Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.35 n.1 Texcoco Jan. 2017
https://doi.org/10.18781/r.mex.fit.1605-2
Scientific articles
Mulberry (Morus alba) leaf extracts as a control alternative of blue mold on postharvest apple fruit
1CONACYT-Universidad Politécnica de Francisco I. Madero. Domicilio conocido. 42660, Tepatepec, Hidalgo, México.
2Colegio de Postgraduados, Campus Montecillo, Carretera México-Texcoco km. 36.5. 56230, Montecillo, Estado de México, México.
3Universidad Politécnica de Francisco I. Madero. Domicilio conocido. 42660,Tepatepec, Hidalgo, México.
Penicillium expansum, causal agent of blue mold on apple, causes significant postharvest losses in apple. This fungus is largely controlled with synthetic fungicides. The goal of this study was to evaluate the effect of leaf extracts of mulberry (Morus alba) as a control alternative of blue mold on postharvest apple fruits. Eleven treatments including a chemical and an untreated control, were evaluated in vitro and in vivo. In the in vitro experiments, mycelial growth and sporulation of the fungus were measured, and in the in vivo experiments severity and fruit decoloration were measured by image analysis. For the leaf extracts, asymptomatic mulberry leaves were collected, crushed and placed in a methanol or ethyl acetate as the solvent phase separation, or distilled water, each at concentrations of 4, 8 and 12%. The results showed that the extract methanol based at 4 % and 12 % inhibited mycelial growth. A treatment with 12 % aqueous extract had the same effect. As for the sporulation of the fungus, it was also observed that the extract methanol based at 4 and 12 % decreased sporulation, and the separated phase with ethyl acetate to 8 %, equaling the chemical control. The severity of disease on inoculated fruits was inhibited by Imazalil with 75.1 %, followed by extract (4 %) with a 40.3 % effectiveness. Only treatment with ethyl acetate at 8 % caused changes on the color of the fruits.
Key words: Malus domestica; Morus alba; Penicillium expansum; postharvest losses
Penicillium expansum, agente causal del moho azul en manzana, causa pérdidas significativas en frutos de manzana en postcosecha, y es controlado mayormente con fungicidas sintéticos. El presente estudio tuvo como objetivo evaluar extractos de hojas de morera (Morus alba) como una alternativa de control del moho azul en frutos de manzana en postcosecha. Once tratamientos, incluyendo un testigo químico (Imazalil) y otro sin tratar, se evaluaron in vitro e in vivo. En los experimentos in vitro se midieron el crecimiento micelial y la esporulación del hongo, y en los experimentos in vivo se midieron la severidad y cambio de color del fruto mediante el análisis de imágenes. Para obtener los extractos de las hojas, se colectaron hojas asintomáticas de morera, se trituraron, y se pusieron en agua destilada esterilizada, metanol y acetato de etilo como solventes de separación de fases, o agua destilada, cada uno a concentraciones de 4, 8 y 12 %. Los resultados demostraron que el extracto obtenido conmetanol a 4 y 12 %, y el extracto acuoso a 12 %, inhibieron el crecimiento micelial. En cuanto a la esporulación del hongo, ésta fue menor en los extractos a base de metanol a 4 y 12 % y el de acetato de etilo a 8 %, igualando al testigo químico. La severidad de la enfermedad en frutos inoculados fue menor en el tratamiento con Imazalil, seguido por el extracto acuoso al 4 %. Solo el tratamiento con acetato de etilo al 8 % cambió la coloración de los frutos.
Palabras clave: Malus domestica; Morus alba; Penicillium expansum; pérdidas postcosecha
The apple (Malus domestica) is one of the most widely produced fruits in the world, with a global harvest volume of approximately 80.8 million tons in 2013 (FAO, 2013). The main producer of this species is China, with a worldwide production of 39.6 million tons (49 % of the worldwide production; FAO, 2013). The European Union and the United States are the second and third largest producers, respectively (FAO, 2013). The production of these amounts of apples demands the use of pesticides (Sauphanor et al., 2009; Simon et al., 2011), both on the field and in storage and transportation (Ticha et al. 2008). One of the fungicides that are most widely used to extend the shelf life of apples is Tiabendazole (He et al., 2014). The tolerance to residues in the fruit of this product is of 5 µg/g in the United States (U.S. Government Publishing Office (GPO), 2015). Many times, with the intention of achieving a better disease control, fungicides are applied in higher doses than are recommended, surpassing the maximum residue tolerance, thus becoming a risk for consumers. Several fungicides have been related to the cause of several diseases in humans, such as cancer, reproductive damages, and endocrine disruption (Baldi et al., 2001). Due to the knowledge of this information, consumers are more aware of their nutrition and demand healthier products, processed at a minimum, and without chemical residues, or treated with products that are not synthetic (Saucedo et al., 2009, Volk et al., 2015). One of the main pathigens that affect apples in postharvest is Penicillium expansum, commonly known as the blue mold. This microorganism casus the greatest losses in apple fruits (Sanzani et al., 2010), and is controlled mainly with the use of synthetic fungicides (Zhang et al., 2011). An alternative to chemical treatments is the use of plant protectors with antimicrobial activity, some of which are effective in controlling diseases in postharvest (Kim et al., 2004; Singh et al., 2007), they have less environmental effects, and don’t damage, or may even improve, human health (Paranagama et al., 2003). The mulberry (M. alba) has been used in human medicie to control fungal pathogens, and its chemicalcomposition has been widely studied (Park et al., 2003; Omidiran et al., 2012). In this regard, Park et al. (2003) found that a chemical known as kuwanon, taken from mullberry roots, inhibited the growth of Streptococcus sobrimus y S. sanguinnis significantly in mouth infections. Other chemical compounds identified in the leaves of this species are alkaloids, saponins, tannins, phenolic compounds, terpenes, glycosides, and flavonoids, all considered to have antimicrobial activity (Cushnie and Lamb, 2006; Soetan et al., 2006; Omidiran et al., 2012; Oh et al., 2013). 2-arilbenzofuran is a compound found in M. alba leaves and is reported as an effective agent in the control of Escherichia coli and Pseudomona aeruginosa (Fukai et al., 2005).
