Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.34 n.3 Texcoco Sep. 2016
https://doi.org/10.18781/r.mex.fit.1603-1
Scientific articles
Bacillus spp. in the Control of Wilt Caused by Fusarium spp. in Capsicum chinense
1Instituto Tecnológico de Conkal, km 16.3, Avenida Tecnológico s/n, CP. 97345 Conkal, Yucatán. México. Teléfono: (999) 9124135
2Facultad de Química, Universidad Autónoma de Querétaro. Cerro de las campanas s/n, Colonia las Campanas, 76010. Querétaro, Querétaro. Teléfono: 01 (442) 192-1200 Extensión: 5531; Fax: 01 (442) 192-1304.
The genus Fusarium is a pathogen associated with chili wilt and reduced crop yield. Rhizobacterias are an alternative to improve agricultural production and protection against plant pathogens. In this work the in vitro antagonism of ten Bacillus strains against Fusarium solani ITCF1 and F. equiseti ITCF2 were evaluated. Our results showed that all the bacterial strains inhibited mycelial growth between 21.28 and 71.70 %, additionally the CBMT2 and CBMT51 strains showed inhibition halos against F. equiseti with halos of 3.76 and 6.37 mm. The two pathogens showed 100 % incidence of disease in seedlings habanero chili and severity of 90.0 % by F. solani and 77.5 % by F. equiseti. In resistance tests wilt four strains of Bacillus based on the antagonistic activity were used, three inoculations were made in base of stem 15, 28 and 35 days after germination, we found that B. subtilis CBMT51and B. cereus BL18 reduced the severity disease caused by F. equiseti and BL18 strain for F. solani, in 47.7, 37.8 and 50.9 % respectively at 28 days of evaluation.
keywords: Rhizobacteria; antagonism; incidence; severity; Fusarium solani; Fusarium equiseti
El género Fusarium es un patógeno asociado al marchitamiento del chile y con reducción del rendimiento del cultivo. Las rizobacterias son una alternativa para mejorar la producción agrícola y protección contra fitopatógenos. En el presente estudio se evaluó el antagonismo in vitro de diez cepas de Bacillus contra Fusarium equiseti ITCF1 y F. solani ITCF2, todas la cepas bacterianas inhibieron el crecimiento micelial entre 21.28 y 71.70 %, adicionalmente las cepas CBMT2 y CBMT51 presentaron halos de inhibición contra F. equiseti con halos de 3.76 y 6.37 mm. Los dos patógenos mostraron 100 % de incidencia de la enfermedad en plántulas de chile habanero y severidad de 90.0 % por F. solani y 77.5 % por F. equiseti. En pruebas de resistencia a la marchitez se utilizaron cuatro cepas de Bacillus con base en la actividad antagónica mostrada, se realizaron tres inoculaciones en la base del tallo a los 15, 28 y 35 días después de la germinación, se obtuvo que B. subtilis CBMT51 y B. cereus BL18 redujeron la severidad de la enfermedad ocasionada por F. equiseti y la cepa BL18 para F. solani, en un 47.7, 37.8 y 50.9 % respectivamente a los 28 días de la evaluación.
