Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.36 n.1 Texcoco Jan./Apr. 2018
https://doi.org/10.18781/r.mex.fit.1710-2
Phytopathological notes
Morphological and molecular identification of Mortierella species associated to rhizosphere of apple trees with symptoms of root diseases
1 Universidad Autónoma de Chihuahua, Facultad de Ciencias Químicas, Campus Universitario #2, Circuito Universitario, Chihuahua. CP. 31125, Chihuahua, Chihuahua, México.
2 Centro de Investigación en Alimentación y Desarrollo, A.C., Campus Cuauhtémoc. Avenida Río Conchos S/N. Parque Industrial, CP. 31570, Cuauhtémoc, Chihuahua, México.
Mortierella species were isolated on Potato Dextrose Agar and V8-agar as well as mature pear fruits and azalea leaves as substrate baits. Four hundred and nineteen Mortierella isolates were obtained from soil samples in Chihuahua, Mexico which were classified into 21 groups, according to their morphological characters. Mortierella was isolated more frequently when pear fruits were used, obtaining 143 isolates (34.1%), followed by the V8-agar-antibiotics medium with 133 isolates (31.7%), and azalea leaves with 95 isolates (22.7%). An isolate from each of the 21 groups was identified molecularly, 12 corresponded to Mortierella alpina, one to M. gamsii, one to M. capitata, and six to Mortierella sp., and one belonged to the Mortierelliales order. In addition, the putative pathogenicity of the 21 Mortierella isolates identified was tested in G30 apple rootstocks under greenhouse conditions and none was pathogenic. These species have not been reported previously in Mexico. The study showed that there are Mortierella species in the rhizosphere of apple trees in Chihuahua Mexico. These fungal isolates might produce polyunsaturated fatty acids and exert effects of elicitation in horticultural plants and fruit trees improving their resistance to multiple pathogens.
Key words: Mortierellales; diversity; substrate baits; isolation medium; pathogenicity
Se aislaron especies de Mortierella en Agar Dextrosa Papa y agar V8, así como peras maduras y hojas de azalea, como sustratos trampa. Se obtuvieron 419 aislados de Mortierella, de muestras de suelo en Chihuahua, México, los cuales se clasificaron en 21 grupos de acuerdo con sus caracteres morfológicos. Mortierella se aisló con mayor frecuencia cuando se emplearon peras, obteniendo 143 aislados (34.1%), seguido del agar V8-antibióticos con 133 aislados (31.7%) y las hojas de azalea, con 95 aislados (22.7%). Se identificó molecularmente un aislado de cada uno de los 21 grupos, 12 correspondieron a Mortierella alpina, uno a M. gamsii, uno a M. capitata y seis a Mortierella sp. y uno perteneció al orden Mortierelliales. Adicionalmente se probó la patogenicidad putativa de los 21 aislados de Mortierella identificados, en porta injertos de manzana G30 bajo condiciones de invernadero y ninguno fue patogénico. Estas especies no habían sido reportadas previamente en México. El estudio demostró que existen especies de Mortierella en la rizosfera de manzanos en Chihuahua, México. Estas podrían producir ácidos grasos poliinsaturados y ejercer efectos elicitores en cultivos hortofrutícolas confiriéndoles resistencia a múltiples patógenos.
Palabras clave: Mortierellales; diversidad; sustratos trampa; medio de aislamiento; patogenicidad
It has been estimated that only 5% of the existing fungi species have been recorded and described (Hawksworth, 2001). The Mortierellales is one of the most abundant and diverse orders of basal fungi, with nearly 100 species described into 13 genera of the Mortierellaceae family (Yadav et al., 2014), in which the Mortierella genus is located. Most of species of this genus are able to produce polyunsaturated fatty acids (PUFAs) by converting excess of sugars and other carbon sources into lipids under different fermentation conditions (Rayaroth et al., 2016). Several studies have shown that some Mortierella species are able to accumulate arachidonic, γ-linolenic, eicosapentaenoic and docosahexaenoic acids in the mycelium (Ho et al., 2007; Dedyukhina et al., 2014). These compounds are involved in induction of resistance to phytopathogens in plants of agricultural importance (Zlotek and Wojcik, 2014). Additionally, these PUFAs are widely used as food supplements and drugs to improve the immune response in humans, giving technological importance to the Mortierella genus as an alternative source of these compounds (Dedyukhina et al., 2014). However, existing information about the diversity of Mortierellales is limited or out of date. The identification of Mortierella species has mainly been based on morphological characters (Watanabe, 2010). Besides, the identity of species of this fungi by molecular techniques has received little attention, especially in México, where currently there is a high interest to find out antagonistic microorganisms to control phytopathogens. Thus, the aim of the study was to isolate and identify native Mortierella species associated to apple trees with apparent symptoms of root diseases.
