Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.34 n.3 Texcoco Sep. 2016
https://doi.org/10.18781/r.mex.fit.1605-1
Scientific articles
Characterization of Phytophthora nicotianae causing vinca blight in urban areas and ornamental nurseries in Culiacan, Mexico
1Centro de Investigación en Alimentación y Desarrollo. CIAD AC. Área de Horticultura. Km 5.5 Carr. Culiacán-Eldorado, Campo El Diez. Culiacán, Sinaloa, México. CP 80110 Tel. 6677605536.
2Instituto Politécnico Nacional-CIIDIR Unidad Sinaloa, Departamento de Biotecnología Agrícola. Juan de Dios Bátiz Paredes No. 250, Col. San Joaquin, Guasave, Sinaloa. CP 81101 Tel. 6878729626. Correo electrónico: msantos@ipn.mx.
The aims of this study were to obtain and characterize, by morphological and molecular techniques, Phytophthora isolates associated with blight symptoms on vinca plants in urban areas and nurseries in Culiacan, Mexico, determine the mating types and evaluate the pathogenicity of isolates. Isolates developed white and cottony colonies with coenocytic mycelium on V8 agar; intercalary and terminal chlamydospores (20 μm) and papillate sporangia, round or oval (34.16 μm and 26.35 μm). The isolates were heterothallic: 21 isolates resulted A1 type and 18 isolates were A2 type. A group of representative isolates showed pathogenicity and caused vinca blight incidence between 13 and 66 %. The identity of the isolates was confirmed by analysis of nucleotidic sequences of the ITS region and nuclear genes as elongation factor (TEF-1α) and beta tubulin (β-Tub). The sequences, compared to the NCBI database, showed a high degree of identity with P. nicotianae (ITS, 98-100 %; TEF-1α, 99-100 %; βTub, 98-100 %) and phylogenetically clustered with reference strains. The utilization of specific primers resulted in a positive amplification for the isolates tested. This is one of the few studies on plant diseases affecting ornamental plants in urban areas in Mexico.
Keywords: Oomycetes; ornamental plant diseases; heterothallism
Los objetivos de este estudio fueron obtener y caracterizar, mediante técnicas morfológicas y moleculares, aislados de Phytophthora asociados con tizón en plantas de vinca en áreas urbanas y viveros en Culiacán, México, determinar los tipos de compatibilidad sexual y evaluar la patogenicidad de los aislados. Los aislados desarrollaron micelio cenocítico y colonias algodonosas de color blanco en agar V8; clamidosporas (20 μm) intercalares y terminales, y esporangios papilados redondos a ovales (34.16 μm y 26.35 μm). Los aislados fueron heterotálicos: 21 del tipo de compatibilidad A1 y 18 del tipo A2. Un grupo de aislados representativos mostraron patogenicidad y causaron incidencia de tizón en vinca entre 13 y 66 %. La identidad de los aislados se confirmó por análisis de secuencias nucleotídicas de las regiones ITS y de los genes nucleares factor de elongación (TEF-1α) y beta tubulina (β-Tub). Las secuencias, comparadas con la base de datos del NCBI, mostraron alto grado de identidad con P. nicotianae (ITS, 98-100 %; TEF-1α, 99-100 %; βTub, 98-100 %) y se agruparon filogenéticamente con cepas de referencia. La utilización de oligonucleótidos específicos resultó en una amplificación positiva para los aislados evaluados. Este es uno de los pocos estudios relacionado con enfermedades en plantas ornamentales en áreas urbanas en México.
Palabras clave: Oomicetes; enfermedades de plantas ornamentales; heterotalismo
The genus Phytophthora, within the oomycetes, includes a diverse group of destructive phytopathogenic microorganisms that infect a wide range of hosts and cause considerable economic losses in natural and agricultural environments worldwide (Erwin and Ribeiro, 1996). Over 100 species of Phytophthora have been described (Martin et al., 2012); some species are highly specialized to one host and others, such as P. nicotianae, have a wide range of hosts in agricultural crops and ornamental plants.