Palabras clave: Rizobacteria; antagonismo; incidencia; severidad; Fusarium solani; Fusarium equiseti
In Mexico, the states with the highest surface dedicated to the production of Capsicum chinense Jacq. are Yucatán, Tabasco, Campeche, and Quintana Roo, Yucatán being the state with the greatest surface of production, with 170 hectáreas, and a production of 1,826 tons (SIAP, 2013). Also, due to its organoleptic characteristics of a longer shelf life and spiciness, the habanero chili produced in the Yucatan is considered of a higher quality than those grwon in other areas of the world (Medina et al., 2008). The reduction of the chili crop yield is related to the presence of plant pathogens. The genus Fusarium is one of the most important plant pathogens related to the wilting and yield reduction of the chili plant. Fusarium spp. and F. oxysporum were the most frequent fungi related to wilting in 100 % of Capsicum annuum L. samples of different types of chilies, such as serrano and jalapeño (Albañil et al., 2015). In C. annuum L., F. oxysporum, F. solani, and F. equiseti have been identified to affect the crop and reducing yield significantly (Martínez et al., 2011). The most common methods of control of these pathogens is the use of synthetic fungicides that pollute the environment. Therefore, an alternative for biological control is the use of antagonistic rhizobacterias, capable of exerting control over pathogens (Guillén et al., 2006). This antagonism is attributed in part to the production of chitinases (Chang et al., 2010) and lipopeptides such as: iturin, surfactin, fengycin, and bacillomycin (Ramarathnam et al., 2007). The most commonly used rhizobacterias belong to the genus Bacillus. The species of B. megaterium and B. lincheniformis not only promote growth and induce a higher yield of C. annuum L., but are also related to the reduction of the incidence of plant pathogens (Amaresan et al., 2014). Applying B. megaterium in C. annuum infected with Phytophthora capsici Leonian reduced the severity of the disease by 50.4 % (Akgül and Mirik, 2008). The rhizobacterias activate defense mechanisms in plants against pathogens (Ahemad and Khan, 2011). In rhizobacterias that promote plant growth, a reduction has been observed of some diseases in plants, and they are related to the capacity of inducing defense enzymes, such asperoxidases, polyphenol oxidase, phenylalanin amonnia lyase, chitinases and β-1,3 glucanase, which can have an effect on the reduction of disease incidence and severity (Saravanakumar et al., 2007). Guillén et al. (2006), applied Bacillus amyloliquefaciens B1, B. licheniformis B3, and B. subtilis B9 and B13 on Fusarium spp., Rhizoctonia solani Kühn and P. capsici Leonian, pathogens that cause rotting of roots in chili plants (C. annuum L.); applying bacteria reduced the incidence by 80 %, and root rotting severity by 39 % in comparison to the control. This study evaluated strains of Bacillus spp. with antifungal activity in vitro and its effect on the response of resistance to wilting in en C. chinense, induced by F. equiseti and F. solani.
Materials and methods
Microorganisms used and preparation of inoculants.
Ten strains were used of Bacillus, isolated, and characterized in earlier works, nine strains with activity in vitro against different pathogenic fungi (Sosa et al., 2012: Mejía et al., 2013; Ruiz et al., 2016) and one strain (Bacillus cereus BL18) reported with properties in the growth enhancement in Capsicum annum L. (Peña et al., 2016). The bacterial inoculants were obtained from broths in a nutrient broth® (CN) stirred for seven days at 200 rpm at 29 °C. The time used was to allow the formation of spores, which was verified with a light microscope. Later, samples were centrifuged at 8,000 x g for 10 min; the cellular package was washed with a 0.9 % NaCl solution, and adjusted to a concentration of 1x108 UFC mL-1 using a Neubauer camera (Luna et al., 2013). Fusarium solani and F. equiseti strains were previously isolated from C. chinense. The pathogens were reactivated in a potato dextrose agar (PDA) medium. For their multiplication, we used oat flakes sterilized in autoclave; for this, 100 g of flakes were placed in 250 mL flasks and 40 mL potato extract were added (300 g of raw potato in 1 L of boiling water for 15 min). Later, we deposited two discs of 0.5 cm of the mycelium in active growth of every pathogenic fungus and they were incubated in a growth chamber at 30 °C for ten days (Bruna, 1991). This was used as an inoculant for later tests.
Inhibition of mycelial growth in vitro of Fusarium spp. by Bacillus spp.
The biotests were carried out by direct confrontation with the widely used dual planting (Li et al., 2011; Essghaier et al., 2012; Rios et al., 2016). In the center of 90 x 15 mm Petri dished with potato dextrose agar (PDA) medium, we placed a disc, 0.5 cm in diameter, of mycelium of the pathogen with 10 d of active growth, and innoculated 6 µL of a 1x108 UFC bacterial solution in four equidistant points around the mycelial growth at a distance of 2 cm. The dishes were incubated at 28 °C for 7 d. The radial growth was measured, and using this data, the percentage of mycelial inhibition was calculated, ttaking the fungal growth in PDA in the absence of bacteria as a control.
Pathogenicity tests for Fusarium equiseti and F. solani in habanero chili.