Other bacteria controlled with M. alba derivatives are Staphylococcus aureus, Micrococcus luteus, (Fukai et al., 2005, Rao et al., 2012), Pseudomonas aeruginosa, S. faecium, Escherichia coli, Neisseria gonorrheae, and Proteus vulgaricus (Omidiran et al., 2012). The effect of some derivatives of M. alba on fungi has been studied in human medicine in Candida albicans, Aspergillus niger (Rao et al., 2012), Aspergillus tamari, Fusarium oxysporum and P. oxalicum (Omidiran et al., 2012). However, little is known of the effect of the extracts of this plant species in the control of plant pathogenic fungi. Bibliography only reports studies on the control of A. niger (Rao et al., 2012), F. guttiforme, Charala paradoxa (Cerqueira et al., 2016), F. oxysporum, and P. oxalycum (Omidiran et al., 2012). Understanding the effect of the mulberry extracts on the control of pathogenic fungi could constitute a sustainable alternative for the control of the blue mold in apples in postharvest, as well as on the field and in other crops.
The aim of this investigation was to evaluate the effect of mulberry (M. alba) leaves on the control of P. expansum in vitro and on apple fruits postharvest (in vivo).
Materials and methods
Investigation work was carried out in the year 2015, in the Francisco I. Madero Polytechnic University, specifically in the experimentation area, located at a lattitude of 20.227044° and a longtitude of -99.089386°, in the municipal area of Tepatepec, Hidalgo, Mexico.
Pathogen isolation and pathogenicity tests. The P. expansum fungus was isolated from apple fruits with typical blue mold symptoms gathered in the Central Market (central de abasto) of Mexico City. Fruit fragments (5 x 5 mm) with approximately 20 % symptomatic tissue and 80 % asymptomatic tissue were cultivated in Petri dished with Potato Dextrose Agar (PDA) and incubated at room temperature (26±2 °C) for 10 days. After incubation, a portion of mycelium was transfered to another Petri dish with PDA to purify the strains. Once the identity of P. expansum was confirmed via the morphological keys by Seifer et al., (2011), the strain was planted in a PDA culture, where it grew for 7 days at 28 °C in the dark in an incubator (Thermo scientific®). At the end of the incubation periodl, a suspension was prepared with sopres (conidia) to innoculate healthy apple fruits and to verify the pathogenicity of the isolation. The conidial suspension was prepared by scraping the mycelium and the conidia, filtering the suspension, and adjusting it to a concentration of 1x106 conidia mL-1 using a Neubauer chamber. Apple (M. domestica) fruits of the variety Golden Delicious were selected in a fruit vending venue in Actopan, Hidalgo, Mexico. All fruits chosen were of approximately the same size and free of damage or signs of infections. Ten fruits wre washed and disinfected with a 3 % v/v sodium hypochlorite solution, and two cuts, each 2 mm in diameter and 2 mm deep, were made using a sterilized dissection needle. Later, using a micropipette, 20 µL were deposited from the conidial suspension in the incision to reproduce the symptoms of the blue mold, in order to corroborate the pathogenicity of P. expansum. Distilled-sterilized water was added to five control fuits. Several Petri, dishes in which the fungus was cultivated, were kept for use in in vitro and in vivo experiments described in this study.