Vinca rosea (L.) is a herbaceous plant, also identified as Catharanthus roseus (L.) G. Don. It is ornamental, resistent to high temperatures, moisture, drought, and soil infertility (Schubert and Leahy, 1989). It is widely used as a garden plant and there are currently more than 100 hybrids developed (McGovern and Begeman, 1996). In addition, vinca plants have been identified as secondary hosts for many pathogenic microorganisms such as viruses, fungi, and oomycetes (Hagan, 2004), which affect fruit and vegetable crops such as papaya, tomato, peppers, and cucumber, among others (Haider et al., 2007).
The disease known as blight and wilting in V. rosea plants is caused by Phytophthora spp., it progresses quickly and is present in warm and humid climates, particularly in summer, when humidity is high (Hao et al., 2010). The symptoms of the disease begin with a gray color in the tips of infected tissues, which quickly become necrotic. Lesions progress quickly and cause a general wilting of the plant (McGovern and Begeman, 1996). Various species, including P. nicotianae, P. citrophthora, P. cryptogea, and P. tropicalis, have been described as causing blight and wilting in vinca plants (McGovern and Begeman, 1996; McMillan and Garofalo, 2004; Orilowski et al., 2011; Hao et al., 2010).
The identification and characterization of Phytophthora spp. is based mainly on morphological characteristics (Waterhouse, 1963): type of mycelia, shape, size, and development of sporangia, production of sexual reproduction structures, as well as the formation of clamidospores (Pintos et al., 2004). Due to the intraspecific morphological variation, the exact identification of isolates is frequently difficult, even for specialists. For this reason, the molecular techniques that analyze genetic sequences have helped to identify and demarcate species. In oomycetes, the genomic regions most commonly used for identification at the species level, are the internal transcribed spacer (ITS) (Cooke et al., 2000) and some codifying regions of molecular genes, that incluye the factor of elongation 1-α (TEF-1α) and beta tubulin (β-Tub) (Kroon et al., 2004). Another promising region to develop specific oligonucleotides for species of Phytophthora is the gene YPT1 related to a RAS protein (Meng and Wang, 2010), since the non-codifying regions of this gene have displayed enough variation support the development of molecular markers for almost all the species of Phytophthora (Schena and Cooke, 2006).
In Culiacán, Sinaloa, ornamental vinca plants are common in parks and/or boulevard strips, home gardens, and ornamental nurseries. During the rainy season in the summer (June-August), it is common to find in plants symptoms of leaf blight and wilting. A previous report mentions the presence of P. nicotianae in vinca causing blight (Alvarez-Rodriguez et al., 2013); however, we are not sure if it is the only species, therefore the aims of this study were to find and characterize, using morphological and molecular techniques, the species of Phytophthora related to blight in vinca plants, to determine the types of sexual compatibility, and to evaluate the pathogenicity in the isolations obtained.
Materials and methods
Isolation of the causal agent of blight and wilting in vinca
Samples of diseased plants were taken from urban gardens and ornamental nurseries in Culiacan. Samples of infected tissues were placed in plastic bags and taken to the laboratory to isolate the Phytophthora species related to the disease. In the lab, small portions of infected plant tissue were washed with distilled water, disinfected with sodium hypochlorite at 0.5 % for 1 min and finally placed in Petri dishes with a V8 agar culture medium (840 ml distilled water, 163 ml V8 juice, 3 g of CaCO3 and 18 g bacteriological agar), which were kept at room temperature for 72 h.
Morphological characterization
The isolations were reactivated in a V8 agar culture medium. Once the mycelium developed, permanent preparations were made using lactophenol and placed for observation under a microscope (Carl Zeiss Axiostar Imager A2). Each isolation was purified with the technique of transference of hyphal tips (Donahoo and Lamour, 2008). To induce the growth of sporangia, cuts were made on the colony under growth and the plate was flooded with sterile distilled water. The plates were incubated for 3 days at room temperature (Martin et al., 2012). The presence of sporangia was observed under a biological microscope adapted with a camera. Once the sporangia were produced, the water was removed and acid fuchsin was added to the plates at 2 % to stain them. Samples were taken after 24 h and observed under the microscope to record the measurements of sporangia and other structures.