Habanero chili cv. native seeds were used after being disinfected with 2 % sodium hypochlorite and washed 3 times with sterile distilled water. They were planted in plastic trays with 98 holes with the commercial sterile substrate Cosmopeat®, sterilized in an autoclave at 120 °C for 15 min. Plantlets were obtained 28 days after germination (DDG) and pathogenicity tests were run on them in order to determine the virulence by action of the pathogenic fungi. Pots were prepared using 32 oz styrofoam cups, with 600 g of substrate of a mixture of the soil K´ancab (regional Mayan name) of the type Luvisol and cow manure (1:1) (Soria et al., 2002). Luvisol soils are reddish brown clay soil and slightly acidic (Bautista, 2005). The substrate was sterilized at 120 °C for 15 min. It was added 10 g of oats with the growth of the pathogens of 1x106 conids per grams of oats, which was quantified by taking a gram of oats with inoculant and adding 9 mL of sterilized distilled water. It was counted with the help of a Neubauer camera, one plantlet was placed in each pot, and in order to allow the pathogen to enter the plant, a 1 cm cut was performed on the apex of the root, in order to determine its virulence (Herrera et al., 2011).
Evaluation of the resistance to wilting by Fusarium spp. in habanero chili plants inoculated with Bacillus spp.
The disinfected habanero chili seeds were planted in plastic trays with 98 holes with the commercial sterile substrate Cosmopeat®. Fifteen days after germination (DDG) an inoculation was carried out, of 1 mL of bacterial suspension of 1x108 UFC mL-1 on the base of the stems of the plantlets. The control was applied 1 mL of 0.9 % of NaCl. At 28 DDG, the transplant was carried out in 32 oz styrofooam cups with 600 g os substrate (mixture of Luvisol and cow manure), previously inoculated with 10 g of oats that contained 1x106 conids·g-1. After 28 and 35 DDG, 3 mL of the bacterial solution were added. Fertilization took place with the formula 125-100-150 of N-P-K (Soria et al., 2002). The experiment was established under macrotunnel-type greenhouse conditions in the Instituto Tecnológico de Conkal (21º 04’ N and 89º 31’ O), with six treatments for each pathogen evaluated, four bacterial strains, a treatment that consisted of no bacterial inoculation, but with the presence of the pathogenic fungi, and a control that consisted in plantlets with no inoculation of pathogens of bacterial strains. It was established in a completely random design with 10 repetitions for each treatment; each pot represented an experimental unit. To associate the resistance induced by the bacterial inoculation, every four days after the appearance of the symptoms, the severity was estimated using a scale of five classes: 1=0 %, 3=10 %, 5=25 %, 7=50 %, and 9=100 % damage (CIAT, 1987). Using the severity data, progress curves of the disease were made, and the model of the Area Under the Disease Progress Curve (AUDPC) was used to calculate the intensity of the disease. On the 28th day after inoculation with the pathogens, the final severity of the disease was calculated with the arcsin instruction; y=arsin (sqrt(y/100)), using the parameter of Yfinal (Campbell and Madden, 1990). Finally, using the Abbott procedure (1925), the efficiency of the bacterial strains as resistance inducers was validated. Averages were compared when the significance between treatments was determined using Tukey (p≤0.05). To verify result reproducibility, the experiments were repeated in two periods (December, 2014 and February, 2015); for the analysis, the averages of both evaluations were used. The statistical analyses were carried out using the program SAS version 9.3 for Windows (SAS Institute Inc. 2010).
Results and Discussion
In vitro activity of Bacillus spp. against Fusarium spp.