Plant material and preparation of extracts. The mulberry leaves used for the extracts were taken from plants grown in 2012 in the planting area of the Francisco I. Madero Polytechnic University. From the middle section of the canopy, healthy-looking and uniform leaves in color and size were selected and washed with distilled water to eliminate surface residues. One kilogram of leaves was weighed, and bit by bit, they were blended in a kitchen blender for one minute until sap was observed. The product was mixed and distributed by weight in four oneliter amber glass jars to avoid the entrance of light. In each jar we poured 600 mL of distilled water, covering all the blended leaves. The mixture was left to settle for 72 hours to drag the metabolites with the polarity of water. After this time, solids were removed using degree 54 Whatman® filter paper, and a mother solution was obtained from the extract (the extracts obtained from the four jars were mixed), which was considered the aqueous extract or the extract without a solvent. Later, the phases of the solution were separated using two different solvents: methanol and ethyl acetate. To do this, 100 mL of aqueous extract and 100 mL of ethyl acetate or methanol (of a reactive degree, 99 % pure) were poured into a phase separation beaker. The solution was left to settle until we noticed the complete separation of both phases. Using the valve of the beaker, we discarded the bottom phase and kept the one which contained the solvent with the possible antimicrobial metabolites (Bresin et al., 2015). The fractions obtained with each solvent (methanol and ethyl acetate) were concentrated in a vacuum rotary evaporator (Ika®) to obtain the extract free of the extraction solvents. The extracts were stored at a temperature of 4 °C until they were to be used for the experiments. Later, for simplicity and clarity of the extracts, they were labeled aqueous extract, extract obtained in methanol and extract obtained in ethyl acetate.
Control of P. expansum in vitro. This phase of the experiment tested 11 treatments in culture medium: PDA + aqueous extract, PDA + extract obtained in methanol, and PDA + extract obtained in ethyl acetate (each at concentrations of 4, 8 and 12 %), PDA + Imazalil 500 mg L-1 as a chemical control, and PDA + distilled water as an absolute control. To prepare these solutions, in separate beakers with the complementary amount of unsolidified PDA, we poured the corresponding amounts of the extracts to obtain the desired concentrations (in the 4 % concentration, we poured 4 mL of the mother solution obtained with each solvent in 96 mL of PDA medium to complete 100 mL, and so on with the other concentrations). Each solution was poured into a Petri dish, properly labelled, and the medium was left to solidify. Next, in the center of each dish, we placed a P. expansum mycelium disc, 5 mm in diameter, obtained from the dishes prepared earlier in the pathogenicity tests. The treatments were laid out in a totally random design with five repetitions. The Petri dishes were incubated at 28 °C (Thermo Scientific® Incubator) for eight days in the dark.
To determine the efficiency of each treatment in the control of P. expansum, the radial growth of the fungal colonies were measured every 24 horas, and the fungal sporulation (concentration of conidia en in the culture medium) was evaluated after 10 días. Each Petri dish was rinsed with distilled-sterilized water, the surface was scraped with a glass rod and filtered through a sterilized cotton mesh. Aliquots of 0.5 mL were transfered from each dish to a Neubauer chamber and the conidia were counted. The complete experiment was repeated 48 hours after the first was concluded.
Control of P. expansum in vivo. We selected apple fruits of the variety Golden Delicious without damages or signs of infections (healthy looking) and similarly sized and colored to be artificially inoculated with P. expansum. The fruits were disinfected with a 2 % sodium hypochlorite solution for three minutes, rinsed with distilled water, and dried at room temperature using paper towels. At the same time, we prepared a suspension of P. expansum spores at a concentration of 1x106 conidia mL-1, as well as concentrations of 4, 8, and 12 % of the aqueous extract and the extracts obtained in methanol and ethyl acetate in sterilized water, and for this, the total amount was determined of solution required per treatment to cover the apples, and based on that, we calculated the amount of each extract to be mixed with the sterilized water and sprayed. As with the experiment in vitro, we included Imazalil 500 mg L-1 as a chemical control and distilled water as an absolute control. The solutions were sprayed on the disinfected fruits in a completely random design with five repetitions per treatment (five fruits, each with two artificil incisions). Twenty four hours after spraying, we inoculated the pathogen on the fruits. For this, and using a sterilized disection needle, two 2 mm deep incisions were made on the fruit (just to ensure the symptom, since the fungus needs no incisions to penetrate) and on these incisions, we deposited 20 µL of the conidial suspension (1x106 conidia mL-1) of the pathogen with a micropipette (20,000 spores). The fruits were placed in a wet chambers (sealed plastic containers) at 26±2 °C, and after incubation, we calculated the diameter of the incision, measuring the radius of the incision every 24 h using a digital caliper. The entire experiment was redone 48 hours after concluding the first one. The effectiveness was calculated using the following formula:
Color change in fruits. To determine if there were any effects of the extracts on the coloring of the fruits treated, photographs were taken on a daily basis of the fruits incubated in the wet chambers (sealed plastic containers) using a 12 megapixel Fujifilm® camera. The photographs were taken at a distance of 30 cm with lighting provided by a 20 w fluorescent lamp. The angle between the lens of the camera and the source of lighting was of approximately 45°. The aperture used in the camera was f/2.8 and the shutter speed was 1/30 s. These parameters were kept constant during the capture of all the images, which were kept in a JPEG format. The averages of the values corresponding to red, green, and blu (RGB) which make up the real color of the apple fruit, were analyzed statistically using the programaAdobe® Photoshop® CS5 version 12.0.4. The values closest to the absolute control (without treating) correspond to the color demanded in markets for the species and the variety (Landero et al., 2013).