Chlamydospores were observed in a carrot-based medium (200 g of carrot, 20 g of bateriological agar, and 1 L of distilled water). The isolates grew in the carrot-based medium and were incubated for 15 days at 25 °C in the dark (Mirsoleimani and Mostowfizadeh-Ghalamfarsa, 2013); after this time, they were observed under the microscope and the presence and size of chlamydospores were recorded.
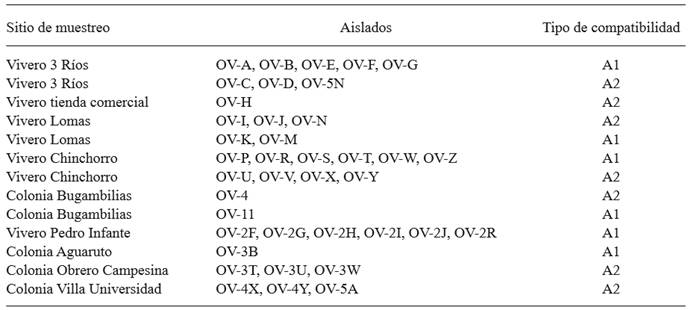
To determine the type of compatibility and record the formation of structures of sexual reproduction, each isolate was confronted with reference strains A1 and A2 of P. nicotianae in V8 culture medium; an agar disc with mycelia in active growth of each isolate, 0.5 cm in diameter, was placed approximately 1 cm away from agar discs with mycelia in active growth of reference strains A1 and A2 of P. nicotianae. The plates were incubated for 15 days at 25 °C and then the presence/absence of oospores was recorded, as well as the characteristics of oogonia and antheridia (Javier-Alva and Mattos, 2006; Waterhouse, 1963).
Molecular identification
The mycelia of the isolates was grown in a PD (potato-dextrose) liquid medium, and stored at -20 °C. The DNA was extracted using the protocol proposed by Zelaya-Molina et al. (2011), which consists of a series of washings with chloroform-isoamyl alcohol. A group of 10 representative isolates that share morphological characteristics were used to amplify the DNA with the pairs of oligonucleotides ELONGF1 (5´-TCACGATCGACATTGCCCTG-3´) and ELONGR1 (5´-ACGGCTCGAGATGACCAT G-3´) for TEF-1α, TUBUF2 (5´-CGGTAACAACTGGGCCAAGG-3´) and TUBUR1 (5´-CCTGGTACTGCTGGTAC TCAG-3´) for beta tubuline (Kroon et al., 2004) and the oligonucleotides DC6 (5´-GAGGGACTTTTGGGTAATCA-3´) and ITS4 (5´-TCCTCCGCTTATTGATATGC-3´) for the region ITS (Bonants et al., 1997).
The conditions to carry out the PCR using the oligonucleotides for TEF-1α and β-Tub consisted of an initial denaturalization at 95 °C for 2 min, followed by 35 denaturalization cycles at 95 °C for 1 min, alignment at 60 °C for 1 min, elongation at 72 °C for 1 min and a final extension at 72 °C for 10 min. The conditions of amplification with the oligonucleotides for ITS were 94 °C for 3 min, followed by 30 cycles at 94 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min and a final extension at 72 °C for 10 min.
The specific oligonucleotides for the species P. nicotianae, Pn1 (5´-GACTTTGTAAGTGCCACCATAC-3´) and Pn2 (5´-CTCAGCTCTTTTCCTTGGATCT-3´) (Meng and Wang, 2010), were used under the following conditions of PCR: 94 °C for 5 min, 35 cycles at 94 °C for 1 min, alignment at 58 °C for 1 min, elongation at 72 °C for 1 min and a final extension at 72 °C for 10 min.