Three apple orchards (Malus x domestica Borkh. Rosales: Rosaceae) were sampled from June through July of 2015 in the four most important apple-producing areas: Cuauhtémoc, Guerrero, Bachiniva and Namiquipa, Chihuahua, Mexico (Table 1). Soil samples (500-600 g) were collected from the rhizosphere of five trees with apparent symptoms of root diseases caused by fungi and Oomycetes in each orchard (Ruiz-Cisneros et al., 2017).
Table 1 Location of apple orchards where samples of rhizosphere soil were collected in Chihuahua, Mexico, in 2015.
| Localidad | Huerto | Localización geográfica | Altitud |
| Cuauhtémoc | Campo 2A | N28°26’40”; O106°59’18” | 2,130 |
| Campana 4 ½ | N28°33’49”; O106°54’24” | 1,995 | |
| Picacho | N28°29’28”; O 106°40’08” | 2,020 | |
| Guerrero | Alberto Gameros | N28°31’58”; O 107°26’57” | 2,096 |
| Alberto Gameros PIG30 | N28°31’58”; O 107°26’57” | 2,096 | |
| Efraín Sandoval | N28º32’59”; O 107º27’10” | 2,099 | |
| Namiquipa | Carlos Márquez | N29°11’20”; O 107°25’14” | 1,877 |
| San Rafael | N29°12’19”; O 107°25’22” | 1,858 | |
| El Terrero | N29°18’84”; O107°44’21” | 2,037 | |
| Bachiniva | La Cienega | N28°46’52”; O 107°15’21” | 2,009 |
| Santa Rosa | N28°50’17”; O 107°14’12” | 1,989 | |
| Los 40 | N28°48’07”; O 107°16’06” | 1,990 |
Two culture media (Potato Dextrose Agar and V8-agar) were used for the isolation of Mortierella strains. The potato-dextrose-agar (PDA; BD Bioxon) and V8-agar [V8 juice, calcium carbonate (CaCO3) - agar], media contained antibiotics [oxytetracycline, 0.01 g/L; rifampicin, 0.03 g/L; piramicin, 0.01 g/L, and 66.8 µL/L of the fungicidal hymexazol formulated (Summit Agro, México]. The isolation of Mortierella strains on V8-agar was performed with and without pear fruits (Pyrus communis L.) and azalea leaves (Rhododendron simsii Planch.) as bait substrates. For isolation on PDA and V8-agar without bait substrates, serial dilutions (1:10) were performed in test tubes containing 9 mL of sterile peptone water (0.1% peptone and 0.85% NaCl in distilled water) by adding 1 g of soil previously sieved, to generate 103, 104 and 105 dilutions. Aliquots (50 µL) of each suspension were spread in triplicate on 90-mm Petri dishes containing one of the two media, using a diffusion technique. The Petri dishes were subsequently incubated at 28 ± 1 °C for 72 h in an environmental chamber without light (Precision Scientific, Winchester, VA, USA). For isolation of Mortierella strains using pear fruits as bait substrate, 200-250 g of moistened soil were placed in 1 L plastic cups with a lid, containing one pear that had been previously washed with 1.5% NaClO for 1 min. The cups were incubated at 26 ± 1 °C in darkness for 72 h. Then, the fruits were rinsed three times with sterile distilled water and dried on sterile brown paper in a biosafety hood (Envirco Corporation, Albuquerque, USA). The fruits were individually incubated at 26 ± 1 °C for 48 h. Three sections (5 mm2) of damaged epidermis (transition zone) or mycelium-containing epidermis, were excised from the fruit and placed on Petri dishes containing V8-agar with antibiotics. The dishes were incubated at 26 ± 1 °C for 72 h. For the isolation using azalea leaves as bait substrate, fresh young leaves were treated in the same way than pear fruits. The leaves were cut in circles of 5 mm of diameter. The circles were placed on Petri dishes (60 × 15 mm) containing 10-15 g of moistened soil and incubated at room temperature for 24 h. The leaf circles were removed, washed with sterile distilled water and dried on sterile brown paper. Leaf circles were placed in the four cardinal points and center of Petri dishes containing V8-agar with antibiotics and incubated at 28 ± 1 °C for 72 h. These experiments were performed in triplicate.