Staistical analysis. Using the data of the experiment in vitro (length of the mycelial growth), we calculated the Area Under the Curve (AUC) using the polygon method (Liengme, 2013). The AUC of each treatment underwent an analysis of variance. For the growth of the fungus with time, we obtained averages of each of the treatments for each of the days in which the evaluation was carried out, undergoing analyses of variance and Tukey multiple average separation tests. For the variable whose data were not normal, these were transformed using the square root √. Using the severity data (diameter of the incision) of the experiment in vivo we determined the AUC of the disease using the method described above. The results underwent analyses of variance and Tukey multiple average separation tests using the program SAS v.9 for Windows®. To evaluate color change in the fruits, the values of R, G, and B were evaluated separately, and they underwent analyses of variance and Tukey multiple average separation tests, using the program mentioned above.
Results and Discussion
Control of P. expansum in vitro. The mycelial growth of the fungus in PDA varied significantly (P<0.0001) between treatments, and was significantly lower in the dishes that contained the methanolic phase at 4 and 12 %, as well as the aqueous phase at these same percentages. The greatest fungal growth rate was observed in the absolute control (Table 1). According to Chon et al. (2009), the greatest content of phenols was determined when methanol was used as a solvent to perform the extraction in mulberry leaves, which is important when considering phenols as secondary metabolites with antimicrobial properties (Wang et al., 2012). However, this study obtained similar results in the tests which used methanol and water as solvents, therefore we can infer that another unknown metabolite or metabolites, different to phenols, could be responsible for the observed antimicrobial effect. In this regard, Ayoola et al. (2011) mention that when they used water and ethanol as solvents to obtain a crude M. alba leaf extract, the analysis indicated the presence of phenolic compounds and flavonoids, which have been reported as having antimicrobial activity, (Cushnie and Lamb, 2006; Soetan et al., 2006). According to Ayoola et al. (2011), the crude extract inhibited the growth of the fungi A. niger, A. tamari, F. oxysporum and P. oxalycum. It is important to mention that for this variable the chemical control no showed control, making it similar to the absolute control.
When the comparison of averages was performed by the group of solvents used for the extraction, we also found a statistical difference between treatments (P<0.0001); showing the least mycelial growth where these were prepared based on an aqueous extract, as well as when the extract was made with methanol (Table 1). The absolute control was the only one that showed a difference with all the treatments, showing the greatest mycelial growth. Asli et al. (2014) reported that the extract obtained with methanol from M. alba leaves presented antimcrobial activity against C. albicans and A. niger, which confirms the results of this study.
Table 1. Mycelial growth of Penicillium expansun in vitro under different treatments based on Morus alba leaves such as aqueous extract, methanolic, and with ethyl acetate.
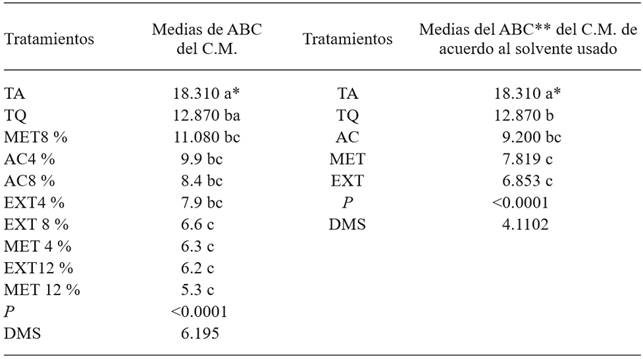
*Same letters indicate and absence of a significant statistical difference (Tukey, P≤0.05). **The area under the curve is presented in adimensional values.
TA= untreated control, TQ= chemical control, MET8 %= Phase separation with methanol, at a concentration of 8 % in a PDA culture medium, AC4 %= Phase separation using ethyl acetate, at a concentration of 4 % in PDA culture medium, AC8 %= Phase separation using ethyl acetate, at a concentration of 8 % in PDA culture medium, EXT4 %= Morus alba extract at a concentration of 4 % in PDA culture medium, EXT8 %= Morus alba extract at a concentration of 8 % in PDA culture medium, MET4 %= Phase separation with methanol, at a concentration of 4 % in PDA culture medium, EXT12 %= Morus alba extract at a concentration of 12 % in PDA culture medium, MET12 %= Phase separation with methanol, at a concentration of 12 % in PDA culture medium. C.M.=Mycelial growth. DMS=Minimum significant difference.
The use of aqueous and methanolic extracts evidently produced the lowest AUC (Table 1), which coincides with the treatments which produced the lowest mycelial growth in the pathogen. Omidirán et al. 2012, mention that when applying aqueous extracts of M. alba on fungi or bacteria, these had a lower growth rate than the applications with ethanolic extracts, which reinforces the results obtained in this investigation.
In the analysis of fungal development in time, it was observed that the increase in the intensity of the disease was homogenous. After the second evaluation, all the treatments showed significant statistical differences between them. The absolute control showed the highest values for mycelial growth in each of the readings taken (Figure 1). The treatments based on aqueous and methanolic extract remained as the ones that showed the least pathogen development in time; only during readings 3 and 5 were all treatments equal, with the exception of the absolute control, which displayed the highest values.