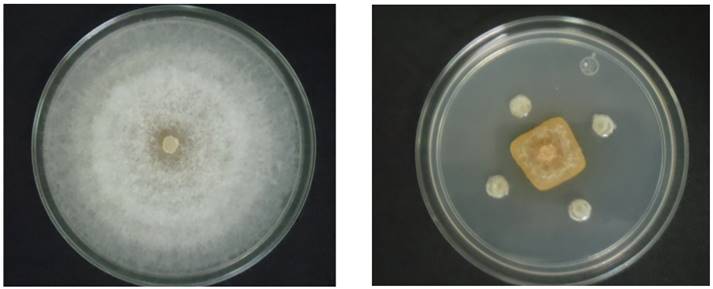
All strains showed inhibition of the mycelial growth of the pathogens; the percentages of inhibition in F. equiseti were between 21.28 and 71.70 %, and for F. solani, it was 37.4 to 69.92 % (Table 1). The greatest effect was observed with B. subtilis CBMT51 on the growth of F. equiseti, and strain CBRF8 showed the greatest effect against both pathogens with values of over 63 %. The strain B. cereus BL8, reported as a growth enhancer, showed percentages of inhibition significantly lower than the two strains mentioned above. The range of inhibition reported by several authors is broad, percentages of inhibition of 90 % are reported for B. subtilis strains against Fusarium sp. (Badía et al., 2011), and, on the other hand, percentages of inhibition of 29.4 % were reported for F. avenacum (Essghaier et al., 2012), and for B. methylotrophicus and B. amyloliquefaciens against F. oxysporum of 42.0 and 51.5 % (Rios et al., 2016); the latter are within the range obtained in this study. Strains CBMT2 and CBMT51 were the only ones to present halos of inhibition of mycelial growth in confrontation with F. equiseti with halos measuring 3.76 and 6.37 mm, respectively, which correspond to 18.3 and 31.5 % of inhibition (Figure 1). The antagonistic activity of Bacillus is due to several mechanisms, such as competition for the colonization of the rhizosphere (Compant et al., 2005), production of lipopeptides such as Iturin, Fengycin, and Surfactin (Kim et al., 2010), and the production of lytic enzymes (Pleban et al., 1997; Chang et al. 2010). The strain B. subtilis Pla10 produces antibiotics of the family Iturin A and Surfactin; the purified fractions showed that only Iturin A presented fungicidal activity (Ragazzo et al., 2011). The purified chitinase from Bacillus cereus YQ 308 inhibits the elongation of F. oxysporum and F. solani hyphae, reducing the biomass of fungi in comparison with the control (Chang et al., 2003). According to the results, four Bacillus strains were selected for the wilting control tests in habanero chili plants based on its antifungal activity; the strain CBRF8, which showed the highest percentage of mycelial inhibition in F. solani, the strain CBMT51, which showed the highest percentage of inhibition and halo of inhibition in F. equiseti, strain CBMT2, with antifungal activity against both pathogens and the presence of inhibition halo in F. equiseti, and the strain BL18 was included, with a moderate antifungal activity, although reported as having plant growth enhancing properties.
Table 1. Activity in vitro of Bacillus spp. against Fusarium spp. after seven days of confrontation.

○Averages with different letters are statistically different (p≤0.05).
Pathogenicity tests for Fusarium equiseti and F. solani in habanero chili.
The pathogenicity tests for Fusarium equiseti ITCF1 and F. solani ITCF2 inoculated in habanero chili plants, showed virulence, since they induced characteristic symptoms such as chlorosis, flaccidity, and partial defoliation partial rotting of roots and neck, which occasionally rose to 3 to 7 cm above the base of the stem. Despite records of 100 % incidence with both species of fungus, severity was greater with F. solani ITCF2, where it recorded 90.0 %, and lower with F. equiseti ITCF1 with 77.5 %, without representing a statistical difference (Table 2). The symptoms of the disease began to manifest themselves after two weeks of inoculation with the pathogen. These results, in terms of incidence and severity, were similar to those obtained in other studies on Capsicum annuum L. and Solanum lycopersicum L., obtaining an incidence of 100 % and severity of 80 to 100 % with two strains of F. oxysporum (Apodaca et al., 2004). In pathogenicity tests on Thevetia peruviana plants inoculated with Fusarium species, F. solani was found to cause symptoms such as necrosis in the stem, chlorotic leaves, and wilting, determining this species as the most virulent (Herrera et al., 2011), results which agree with those in this study. An analysis of variance was carried out with the Area Under the Disease Progress Curve (AUDPC) model to determine the pathogenicty and aggressiveness of the two fungi, and for F. equiseti and F. solani, 1120.0 and 1201.0 units day-1 were obtained, respectively. There was no significant difference in the virulence of the fungi (Table 2), although a greater unit can be observed of plants diseased with F. solani. For the control, without the presence of fungi, there were 0.0 diseased units, keeping the plants healthy and vigorous until the end of the experiment.
Evaluation of the resistance to wilting by Fusarium spp. in habanero chili plants inoculated with Bacillus spp.