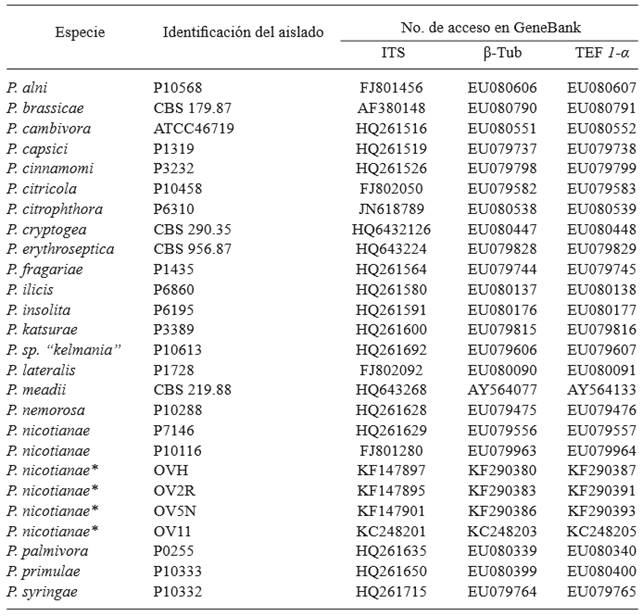
Phylogenetic analysis and sequencing
The PCR products obtained from the genes ITS, TEF-1α and β-Tub were purified using the kit Wizard SV Gel and PCR Clean-Up System (Promega), following the specifications described by the supplier. Sequencing was carried out by capillary electrophoresis with an automatic sequencer ABI PRISM 310 (Genetic Analizer PE Applied Biosystems), in the Unit of Molecular Biology of the Cell Physiology Institute of the UNAM. The sequences were edited using the program CHROMAS Pro 1.6 (Technelysium Pty Ltd, South Brisbane, Qld, Australia), and compared with sequences of the GenBank of the National Center for Biotechnology Information (NCBI) using the Nucleotide Basic Local Alignment Search Tool (BLAST-N) and the Megablast algorithm. For the multiple alignment and phylogenetic analysis of four sequences of representative isolations, we used the software MEGA 6.06 (Tamura et al., 2013). The sequences were aligned with another 22 belonging to different species of Phytophthora (Table 1) using the program MUSCLE integrated in MEGA (Edgar, 2004), using the default values for the penalization for opening and extension of gaps and with the later manual correction of the alignment. The alignments underwent the nucleotide substitution model selection program in order to select the evolutionary model that best fit the data. For the phylogenetic reconstruction we used the maximum verisimilitude model and the Tamura-Nei model. The variation of rates between sites was modeled by a gamma distribution plus invariable sites (4 categories). The support of the internal topology of the dendrograms was determined by bootstrap analysis with 1000 re-sampling (Felsenstein, 1985). Linked alignments were carried out on the 3 markers using the software DAMBE 6.1.23 (Xia, 2013) and a phylogenetic tree was constructed as described earlier.
Pathogenicity evaluation
Ten-week old vinca plants were inoculated by spraying a suspension of zoospores of 1 x 104 zoospores/ml of 10 representative isolates from different sampling sites. Three pots containing 5 plants each were inoculated with isolate. Plants inoculated with only sterile distilled water were used as a control treatment. All plants were kept in a shade mesh area at 25 °C and 80-90 % relative humidity. Once the symptoms developed in the inoculated plants, the pathogen was reisolated and the incidence of the disease was quantified.
Results and Discussion
Morphological identification
Thirty-nine Phytophthora sp. isolates were obtained from vinca plants with symptoms of blight and wilting (Figure 1A); 77 % came from ornamental nurseries and 23 % came from urban areas. The isolations produced coenocytic mycelia and white cotton-like colonies in V8 agar; they grew between 7 and 30 °C (optimum temperature 25 °C); they developed persistent sporangia, oval or lemon shaped terminals, mainly papillate (Figure 1B) with a average of 34.16 μm in length and 26.35 μm in width (n=24). Waterhouse (1963) mentions that the range of the size of P. nicotianae sporangia is around 25-50 x 20-40 μm; meanwhile, Erwin and Ribeiro (1996) reported ranges of 11-60 x 20-45 μm. A proportion in length/width of 1.29 μm (n=24) was observed in the sporangia, similar to the proportion of 1.3 described by Erwin and Ribeiro (1996) for P. nicotianae. All isolations displayed intercalary and terminal chlamydospores (Figure 1D), which is a characteristic of P. nicotianae (Waterhouse, 1963; Erwin and Ribeiro, 1996).
