
Chen C, Harel A, Gorovoits R, Yarden O and Dickman MB. 2004. MAPK Regulation of sclerotial development in Sclerotinia sclerotiorum in linked with pH and cAMP sensing. Molecular Plant-Microbe Interactions 17:404-413. https://doi.org/10.1094/MPMI.2004.17.4.404 [ Links ]
Fallik E, Ziv O, Grinberg S , Alkalai S andKlein JD . 1997. Bicarbonate solutions control powdery mildew (Leveillula taurica) on sweet red pepper and reduce the development of postharvest fruit rotting. Phytoparasitica 25:41-43. https://doi.org/10.1007/BF02981478 [ Links ]
Fernando WGD, Nakkeeran S and Zhang Y. 2004. Ecofriendly methods in combating Sclerotinia sclerotiorum (Lib.) de Bary. Pp: 329-347. In: Pandalai SG (ed). Recent Research Developments in Environmental Biology. Research Signpost. India. Disponible en línea: https://www.researchgate.net/publication/238111476_Ecofriendly_methods_in_combating_Sclerotinia_sclerotiorum_Lib_de_Bary [ Links ]
Georgiou DC, Patsoukis Ν, Papapostolou Ι and Zervoudakis G. 2006. Sclerotial metamorphosis in filamentous fungi is induced by oxidative stress. Integrative and Comparative Biology 46:691-712. https://doi.org/10.1093/icb/icj034 [ Links ]
Giri B, Giang PH, Kumari R, Prasad R and Varma A. 2005. Microbial diversity in soils. Pp: 19-55. In: Buscot F and Varma A. (eds.). Microorganisms in soils: roles in genesis and functions. Springer-Verlag Berlin Heidelberg. 422p. DOI: 10.1007/b137872 [ Links ]
Hansberg W and Aguirre J. 1990. Hyperoxidant states cause microbial cell differentiation by cell isolation from dioxygen. Journal of Theoretical Biology 142:201-221. https://doi.org/10.1016/S0022-5193(05)80222-X [ Links ]
Harel A , Gorovits R andYarden O . 2005. Changes in protein kinase A activity accompany sclerotial development in Sclerotinia sclerotiorum. Phytopathology 95:397-404. https://doi.org/10.1094/PHYTO-95-0397 [ Links ]
Hegedus DD and Rimmer SR. 2005. Sclerotinia sclerotiorum: When “to be or not to be” a pathogen?. FEMS Microbiology Letters 251:177-184. https://doi.org/10.1016/j.femsle.2005.07.040 [ Links ]
Humpherson-Jones FM and Cooke RC. 1977. Morphogenesis in sclerotium-forming fungi. II. Rhythmic production of sclerotia by Sclerotinia sclerotiorum (Lib.) de Bary. New Phytology 78:181-187. https://doi.org/10.1111/j.1469-8137.1977.tb01556.x [ Links ]
Igwegbe ECK, King V and Salary J. 1977. 6-methylpurine-induced inhibition of sclerotia morphogenesis in Sclerotium rolfsii and its reversal by adenosine. Mycopathologia 62:153-159. https://doi.org/10.1007/BF00444108 [ Links ]
Jabnoun-Khiareddine H, Abdallah R, El-Mohamedy R, Abdel-Kareem F, Gueddes-Chahed M, Hajlaoui A and Daami-Remadi M. 2016. Comparative efficacy of potassium salts against soil-borne and air-borne fungi and their ability to suppress tomato wilt and fruit rots. Journal of Microbial and Biochemical Technology 8:45-55. http://dx.doi.org/10.4172/1948-5948.1000261 [ Links ]
Karabulut OA, Smilanick JL, Mlikota F, Mansour M and Droby S. 2003. Near-harvest applications of Metschnikowia fructicola, ethanol, and sodium bicarbonate to control postharvest diseases of grape in central California. Plant Disease 87:1384-1389. https://doi.org/10.1094/PDIS.2003.87.11.1384 [ Links ]
Knorev EA, Zhang H, Joseph J, Kennedy MC and Kalyanaraman B. 2000. Bicarbonate exacerbates oxidative injury induced by antitumor antibiotic doxorubicin in cardiomyocytes. American Journal of Physiology Heart and Circulatory Physiology 279:2424-2430. https://doi.org/10.1152/ajpheart.2000.279.5.H2424 [ Links ]
Le Tourneau D. 1979. Morphology, cytology and physiology of Sclerotinia species in culture. Phytopathology 69:887-890. http://dx.doi.org/10.1094/Phyto-69-887 [ Links ]
Lowry R. 2001-2018. VassarStats. A Web Site for Statistical Computation. http://vassarstats.net/csfit.html [ Links ]