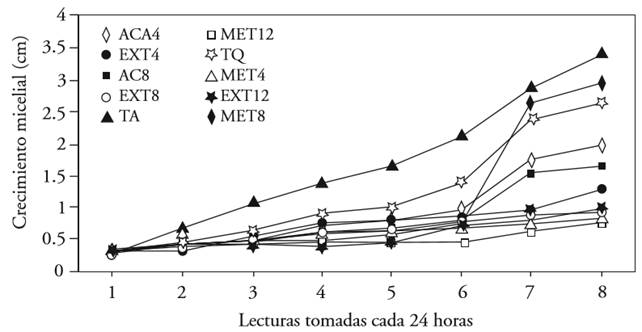
Figure 1. Mycelial growth by reading of Penicillium expansum under different concentrations of Morus alba extracts obtained with three solvents. Each line with a different color indicates a treatment.
The treatments that showed the lowest number of conidia per mL were those for which the culture medium was mixed with a 4 % aqueous extract, ethyl acetate at 8 % and aqueous extract at 12 %, statistically equaling the chemical control (DMS=63.01; Table 2). This is relevant because spores are an effective dispersal and infection mechanism, and with the application of the treatments mentioned earlier, these are reduced. This may be due to the fact that methanol was the solvent with one of the best results, and used for the extraction of M. alba leaves, it presents an drag of phenolic compounds, which, as we mentioned earlier, have antimicrobial activity (Chon et al., 2009). This is also confirmed with the mycelial inhibition of the pathogen when the same solvent was used. On the other hand, the greatest amount of conidia was observes in the absolute control. As for the favorable result with the application of the aqueous extract, Cerqueira et al. (2016) found that the inhibition halos on F. guttiforme were observed precisely in the treatment based on aqueous extract of the Bonetia stricta leaf. Regarding this, we can mention that an extract is the complete mixture of the metabolites that plant leaves contain, and this condition may be what causes the lower mycelial growth or the lower number of spores in the pathogen; although the application of only one metabolite may be less effective, this is not the case in all extracts.
Table 2. Average number of Penicillium expansum spores due to different treatments based on Morus alba leaves such as aqueous extract, methanolic, and with ethyl acetate.
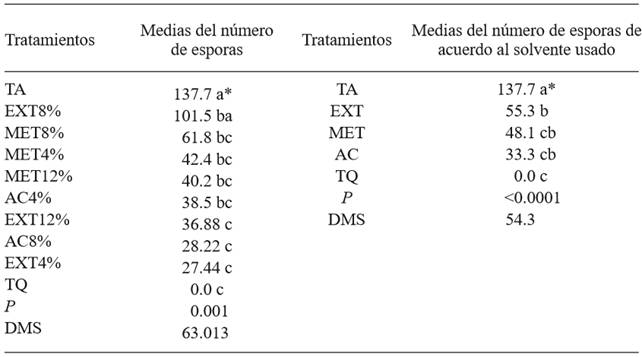
*Same letters do not indicate significant difference (Tukey, P≤0.05). Data were converted to √, real values showed for a better comprehension.
The sporulation of P. expansum was significantly different between the treatments with two solvents (ethyl acetate and methanol). However, sporulation was lower in both solvents than in the untreated control. Results indicate that the aqueous extract was statistically different to the absolute control, though no better than the extract obtained with methanol and with ethyl acetate (Table 2). In this case, the chemical control displayed the greatest effectiveness, with no spores presented.
Control of P. expansum in vivo. The results showed statistical differences between treatments (P<0.0001). The treatment with the lowest area under the disease progression curve was the one which used 4 % aqueous extract (Table 3), surpassed only by the chemical control. The extract of the leaves from the species under study was tested against A. niger and C. albicans, revealing that all concentrations presented a notorious antimicrobial activity (Rao et al., 2012). It is worth mentioning that M. alba has been widely studied in its phytochemical composition, as well sa its effect on pathogenic human microorganisms (Cushnie and Lamb, 2006; Soetan et al., 2006; Rao et al., 2011 Omidiran et al., 2012; Oh et al., 2013). However, the information on its effect on microorganisms that cause losses in agriculture is precarious. This work documents information on the effect of leaf extracts on an economically important plant pathogen. Likewise, antibacterial activity has also been reported, due to Kuwanon G, a compound isolated from M. alba roots (Rao et al.,2012).
Table 3. Average severity caused by Penicillium expansum in apple fruits with extracts of Morus alba leaves as aqueous, methanolic, and with ethyl acetate.
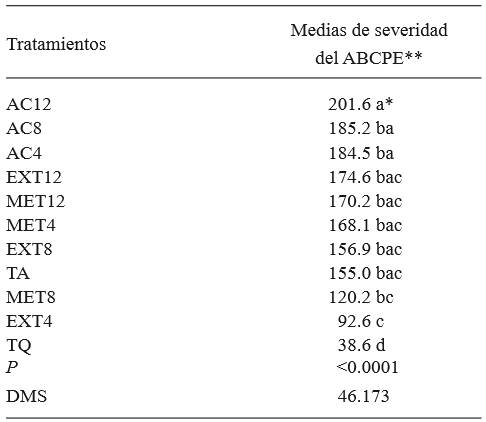
*Same letters do not indicate statistic significance (Tukey, P≤0.05).