Sexual compatibility
The isolations were heterothallic, 21 of a compatibility type A1 and 18 of compatibility type A2 (Table 2). The interaction between compatibility types A1 and A2 produced anaplerotic oospores (Figure 1C), with spherical and flat oogonia, and amphiginous antheridia. These characteristics are consistent with P. nicotianae according to Waterhouse (1963), and Erwin and Ribeiro (1996). In some sampling sites, both types of compatibility were found, which suggests the presence of sexual recombination; this would increase the genetic variability of the population and could cause the appearance of highly pathogenic isolates (Rodríguez-Tovar et al., 2004; Blaya et al., 2015). An important observation was the presence of both types of compatibility in three out of the four nurseries observed, which means that recombination could take place in this site, and that if plants with initial infections were to be sold, they would be a factor of dispersion in the region.
Molecular identification
A fragment of approximately 1300 pb was obtained when oligonucleotides DC6/ITS4 were used in the ten isolates selected. The products were sequenced and compared with sequences deposited in the NCBI. Homology percentages ranged between 98 and 100 % with the species P. nicotianae. Oligonucleotides ELONGF1/ ELONGR1 generated a fragment of approximately 1000 pb; the products were sequenced and when the sequences were compared, a homology was obtained between 99 and 100 % with P. nicotianae. Oligonucleotides TUBUF2/TUBUR1 generated a fragment of approximately 1000 pb, and its sequences displayed a homology between 98 and 100 % with P. nicotianae. The sequences of the different amplified regions were deposited in the NCBI (Table 3).
Table 3. Accesions of sequences of three loci deposited in the GenBank of P. nicotianae isolates obtained from V. rosea in urban areas in Culiacan and incidence of infection in pathogenicity experiment.
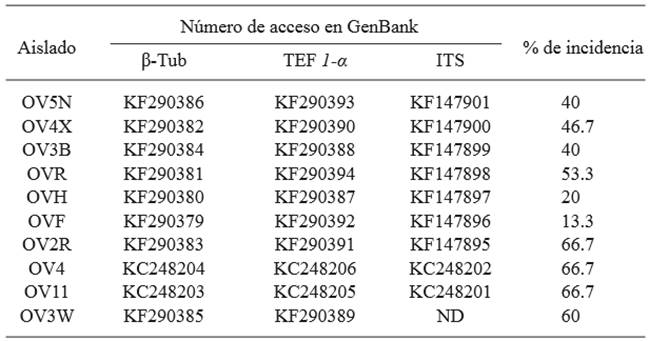
Β-Tub = beta tubulin, TEF 1-α = elongation factor, ITS= internal transcribed spacer region, ND= not determined.
The oligonucleotides Pn1/Pn2 specific to P. nicotianae amplified a fragment of approximately 400 pb in all the isolates (Figure 2). These results were expected for P. nicotianae (Meng and Wang, 2010). The use of these oligonucleotides can be a quick strategy to identify this species.
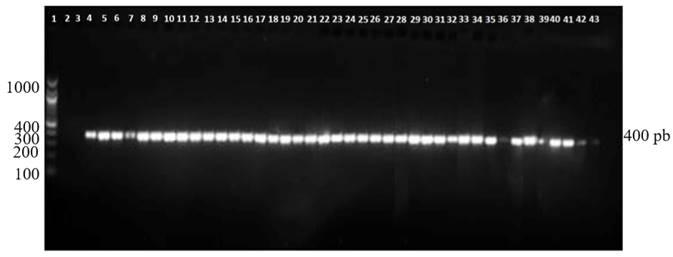
Figure 2. Amplified DNA fragment with the oligonucleotides Pn1-Pn2 specific to P. nicotianae. Line 1, Molecular marker 100 bp; Line 2, water; Line 3, negative control (P. capsici); Line 4, P. nicotianae positive control, Lines 5-43 P. nicotianae isolated from Vinca rosea.