Lushchak OV, Bayliak MM, Korobova OV, Levine RL and Lushchak VI. 2009. Buffer modulation of menadione-induced oxidative stress in Saccharomyces cerevisiae. Redox Report 14:214-220. https://doi.org/10.1179/135100009X12525712409454 [ Links ]
Marcet-Houben M and Gabaldón T. 2011. Evolution of fungi and their respiratory metabolism. Pp: 257-272. In: Pontarotti P (ed). Evolutionary Biology-Concepts, Biodiversity, Macroevolution and Genome Evolution. Heidelberg: Springer-Verlag, Berlin. 345p. https://doi.org/10.1007/978-3-642-20763-1_15 [ Links ]
Mónaco CI, Rollán MC y Nico AI. 1998. Efecto de micoparásitos sobre la capacidad reproductiva de Sclerotinia sclerotiorum. Revista Iberoamericana de Micología 15:81-84. Disponible en línea: https://www.researchgate.net/publication/237685229_Efecto_de_micoparasitos_sobre_la_capacidad_reproductiva_de_Sclerotinia_sclerotiorum [ Links ]
Olivier C, Halseth ED and Mizubuti ESGm, Loria R. 1998. Postharvest application of organic and inorganic salts for suppression of silver scurf on potato tubers. Plant Disease 82:213-217. https://doi.org/10.1094/PDIS.1998.82.2.213 [ Links ]
Ordóñez-Valencia C, Alarcón A, Ferrera-Cerrato R and Hernández-Cuevas LV. 2009. In vitro antifungal effects of potassium bicarbonate on Trichoderma sp. and Sclerotinia sclerotiorum. Mycoscience 50:380-387. https://doi.org/10.1007/S10267-009-0495-Z [ Links ]
Palmer CL, Horst RK and Langhans RW. 1997. Use of bicarbonates to inhibit in vitro colony growth of Botrytis cinerea. Plant Disease 81:1432-1438. https://doi.org/10.1094/PDIS.1997.81.12.1432 [ Links ]
Punja ZK and Damiani A. 1996. Comparative growth, morphology and physiology of three Sclerotium species. Mycologia 88:694-706. http://dx.doi.org/10.2307/3760963 [ Links ]
Riddell RW. 1950. Permanent stained mycological preparation obtained by slide culture. Mycologia 42:265-270. http://dx.doi.org/10.2307/3755439 [ Links ]
Rollins JA and Dickman MB. 2001. pH signaling in Sclerotinia sclerotiorum: identification of a pacC/RIM1 homolog. Applied and Environmental Microbiology 67:75-81. http://dx.doi.org/10.1128/AEM.67.1.75-81.2001 [ Links ]
Saharan GS and Mehta N. 2008. Reproduction and reproductive structures. Pp: 113-161. In: Saharan GS and Mehta N (eds). Sclerotinia diseases of crop plants: biology, ecology and disease management. Springer. India. 418p. https://doi.org/10.1007/978-1-4020-8408-9_8 [ Links ]
Sentandreu R, Mormeneo S and Ruiz-Herrera J. 1994. Biogenesis of the fungal cell wall. Pp: 111-124. In: Wessels JGH, Meinhardt F (eds). The Mycota I Growth, differentiation and sexuality. Springer-Verlag. Berlin Heidelberg. 521p. https://doi.org/10.1007/978-3-662-11908-2_6 [ Links ]
Sideri M andGeorgiou DC . 2000. Differentiation and hydrogen peroxide production in Sclerotium rolfsii are induced by the oxidizing growth factors, light and iron. Mycologia 92:1033-1042. http://dx.doi.org/10.2307/3761468 [ Links ]
Smilanick JL , Margosan DA, Mlikota F , Usall J and Michael IF. 1999. Control of citrus green mold by carbonate and bicarbonate salts and the influence of commercial postharvest practices on their efficacy. Plant Disease 83:139-145. https://doi.org/10.1094/PDIS.1999.83.2.139 [ Links ]
Smith ME, Henkel TW andRollins JA . 2015. How many fungi make sclerotia? Fungal Ecology 13:211-220. https://doi.org/10.1016/j.funeco.2014.08.010 [ Links ]
Smits GB and Noguera R. 1988. Ontogeny and morphogenesis of sclerotia and pycnidia of Macrophomina phaseolina. Agronomia Tropical Maracay 38:69-78. [ Links ]
Türkkan M, Özcan M and Erper I. 2017. Antifungal effect of carbonate and bicarbonate salts against Botrytis cinerea, the causal agent of grey mould of kiwifruit. Akademik Ziraat Dergisi 6:107-114. http://dx.doi.org/10.29278/azd.371066 [ Links ]
Townsend BB and Willetts HJ. 1954. The development of sclerotia of certain fungi. Transactions of the British Mycological Society 37:213-221. https://doi.org/10.1016/S0007-1536(54)80003-9 [ Links ]
Received: March 28, 2018; Accepted: June 27, 2018











 text in
text in 