The colonies with typical morphology of Mortierella were isolated and purified on V8-agar or PDA medium without antibiotics, using a monosporic culture technique followed by incubation at 28 ± 1 °C, without light for 120 h.
Putative Mortierellales isolates were grouped according to their macroscopic morphological characters and then an isolate of each group was taken for identification (Watanabe, 2010) according to its microscopic characters seen at an optical microscope (Carl Zeiss, Germany).
Genomic DNA (gDNA) was extracted according to Raeder and Broda (1985) and Ruiz-Cisneros et al. (2017). This gDNA was used to amplify the internal transcribed spacer (ITS) region of 18S rDNA gene using the universal primers ITS5 (5’- GGAAGTAAAAGTCGTAACAAGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) (White et al., 1990). The amplification was performed according to the methodology described by Ruiz-Cisneros et al. (2017). The PCR products were examined by electrophoresis on a 1% agarose gel. Subsequently, these products were sequenced at Macrogen Company (Rockville, MD, USA). The obtained sequences were compared against the NCBI database using the BLAST algorithm (Altschul et al., 1990) to verify the percent identity corresponding to the analyzed species. Additionally, a phylogenetic tree by maximum likelihood method, to observe the grouping of Mortierellales fungi was constructed, using Mega software version 6.0 (Tamura et al., 2013).
The pathogenicity of 21 Mortierella strains was tested in G30 apple rootstocks (one of the most planted in Mexico) under greenhouse conditions, according to Ruiz-Cisneros et al. (2017) with modifications. Ten G30 rootstocks (1 year old) were tested for each Mortierella strain, along with ten control trees (without inoculum). Two months after planting, each tree was inoculated with 10 mL of unquantified Mortierella inoculum. The inoculum was 6-7 d old, grown in vegetable broth V8 [V8 juice (Campbell’s™) and calcium carbonate (CaCO3)] and maintained at 28 °C with constant orbital shaking at 140 rpm (Orbit 1900, Labnet International Inc.). Subsequently, the inoculated rootstocks were maintained for another two months under greenhouse conditions and during this time were monitored weekly.
Four hundred and nineteen Mortierella isolates were obtained from tested soil samples (Table 1, Figure 1a-b). The use of pear fruits as bait substrate allowed to obtain the highest number of isolates (143 isolates, 34.1%), followed by the V8-agar-antibiotics medium (133 isolates, 31.7%) and the method involving leaves of azalea as bait substrate (95 isolates, 22.7%). The least effective isolation method was the PDA-antibiotics medium (48 isolates, 11.5%) (Figure 1a). The high number of isolated microorganisms using pear fruits might be consequence of a higher contact area of this fruits with soil, and longer exposure. Azalea leaves and mature fruits have been used as bait substrates to isolate other microorganisms with favorable results. In contrast to our results, Yadav et al. (2014) efficiently isolated Mortierella alpina on PDA medium.
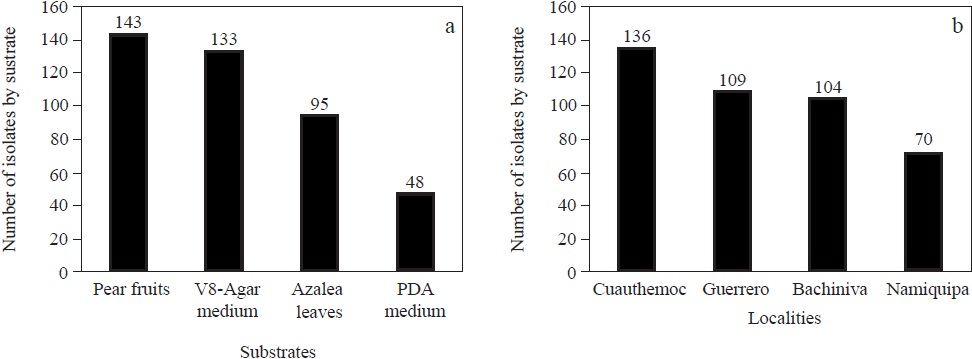
Figure 1 Mortierella isolates associated to diseased apple trees rhizosphere in four main producing localities of Chihuahua State, Mexico; a) number of isolates obtained by substrate; b) number of isolates obtained by locality.