The phylogenetic reconstruction resulting from linking the three markers showed that the four isolates analyzed in this study were grouped in the same clade as the reference strains of P. nicotianae (Figure 3; 100 % bootstrap), belonging to clade 1 of species of Phytophthora (Cooke et al., 2000; Kroon et al., 2004; Blair et al., 2008). The analyses of the individual partial sequences of each marker showed low percentages of support of the internal topology of the dendrograms compared with the values resulting from linking the 3 loci under analysis. With the sequences obtained in this study, individual alignments were carried out for each marker used (ITS, β Tub and TEF 1-α). Later, the 3 alignments were linked (1589 pb) and a similarity was found of 99.6 to 100 % between sequences, therefore not all sequences are equal; 11 differences were found between nucleotides and 2 gaps per insertions of nucleotides (Data not shown). Future studies could estimate thte genetic diversity of Phytophthora nicotianae populations by analyzing a greater number of isolates from different regions and years of collection in order to enrich the study and correlate it with the phenotypical characteristics of the pathogen.
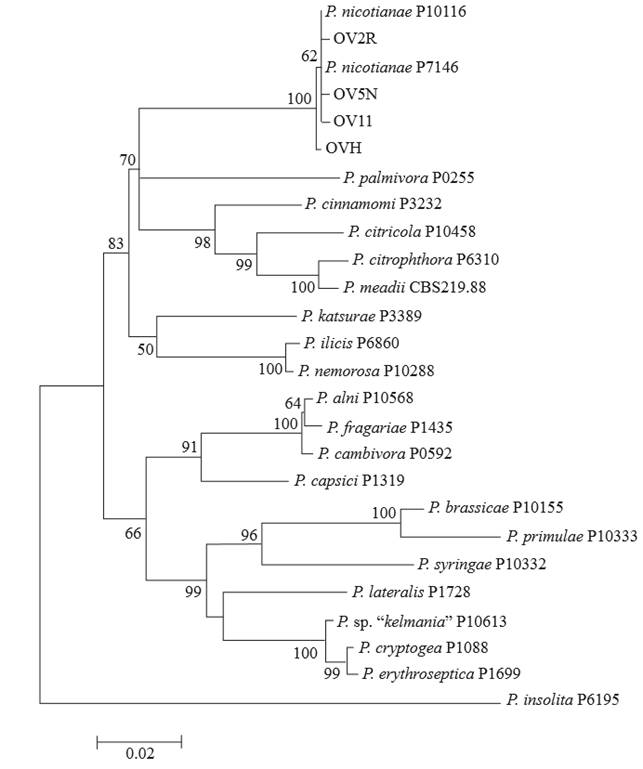
Figure 3. Phylogenetic tree diagram of representative isolates of this study (OV2R, OV5N, OV11, and OVH) and 22 reference strains of Phytophthora derived from the partial sequences of three loci (ITS, β Tub, and TEF 1-α). The tree was created using the software MEGA 6.06 (bootstrap=1000), using the Tamura-Nei substitution model (TN93 G+I). The bootstrap values are shown on the branches.
Pathogenicity evaluation
The 10 isolates evaluated in the pathogenicity tests caused the symptoms of the disease in V. rosea plants. The initial symptoms of the disease were observed 4 days after inoculation and included leaf blight. Afterwards, plants developed symptoms of wilting and died 12 days after inoculation. All isolations were virulent, they infected healthy vinca plants, although there was a variation in the incidence level, since the percentage of plants with symptoms fluctuated between 13 and 66.7 % (Table 3). Inoculated strains were reisolated from inoculated plants.
It is important to mention that P. nicotianae infects more than 300 different hosts, including Solanum lycopersicum and Capsicum annuum (Erwin and Ribeiro 1996). For this reason, we cannot discard that the species P. nicotianae found in vinca plants in urban areas is also causing some infections in the vegetable-growing area of the Culiacan valley, which is based largely on the production of tomatoes and peppers. Although diverse species of Phytophthora have been reported as causal agents of blight and wilting in vinca (McGovern and Begeman, 1996; McMillan and Garofalo, 2004; Orilowski et al., 2011; Hao et al., 2010), the morphological, phylogenetic, and pathogenic analysis performed in this investigation shows that P. nicotianae is responsible for the damages inflicted on this ornamental plant in the samples gathered in the city of Culiacan.