**The values showed for ABCPE are adimensional. AUDPC= area under the disease progress curve.
On the other hand, the treatment with the highest area concerned the extract obtained with ethyl acetate at 12 %, which favored the development of the pathogen, while the rest of the treatments and the untreated control displayed a similar behavior amongst them, including the extract obtained with methanol, which, in mycelial growth and production of spores, was one of the treatments which produced the lowest growth values. Ethyl acetate is a solvent of medium polarity that extracts more compounds than methanol or other solvents. Among these are carbohydrates (Mumammad et al., 2014), which function as a source of food and growth for microorganisms, such as fungi. The chemical control appeared to have the lowest development of the disease (75.09% de efectividad), followed by the treatment in which aqueous extract at 4% was used for the control of the disease (40.26% effectiveness). These results coincide with those obtained by Omidiran et al. (2012), who found that applying aqueous extracts of M. alba on certain fungi and bacteria causes the inhibition of their growth.
The analysis of images for the evaluation of the change in coloring of the fruits showed that the treatment with the extract obtained with ethyl acetate at 8% caused the decoloring of fruit in comparison with the absolute control, which did not induce any color change and has the color required by the market. The rest of the treatments proved to be statistically similar to the untreated control, indicating that there are no changes in the color due to treatments. The change in the coloring of the fruits due to the extract obtained with ethyl acetate at 8% is explained by the use of this solvent, which extracts different compounds to methanol, such as anthraquinones, carbohydrates, and flavonoids (Mumammad et al., 2014), possibly in greater amounts than other solvents.
Tabla 4. Penicillium expansum and RVA averages on apple fruits after appplying treatments based on Morus alba leaves such as aqueous extract, methanolic, and ethyl acetate.
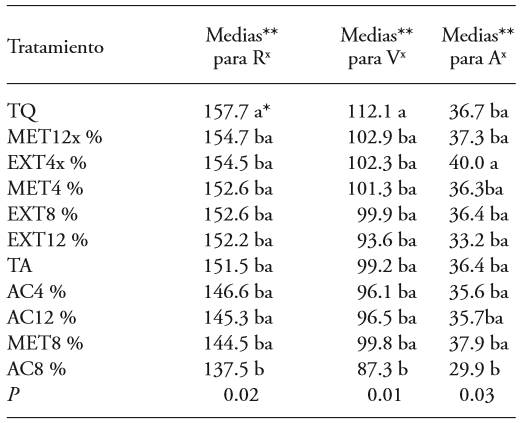
*Same letters do not indicate statistic significance (Tukey, P≤0.05).
**Averages for RVA are adimensional. R=red, V=green, A=blue.
x The valuesnearest to those obtained for the absolute control correspond to the coloring normally required in markets for the species and variety, while those furthest could indicate a normal change in the coloring of the fruit.
The above suggests that if mulberry leaf extracts are used to control blue mold in harvested fruits, the use of ethyl acetate is not remcommended for use as an extraction solvent, since it changes the color of the fruit and stimulates fungal development (Table 3). The results show that the use of methanol or water are less influential on the coloring of the fruit. Also, as entioned earlier, methanol is the solvent used to extract the most phenols (Wang et al., 2012) and is preferred since it is less pollutant and costly than other solvents.
Conclusions
The results of this study suggest that the mulberry leaf extracts have the potential to be used as an alternative in the control of P. expansum in apple fruits in postharvest. In the extraction of the antimicrobial metabolites of this plant, we recommend using methanol at 4% or 12% and not ethyl acetate, since the latter could decolor the fruits and stimulate fungal development. This investigation motivates further studies on the effect of M. alba extracts in pathogens in other economically important crops. It is worth pointing out that for a more effective control of the blue mold, the application of mulberry extract must be complemented with other sustainable control measures, such as the control of weather conditions in controlled environments. Other more effective solvents for the extraction of M. alba metabolites and of a lower risk when using or toxicity must be tested for the extraction. This investigation did not consider the determination of the chemical composition of M. alba under the weather, soil and plant iversity conditions of the place of study. This part of the investigation would be of interest for future investigations in fungal control with organic products. The decoloring of fruits observed by the effect of the ethyl acetate is a topic of investigation to know what compounds or metabolites are responsible for this effect.
Acknowledgements
We would especially like to thank Dra. Julia María Domínguez Soto, who works in the UPFIM, for her support and willingness with the material required for the experiments. Likewise, we would like to thank everyone who directly or indirectly contributed in the completion of this study.