The number of Mortierella isolates obtained from each region is shown in Figure 1b. The highest number of isolates (136, 32%) was obtained from the Cuauhtémoc area. Webster and Weber (2007) demonstrated that the isolation frequency for each fungus is highly variable, depending on the isolation method, culture medium, temperature and other factors during processing of samples. The evident variation in abundance of Mortierella in tested orchards might be due to multiple factors such as the geographical location, soil type, rootstock type, age of trees, climatic conditions, technification level, organic matter content, existent vegetation, among other factors. Bosso et al. (2017) found Mortierella species in soils with different uses and they attributed the high presence of Mortierella to the possible control of phytopathogens, since this genus may have disease suppressive properties (Dedyukhina et al., 2014; Zlotek and Wojcik, 2014).
Four hundred and nineteen Mortierellales isolates were differentiated and identified according to their macro- and microscopic distinctive characters, such as the flower-shaped radial growth of young colonies of white color (Figure 2). However, some of these colonies turned yellow when they grew up. The mycelium of the isolates was hyaline and coenocytic but some hyphae became septated as they aged (Watanabe, 2010). In addition, the microscopic structures typical of asexual reproduction of this genus were observed, besides, the resistance structures as catenulate and intercalary chlamydospores, were also observed in most of the isolates (Figure 2, Table 2; Watanabe, 2010). Sexual reproductive structures such as zygospores, were absent in the isolates. Park et al. (2001) demonstrated that this structure type is uncommon and often surrounded by coenocytic mycelia and that the morphological changes of Mortierella, were dependent on culture conditions and strain genotype.
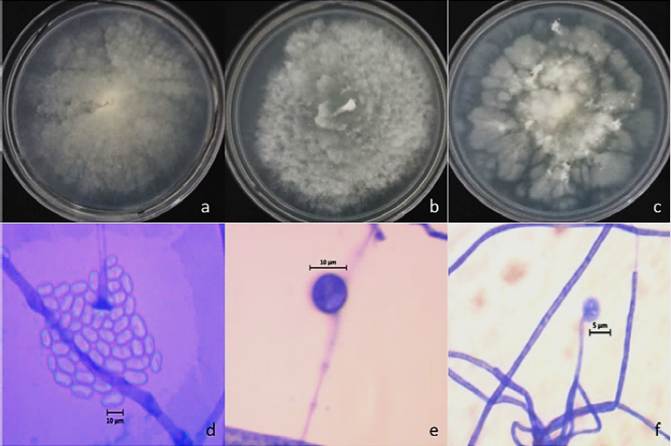
Figure 2 Morphology of Mortierella spp., a-c) macroscopic characteristics grown of M. alpina, M. alpina and Mortierella sp. on PDA culture medium; d-f) microscopic characters, d) sporangiospores hyaline and ovoid, e) intercalary chlamydospore, f) terminal sporangia.
Table 2 Main microscopic characters of Mortierella isolates associated to diseased apple trees rhizosphere in Chihuahua State, Mexico.