Conclusions
Phytopthora nicotianae was the only species associated with blight and death of vinca plants in the city of Culiacan. The molecular characterization indicated that the nucleotidic sequences in the regions TEF 1-α, β-Tub, and ITS showed a homology of 98-100 % with the species P. nicotianae and they were grouped phylogenetically with reference strains of this species. All the isolates were detected by the oligonucleotides Pn1/Pn2 specific to P. nicotianae. The Koch postulates showed the pathogenicity of the isolations on vinca. This is one of the few studies focused on ornamental plant diseases in urban areas in Northwestern Mexico.
Acknowledgements
To CONACYT for funding Brando Álvarez Rodríguez’s studies.
REFERENCES
Alvarez-Rodriguez B, Ortiz-Meza JA, Rojo-Baez I, Márquez-Zequera I, García-Estrada RS, Carrillo-Fasio JA and Allende-Molar R. 2013. First report of vinca blight caused by Phytophthora nicotianae in Northwestern Mexico. Plant Disease 97:1257. http://dx.doi.org/10.1094/PDIS-04-13-0400-PDN [ Links ]
Blair JE, Coffey MD, Park S-Y, Geiser DM and Kang S. 2008. A multi-locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genetics and Biology 45:266-277. http://dx.doi.org/doi:10.1016/j.fgb.2007.10.010. [ Links ]
Blaya J, Lacasa C, Lacasa A, Martínez V, Santísima-Trinidad AB, Pascual JA and Ros M. 2015. Characterization of Phytophthora nicotianae isolates in southeast Spain and their detection and quantification through a real-time TaqMan PCR. Journal of Science Food and Agriculture 95:1243-1251. http://dx.doi.org/doi:10.1002/jsfa.6813. [ Links ]
Bonants P, Hagenaar-de Weerdt M, van Gent-pelzer M, Lacourt I, Cooke D and Duncan J. 1997. Detection and identification of Phytophthora fragariae Hickman by the polymerase chain reaction. European Journal of Plant Pathology 103:345-355. http://dx.doi.org/doi:10.1023/A:1008640227432 [ Links ]
Cooke DEL, Drenth A, Duncan JM, Wagels G and Brasier CM. 2000. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genetics and Biology 30:17-32. http://dx.doi.org/10.1006/fgbi.2000.1202 [ Links ]
Donahoo RS and Lamour KH. 2008. Characterization of Phytophthora species from leaves of nursery woody ornamentals in Tennessee. HortScience 43:1833-1837. Disponible en línea: http://hortsci.ashspublications.org/content/43/6/1833.full.pdf+html [ Links ]
Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32:1792-1797. http://dx.doi.org/10.1093/nar/gkh340 [ Links ]
Erwin D, and Ribeiro O. 1996. Phytophthora Diseases Worldwide. Minnesota. The American Phytopathological Society. 562 Pp. [ Links ]
Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. Disponible en línea: http://statweb.stanford.edu/~nzhang/Stat366/Felsenstein85.pdf [ Links ]
Hagan A. 2004. Diseases of anual vinca in the greenhouse and landscape. Alabama Cooperative Extension System ANR-1023 6 pag. Disponible en línea: http://www.aces.edu/pubs/docs/A/ANR-1023/ANR-1023.pdf [ Links ]
Haider M, Tahir M, Saeed A, Shah A, Rashid N, Javed M and Iqbal J. 2007. Vinca minor, another host of a tomato infecting begomovirus in Pakistan. African Crop Science Conference Proceedings 8:905-907. Disponible en línea: http://www.acss.ws/acw/Upload/XML/Research/425.pdf [ Links ]
Hao W, Richardson P and Hong C. 2010. Foliar blight of annual vinca (Catharanthus roseus) caused by Phytophthora tropicalis in Virginia. Plant Disease 94:274. http://dx.doi.org/10.1094/PDIS-94-2-0274A [ Links ]
Javier-Alva J y Mattos L. 2006. Nuevo método para aislar Phytophthora parasitica Dastur de raicillas de limonero patrón rugoso Citrus jambhiri Lush bajo riego por aspersión. Universalia 11:23-35. Disponible en línea: https://dialnet.unirioja.es/servlet/articulo?codigo=2916267 [ Links ]