REFERENCES
Andallu B., Suryakantham V., Srikanthi B.L. and Reddy G.K. 2001. Effect of mulberry (Morus indica L.) therapy on plasma and erythrocyte membrane lipids in patients with type 2 diabetes. Clinica Chemical Acta 314:47. Doi:10.1016/S0009-8981(01)00632-5 [ Links ]
Asli E. A., Avci E., Ozcelik B., Alp A. G. and Ali K. D. 2014. Antioxidant and antimicrobial activities with GC/ MS analysis of the Morus alba L. leaves. Hittite Journal of Science and Engineering 1:137-141. Doi: 10.17350/HJSE19030000006 [ Links ]
Ayoola O.A., Baiyewu R.A., Ekunola J.N., Olajire B.A., Egunjobi J.A., Ayeni E.O. and Ayodele O.O. 2011. Phytoconstituent screening and antimicrobial principles of leaf extracts of two variants of Morus alba. African Journal of Pharmacy and Pharmacology 19:2161-2165. Doi: 10.5897/AJPP11.590 [ Links ]
Baldi I., Filleul L., Mohammed-Brahim B., Fabrigoule C., Dartigues J.F., Schwall S. 2001. Neuropsychologic effects of long-term exposure to pesticides: results from the French Phytoner study. Environmental Health Perspectives 109:839-44. Doi: 10.1136/oem.2009.047811 [ Links ]
Bresin P., Piol M., Fabbro D., Mancini M.A., Casseta B. and Del Bianco C. 2015. Analysis of organo-chlorine pesticides residue in raw coffee with a modified “quick easy cheap effective rugged and safe” extraction/clean up procedure for reducing the impact of caffeine on the gas chromatography-mass spectrometry measurement. Journal of Chromatography A, 1376:167-171. http://dx.doi.org/10.1016/j.chroma.2014.12.016 0021-9673 [ Links ]
Cerqueira S.M.D., Barcellos C.H., Bueno F.P.M., Aires V.J. and Dummer M.D. 2016. Antifungal activity of plant extracts with potential to control plant pathogens in pineapple. Asian Pacific Journal of Tropical Biomedicine 6:26-31. Doi:10.1016/j.apjtb.2015.09.026. [ Links ]
Chon S.U., Kim Y.M., Park Y.J., Heo B.G., Park Y.S. and Gorinstein S. 2009. European Food Research and Technology 2:231-237. Doi:10.1007/s00217-009-1165-2 [ Links ]
Cushnie T.P. and Lamb A.J. 2006. Antimicrobial activity of flavonoid. International Journal of Antimicrobial Agents 26:342-356. Doi: 10.1016/j.ijantimicag.2005.09.002 [ Links ]
FAO (Food and Agriculture Organization of the United Nations). 2012. FAOSTAT Production Database. [2016-07.21]. http://faostat.fao.org [ Links ]
FAO (Food and Agriculture Organization of the United Nations). 2016. FAOSTAT Production Database. [2016-3-15]. http://faostat.fao.org [ Links ]
Fukai T., Kaitou K. and Terada S. 2005. Antimicrobial activity of 2-arylbenzofurans from Morus species against methicillin-resistant Staphylococcus aureus. Fitoterapia 76:708-711. Doi:10.1016/j.fitote.2005.06.012 [ Links ]
He L., Chen T., and Labuza T. P. 2014. Recovery and quantitative detection of thiabendazole on apples using a surface swab capture method followed by surface-enhanced Raman spectroscopy. Food Chemistry 148: 42-46. Doi:10.1016/j.foodcont.2016.04.003 [ Links ]
Kim H. O., Park S. W., and Park H. D. 2004. Inactivation of Escherichia coli O157:H7 by cinnamic aldehyde purified from Cinnamomum cassia shoot. Food Microbiology 21:105-110. Doi:10.1016/S0740-0020(03)00010-8 [ Links ]
Landero V.N., Nieto A. D., Téliz O. D., Alatorre R. R. y Ortíz G. C. F. 2013. Potencial antifúngico de extractos de cuatro especies vegetales sobre el crecimiento de Colletotrichum gloeosporioides en papaya (Carica papaya) en poscosecha. Revista Venezolana de Ciencia y Tecnología de Alimentos. 1:047-062. https://doaj.org/article/14a83e0b1ce841a29a0d995d1af3bfe9 [ Links ]
Liengme B. 2013. A Guide to Microsoft Excel 2013 for Scientists and Engineers. Elsevier Science and technology. 382 p. [ Links ]
Mumammad A.M., Ismail M.A., Jaafar, K.M. and Garba M.M. 2014. Phytochemical screening and anticonvulsant studies of ethyl acetate fraction of Globimetula braunii on laboratory animals. Asian Pacific Journal of Tropical Biomedicine 4:285-289 Doi:10.12980/APJTB.4.2014C925 [ Links ]
Oh J, Jo H., Cho A.R., Kim S.J. and Hn J. 2013 Antioxidant and antimicrobial activities of various leafy herbal teas. Food control 31:403-409. Doi:10.1016/j.foodcont.2012.10.021 [ Links ]
Omidiran M. O., Baiyewu R. A., Ademola I. T., Fakorede O. C., Toyinbo E. O., Adewumi O. J. and Adekunle E. A. 2012. Phytochemical Analysis, Nutritional Composition and Antimicrobial Activities of White Mulberry (Morus alba). Pakistan Journal of Nutrition 11: 456-460. http://scialert.net/abstract/?doi=pjn.2012.456.460 [ Links ]