| Código del aislado |
Identificación molecular | Forma de los esporangios |
Tamaño (µm) |
Esporangióforo (µm) |
Clamidospora (µm) |
Esporangiosporas (forma) |
| G11 | Mortierella sp. (TR065) | Ovalado | 5 | 43 | Ausente | |
| G3-2 | M. alpina (FCF20120803) | Esférico | 5 | 50 | Intercalar (5-10) | |
| G17 | M. alpina (ATT234) | Esférico | 5-7 | 35-50 | Intercalar (5) | |
| G18 | M. alpina (C051D16) | Ovalado | 10 | 50 | Intercalar (5-7) | |
| G21 | Mortierella sp. (FMR13-4) | Ovalado | 8-12 | 20 | Intercalar (5) | |
| G26 | Mortierella sp. (MEL2385001) | Esférico | 5-10 | 40-55 | Catenulada (7-10) | |
| G29-1 | M. alpina C08ID17 | Esférico | 10-15 | 30-50 | Intercalar (7-10) | |
| G61 | M. alpina MUT: 5194 | Ovalado | 5-7 | 20-30 | Intercalar (5) | |
| G31 | M. alpina strain xds08088 | Esférico | 5-10 | 50-60 | Ausente | |
| G12 | M. alpina voucher RIFA 12B | Esférico | 10-7 | 40-70 | Intercalar (8-10) | |
| G54 | M. alpina A03ID2 | Esférico | 10-7 | 30-50 | Intercalar (5-7) | Ovalada |
| G52 | M. alpina ATCC16266 | Esférico | 7-10 | 30-50 | Intercalar (5-7) | |
| G14 | M. alpina A01ID1 | Ovalado | 5-10 | 40-50 | Ausente | Ovalada |
| G44 | Mortierella sp. FMR23-12 | Esférico | 10-15 | 30-40 | Intercalar (5-10) | |
| G16 | M. gamsii aurium 1205 | Esférico | 5-10 | 20-40 | Intercalar (7-10) | |
| G2 | Uncultured Mortierellales | Esférico | 5-10 | 30-50 | Intercalar (5-10) | Ovalada |
| G9 | Mortierella sp. 11MA04 | Esférico | 5-10 | 20-40 | Intercalar (7-10) | |
| G25 | M. capitata | Ovalado | 10 | 30-40 | Catenulada (5-7) | |
| G32 | Mortierella sp. (FMR23-12) | Esférico | 5 | 40-50 | Intercalar (5-10) | |
| G35 | Mortierella sp. F0210-20S2 | Esférico | 7-10 | 40-50 | Intercalar (10-15) | |
| G43 | Mortierella sp. SD006 | Ovalado | 8-10 | 30-40 | Ausente |
gDNA fragments obtained after amplification of the fungal samples, with ITS4 and ITS5 primers, showed high homogeneity. According to the morphological characters and sequences obtained from the PCR products, 12 of the 21 Mortierellales isolates belonged to Mortierella alpina, six to Mortierella sp., one to Mortierella gamsii, one to Mortierella capitata and one to the Mortierellales order (Figure 3). All isolates had 99-100% identity and a maximum similarity with the molecular scores and taxonomic keys, corresponding to each strain and according to the sequences available in the GenBank database (NCBI) (Altschul et al., 1990). Melo et al. (2014) found M. alpina from tissues of the antarctic moss Schistidium antarctici, demonstrating that Mortierella can be found in high frequency. These differences could be due to the sampling source, since in our study, soil samples were taken from agroecosystems disturbed by anthropogenic activities, mainly by intensive use of pesticides for agricultural pests and diseases management. Nicola et al. (2017) found that Mortierella is a fungus that is usually found colonizing plant roots and is normally associated with apple replant diseases. In our study, this replanting process was detected in some sampled orchards.

Figure 3 Maximum likelihood tree of Mortierella isolates, obtained in diseased apple trees rhizosphere in Chihuahua, Mexico, based on BLAST results from sequences of the ITS4 and ITS5 region of each isolate, the scale bar represents one nucleotide substitutions and numbers branch points indicate values support as percentage based on 1,000 bootstrap replicates (only values > 50% are shown).
The maximum likelihood tree showed genetic differences between Mortierella isolates. According to O’Donnell et al. (2001), some genera and even some species of the Mucorales order are polyphyletic, as can be observed in the phylogenetic tree obtained from the different isolates. Mortierellales isolates were not pathogenic to apple rootstocks G30. Bosso et al. (2017) demonstrated that these fungi species can be considered antagonistic due to the ability to produce different substances which can help in the defense of plants, as systemic resistance inducer.
There are several Mortierella species associated to the rhizosphere of apple trees with apparent symptoms of root diseases in Chihuahua. The most efficient method for the isolation of Mortierella involved the use of pear fruits as bait substrate.