Kroon LPNM, Bakker FT, Van den Bosch GBM, Bonants PJM and Flier WG. 2004. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genetics and Biology 41:766-782. http://dx.doi.org/doi:10.1016/j.fgb.2004.03.007 [ Links ]
Martin F, Abad Z, Balci Y and Ivors K. 2012. Identification and detection of Phytophthora: Reviewing our progress, identifying our needs. Plant Disease. 96:1080-1103. http://dx.doi.org/10.1094/PDIS-12-11-1036-FE [ Links ]
McGovern R and Begeman J. 1996. Reduction of Phytophthora blight of Madagascar periwinkle in the landscape by soil solarization. Proceedings of the Florida State Horticultural Society 108:58-60. Disponible en línea: http://fshs.org/proceedings-o/1995-vol-108/58-60%20(MCGOVERN).pdf [ Links ]
McMillan R and Garofalo J. 2004. Phytophthora parasitica wilt of new cultivars of Catharanthus roseus. Proceedings of the Florida State Horticultural Society 117:316-317. Disponible en línea: http://journals.fcla.edu/fshs/article/view/85918/82834 [ Links ]
Meng J and Wang Y. 2010. Rapid detection of Phytophthora nicotianae in infected tobacco tissues and samples based on its Ypt1 gene. Journal of Phytopathology. 158:1-7. http://dx.doi.org/doi:10.1111/j.1439-0434.2009.01548.xo [ Links ]
Mirsoleimani Z and Mostowfizadeh-Ghalamfarsa R. 2013. Characterization of Phytophthora pistaciae, the causal agent of pistachio gummosis, based on host range, morphology, and ribosomal genome. Phytopathologia Mediterranea 52:501-516. http://dx.doi.org/10.14601/Phytopathol_Mediterr-11549 [ Links ]
Orilowski L, Ptaszek M and Trzewik A. 2011. Phytophthora shoot blight of periwinkle in polish hardy ornamental nursery stock. Journal of Plant Protection Research 51:1-6. Disponible en línea: http://www.plantprotection.pl/PDF/51(4)/JPPR_51(4)_22_Orlikowski.pdf [ Links ]
Pintos VC, Mansilla VJ y Aguín CO. 2004. Phytophthora ramorum nuevo patógeno en España sobre Camellia japonica y Viburnum tinus. Boletin de Sanidad Vegetal Plagas. 30:97-111. Disponible en línea: http://www.magrama.gob.es/ministerio/pags/biblioteca/plagas/BSVP-30-01-01-097-111.pdf [ Links ]
Rodríguez-Tovar A, Xoconostle-Cásarez B y Valdés M. 2004. Ecología molecular de los hongos ectomicorrízicos. Revista Fitotecnia Mexicana 27:267-278. Disponible en línea: http://www.redalyc.org/pdf/610/61027307.pdf [ Links ]
Schena L and Cooke DEL. 2006. Assessing the potential of regions of the nuclear and mitochondrial genome to develop a “molecular tool box” for the detection and characterization of Phytophthora species. Journal of Microbiological Methods 67:70-85. http://dx.doi.org/10.1016/j.mimet.2006.03.003 [ Links ]
Schubert T and Leahy R. 1989. Phytophthora blight of Catharanthus roseus. Florida Department of Agriculture and Consumer Service Division of Plant Industry. Plant Pathology Circular 321. Disponible en línea: http://www.freshfromflorida.com/content/download/11327/144253/pp321.pdf [ Links ]
Tamura K, Stecher G, Peterson D, Filipski A and Kumar S. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30:2725-2729. http://dx.doi.org/10.1093/molbev/mst197 [ Links ]
Waterhouse G. 1963. Key to the species of Phytophthora de Bary. Mycological Papers. No. 92. [ Links ]
Xia X. 2013. DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Molecular Biology and Evolution 30:1720-1728. http://dx.doi.org/10.1093/molbev/mst064 [ Links ]
Zelaya-Molina LX, Ortega MA and Dorrance AE. 2011. Easy and efficient protocol for oomycete DNA extraction suitable for population genetic analysis. Biotechnology Letters 33:715-720. http://dx.doi.org/10.1007%2Fs10529-010-0478-3 [ Links ]
Received: May 06, 2016; Accepted: July 08, 2016











 text in
text in