Paranagama P. A., Abeysekera K. H. T., Abeywickrama K. and Nugaliyadd L. 2003. Fungicidal and anti-aflatoxigenic effects of the essential oil of Cymbopogon citratus Stapf. (lemongrass) against Aspergillus flavus Link. isolated from stored rice. Letters in Applied Microbiology 37:86-90. http://www.ncbi.nlm.nih.gov/pubmed/12803563 [ Links ]
Park K. M., You J. S., Lee H.Y., Baek N.I. and Hwang J.K. 2003. Kuwanon G: an antibacterial agent from the root bark of Morus alba against oral pathogens. Journal of Ethnopharmacology 84:181-185. Doi:10.1016/S03788741(02)00318-5 [ Links ]
Rao T. G., Baku K.S., Ujwal K. J., Sujana P., Veerabhadr R. A. and Sreedhar A. S. 2011. Anti-microbial principles of selected remedial plants from Southern India. Asian Pacific Journal of Tropical Biomedicine 1:298-305. Doi:10.1016/S2221-1691(11)60047-6 [ Links ]
Rao S.J.A., Ramesh C.K. and Mahmood R. 2012. Anthelmintic and antimicrobial activities in some species of mulberry. International Journal of Pharmacy and Pharmaceutical Science 4:335-338. http://www.ijppsjournal.com/Vol4Suppl5/4992.pdf [ Links ]
Saucedo P. S., Jasso C. D., Ventura S. J., Sáenz G. A., Rodríguez H. R. and Aguilar C. N. 2007. Effect of candelilla wax with natural antioxidants on the shelf life quality of fresh-cut fruits. Journal of Food Quality 30:823-836. Doi: 10.1111/j.1745-4557.2007.00165.x [ Links ]
Saucedo P. S., Rojas M. R., Aguilera C. A. F., Sáenz G. A., Garza H. L. and Jasso C. D. 2009. Edible film based on candelilla wax to improve the shelf life and quality of avocado. Food Research International 42:511-515. Doi:10.1016/j.foodres.2009.02.017 [ Links ]
Sauphanor B., Dirwimmer C., Boutin S., Chaussabel A.L., Dupont N., Fauriel J. 2009 Analyse comparative de différents systèmes en arboriculture fruitière. In: INRA, editor. Ecophyto R&D: vers des systèmes de culture économes en produits phytosanitaires. Rapport d’Expertise Collective Inra, Tome IV. [ Links ]
Seifert K., Morgan J. G., Gams W. and Kendric B. 2011. The genera of Hyphomycetes. CBS-KNAW Fungal Biodiversity Centre. Ultrecht, Netherlands. 997 p. [ Links ]
Simon S., Brun L., Guinaudeau J., Sauphanor B. 2011. Pesticide use in current and innovative apple orchard Systems Agronomy. Agronomy Sustainability Development 31:541-55. Doi 10.1007/s13593-011-0003-7 [ Links ]
Singh G., Maurya S., Lampasona M.P. and Catalán A. N. C. 2007. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food and Chemical Toxicology 45:1650-1661. Doi:10.1016/j.fct.2007.02.031 [ Links ]
Sanzani S. M., Schena L., Girolamo A., Ippolito A., and Gonzalez C. L. 2010. Characterization of genes associated with induced resistance against Penicillium expansum in apple fruit treated with quercetin. Postharvest Biology and Technology 56:1-11. Doi:10.1016/j.postharvbio.2009.11.010 [ Links ]
Soetan K., Oyecunle M.A., Aiyelaagbe O.O. and Fafunso M.A. 2006. Evaluation of antimicrobial activity of saponins extract of Sorghum bicolor. African Journal of Biotechnology 23:2405-2407. http://www.academicjournals.org/AJB [ Links ]
Ticha J., Hajslova J., Jech M., Honzicek J., Lacina O., Kohoutkova J. 2008. Changes of pesticide residues in apples during cold storage. Food Control 19:247-56. Doi:10.1016/j.foodcont.2007.03.011 [ Links ]
U.S. Government Publishing Office (GPO). 2015. Part-180 Tolerances and exemptions for pesticide chemical residues in food. 2016-07-21 2016-07-21 http://www.ecfr.gov/cgi-bin/textidx?SID1⁄45370c30aabced019b313754fe0b0c682&node1⁄4pt40.24.180&rgn1⁄4div5#_top . [ Links ]
Volk G.M.C., Chao T., Norelli J., Brown S.K., Fazio G., Peace C., McFerson J., Zhong G.-Y., Bretting P. 2015. The vulnerability of US apple (Malus) genetic resources. Genetic Resources and Crop Evolution 62:765-794. Doi:10.1007/s10722-014-0194-2 [ Links ]
Wang W., Zu Y., Fu Y. and Effert T. 2012. In Vitro Antioxidant and Antimicrobial Activity of Extracts from Morus alba L. Leaves, Stems and Fruits. American Journal of Chinese Medicine 40:349. Doi: http://dx.doi.org/10.1142/S0192415X12500279 [ Links ]
Zhang C., Wang J., Zhang J., Hou C. and Wang G. 2011. Effects of β-aminobutyruc acid on control of postharvest blue mold of apple fruit and its possible mechanisms of action. Postharvest Biology and Technology 61:145-151. Doi:10.1016/j.postharvbio.2011.02.008. [ Links ]
Received: May 10, 2016; Accepted: August 05, 2016











 text in
text in 