Literatura citada
Altschul SF, Gish W, Miller W, Myers EW and Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology, 215:403-410. https://dx.doi.org/10.1016/S0022-2836(05)80360-2 [ Links ]
Bosso L, Lacatena F, Varlese R, Nocerino S, Cristinzio G and Russo D. 2017. Plant pathogens but not antagonists change in soil fungal communities across a land abandonment gradient in a Mediterranean landscape. Acta Oecologica, 78:1-6. https://doi.org/10.1016/j.actao.2016.11.002 [ Links ]
Dedyukhina EG, Chistyakova TI, Mironov AA, Kamzolova SV, Morgunov IG and Vainshtein MB. 2014. Arachidonic acid synthesis from biodiesel-derived waste by Mortierella alpina. European Journal of Lipid Science and Technology, 116:429-437. https://doi.org/10.1002/ejlt.201300358 [ Links ]
Hawksworth DL. 2001. The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycological Research, 105:1422-1432. https://doi.org/10.1017/S0953756201004725 [ Links ]
Ho SY, Jiang Y and Chen F. 2007. Polyunsaturated fatty acids (PUFAs) content of the fungus Mortierella alpina isolated from Soil. Journal of Agricultural of Food Chemistry, 55:3960-3966. https://doi.org/10.1021/jf0700071 [ Links ]
Melo IS, Santos SN, Rosa LH, Parma MM, Silva LJ, Queiroz SCN and Pellizari VH. 2014. Isolation and biological activities of an endophytic Mortierella alpina strain from the antarctic moss Schistidium antarctici. Extremophiles, 18:15-23. https://doi.org/10.1007/s00792-013-0588-7 [ Links ]
Nicola L, Turco E, Albanese D, Donati C, Thalheimer M, Pindo M, Cavalieri ID and Pertot I. 2017. Fumigation with dazomet modifies soil microbiota in apple orchards affected by replant disease. Applied Soil Ecology, 113:71-79. https://doi.org/10.1016/j.apsoil.2017.02.002 [ Links ]
O’Donnell KL, Lutzoni FM, Ward TJ and Benny GL. 2001. Evolutionary relationships among Mucoralean fungi (Zygomycota): Evidence for family polyphyly on a large scale. Mycologia, 93:286-296. https://doi.org/10.2307/3761650 [ Links ]
Park EY, Koike Y, Cai HJ, Higashiyama K and Fujikaya S. 2001. Morphological diversity of Mortierella alpina: Effect of consumed carbon to nitrogen ratio in flask culture. Biotechnology and Bioprocess Engineering, 6:161-166. https://doi.org/10.1007/BF02932544 [ Links ]
Raeder U and Broda P. 1985. Rapid preparation of DNA from filamentous fungi. Letters in Applied Microbiology, 1:17-20. http://dx.doi.org/10.1111/j.1472-765X.1985.tb01479.x [ Links ]
Rayaroth A, Tomar RS and Mishra RK. 2017. Arachidonic acid synthesis in Mortierella alpina: Origin, evolution and advancements. Proceedings of the National Academy of Sciences, India, Section B: Biological Sciences, 87:1053-1066. https://doi.org/10.1007/s40011-016-0714-2 [ Links ]
Ruiz-Cisneros MF, Rios-Velasco C, Berlanga-Reyes D.I, Ornelas-Paz JJ, Acosta-Muñiz CH, Romo-Chacón A, Zamudio-Flores PB, Pérez-Corral DA, Salas-Marina MÁ, Ibarra-Rendón JE, and Fernández-Pavía SP. 2017. Incidence and causal agents of root diseases and its antagonists in apple orchards of Chihuahua, Mexico. Revista Mexicana de Fitopatología, 35:437-462. https://doi.org/10.18781/R. MEX.FIT.1704-3 [ Links ]
Tamura K, Glen S, Peterson D, Filipski A and Sudhir K. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30:2725-2729. https://doi.org/10.1093/molbev/mst197 [ Links ]
Watanabe T. 2010. Pictorial atlas of soil and seed fungi: Morphologies of cultured fungi and key to species (3rd ed.):153-155. CRC Press. [ Links ]
Webster J and Weber R. 2007. Introduction to fungi. Cambridge University PresWhite TJ, Bruns, T, Lee SB and Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ and White TJ, Eds. PCR protocols: A guide to methods and applications, Academic Press, New York, 315-322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1 [ Links ]
Yadav DR, Kim SW, Babu AG, Adhikari M, Kim C, Lee HB and Lee YS. 2014. First report of Mortierella alpina (Mortierellaceae, Zygomycota) isolated from crop field soil in Korea. Mycobiology, 42:401-404. https://doi.org/10.5941/MYCO.2014.42.4.401 [ Links ]
Zlotek U and Wójcik W. 2014. Effect of arachidonic acid elicitation on lettuce resistance towards Botrytis cinerea. Scientia Horticulturae, 179:16-20. https://doi.org/10.1016/j.scienta.2014.08.026 [ Links ]
Received: October 09, 2017; Accepted: December 13, 2017











 text in
text in 