The appearance of symptoms in plants inoculated with F. equiseti and F. solani were observed eight days after inoculation. In general, in inoculated plants, F. solani displayed more virulence than F. equiseti when inducing greater severity during the progress of the disease; in control plants, without the inoculation of pathogens, the disease was not present (Figure 2). The analysis of the intensity of the disease showed less AUDPC with the inoculations of the strains CBMT51 and BL18 against F. equiseti, although only BL18 allowed less severity in plants infected with F. solani. At the end of the experiment, the parameter Yfinal displayed, with CBMT51, a severity of 39.7 % in F. equiseti, while BL18 against F. solani gave 41.7 %. This helped calculate the higher levels of disease control with 47.8 and 50.9 % efficiency, respectively (Table 3). In trials with B. subtilis CAS15 applications on C. annuum, it reduced the severity of the disease caused by F. oxysporum, with a control efficiency of 56.9 %. Similar to that obtained in this study, this efficiency was attributed to a systemic resistance induction (SRI) (Yu et al., 2011). The SRI has been observed in C. annuum against Phytophthora capsici when B. megaterium was used, when reducing the severity of the disease between 18.3 and 33.3 %, and a control efficiency of 23.1 to 57.7 % (Akgül and Mirik, 2008). In the Cucumis sativus crop with F. oxysporum problems, evaluations with Bacillus B068150, reduced the severity by 49.32 %, which meant a control of 50.68 % (Li et al., 2011). In S. lycopersicum, resistance was conferred against Ralstonia solanacearum when using B. subtilis (CYBS-5) with a control efficiency of over 50 % (Chen et al., 2012). Likewise, in S. lycopersicum L. affected by F. oxysporum, the implementation of Pseudomonas fluorescens significantly reduced the incidence and severity of wilting between 4.86 and 74.49 %, where the preinoculation with bacteria tends to improve the activation of the SRI (Yu et al., 2011). Som rhizobacteria ar capable of producing salicylic acid, responsible for SRI, as shown with P. aeruginosa 7NSK2 against Botrytis cinerea (De Meyer and Hofte, 1997). These attributes can be used for the biological control of pathogens that originate in the soil, which can reduce the use of synthetic pesticides.
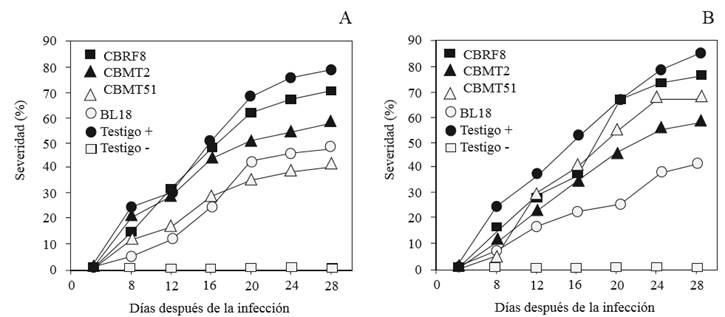
Figure 2. Disease progress curves of the severity by wilting induced by Fusarium equiseti (A) and F. solani (B) 28 days after the infection in habanero chili plants innoculated with Bacillus spp.
Table 3. Area under the disease progress curve (AUDPC), severity and control efficiency for Fusarium equiseti and F. solani in habanero chili plants innoculated with Bacillus spp.

○Averages with different letters in each column are statistically different (Tukey P≤ 0.05). DMS = minimum significant difference; Control + with presence of the pathogen; Control - without pathogens or bacterias.
Conclusions
The bacterial strains in this study presented antagonistic properties in vitro against Fusarium solani and F. equiseti, both of which displayed virulence in Capsicum chinense, causing wilting in plants. For the resistance to wilting in C. chinense, the inoculation of the strains Bacillus subtilis CBMT51 and B. cereus BL18 reduced the severity caused by F. equiseti, although only BL18 decreased it in F. solani. The capacity to control the disease was observed to be related to the type of bacterial strain and the species of the pathogen. Strains CBMT51 and BL18 displayed properties un the biocontrol of witling caused by Fusarium, therefore they can be used to protect habanero chili crops.
Acknowledgements
Project partially funded by TecNM, the authors wish to thank Alejandro García Ramírez for his technical support.
REFERENCES
Albañil-Juárez J A, Mariscal-Amaro LA, Martínez-Martínez TO, Anaya-López JL, Cisneros-López HC y Pérez-Ramírez HA. 2015. Estudio regional de fitopatógenos asociados a la secadera del chile en Guanajuato, México. Revista Mexicana de Ciencias Agrícolas 11: 2191-2197. Disponible en línea: http://cienciasagricolas.inifap.gob.mx/editorial/index.php/Agricolas/article/view/4044/3378 [ Links ]
Abbott WS. 1925. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology 18: 265-267. http://dx.doi.org/10.1093/jee/18.2.265a [ Links ]
Ahemad M and Khan MS. 2011. Functional Aspects of Plant Growth Promoting Rhizobacteria: Recent Advancements. Insight Microbiology 1: 39-54. http://dx.doi.org/10.5567/IMICRO-IK.2011.39.54 [ Links ]
Akgül DS and Mirik M. 2008. Biocontrol of Phytophthora capsici on pepper plants by Bacillus megaterium strains. Journal of Plant Pathology 90: 29-34. http://dx.doi.org/10.4454/jpp.v90i1.588 [ Links ]
Amaresan N, Jayakumar V and Thajuddin N. 2014. Isolation and characterization of endophytic bacteria associated with chilli (Capsicum annuum) grown in coastal agricultural ecosystem. Indian Journal of Biotechnology 13: 247-255. Disponible en línea: http://nopr.niscair.res.in/bitstream/123456789/29149/1/IJBT%2013%282%29%20247-255.pdf [ Links ]
Apodaca-Sánchez MA, Zavaleta-Mejía E, Osada-Kawasoe S, García-Espinosa R y Valenzuela-Ureta JG. 2004. Pudrición de la corona del chile (Capsicum annuum L.) en Sinaloa, México. Revista Mexicana de Fitopatología 22: 22-29. Disponible en: http://www.redalyc.org/articulo.oa?id=61222104. [ Links ]
Badía RMM, Hernández TB, Murrel LJA, Mahillon J y Pérez M. 2011. Aislamiento y caracterización de cepas de Bacillus asociadas al cultivo del arroz (Oryza sativa L.). Revista Brasileira de Agroecologia 6: 90-99. Disponible en línea: http://www.aba-agroecologia.org.br/revistas/index.php/rbagroecologia/article/view/9924/6778 [ Links ]
Bautista F, Palma-López D y Huchim-Malta W. 2005. Actualización de la clasificación de los suelos del estado de Yucatán. p. 105-222. En: F. Bautista y G. Palacios (Eds.) Caracterización y Manejo de los Suelos de la Península de Yucatán. Implicaciones Agropecuarias, Forestales y Ambientales. Universidad Autónoma de Campeche, Universidad Autónoma de Yucatán. 282. [ Links ]
Bruna A. 1991. Marchitez y pudrición de corona y raíces de espárrago (Asparagus officinalis L.) causado por Fusarium oxysporum Schlechtf. sp. asparagi Cohen. Agricultura Técnica (Chile) 51: 52-54. Disponible en línea: http://www.chileanjar.cl/files/V51I1A08_es.pdf [ Links ]
Campbell CL and Madden LV. 1990. Introduction to plant epidemiology. John Wiley and Sons. New York. 532 p. [ Links ]
Chang WT, Chen CS and Wang SL. 2003. An Antifungal Chitinase Produced by Bacillus cereus with Shrimp and Crab Shell Powder as a Carbon Source. Current Microbiology 47: 102-108. http://dx.doi.org/10.1007/s00284-002-39557 [ Links ]
Chang WT, Chen ML and Wang SL. 2010. An antifungal chitinase produced by Bacillus subtilis using chitin waste as a carbon source. World Journal of Microbiology and Biotechnology 26: 945-950. http://dx.doi.org/10.1007/s11274009-0244-7 [ Links ]
Chen Y, Yan F, Chai Y, Liu H, Kolter R, Losick R and Guo J. 2012. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environmental Microbiology 10: 1-17. http://dx.doi.org/10.1111/j.14622920.2012.02860.x [ Links ]
CIAT. 1987. Sistema estándar para la evaluación de germoplasma de fríjol. Art van Schoohoven y Marcial Pastor-Corrales (comp). Cali, Colombia. 42 p. Disponible en línea: https://books.google.com.mx/books?id=mpgIE_jDedMC&pg=PA5&lpg=PA5&dq=Sistema+estándar+para+la+evaluación+de+germoplasma+de+fr%C3%ADjol.&source=bl&ots=CLo9IQyb3M&sig=PAt4zRe7HGCi9C5rNeK55kwA8XU&hl=es419&sa=X&ved=0ahUKEwi5wr_Gr9_KAhWI1h4KHaJeA_4Q6AEIIzAC#v=onepage&q=Sistema%20estándar%20para%20la%20evaluación%20de%20germoplasma%20de%20fr%C3%ADjol.&f=false [ Links ]
Compant S, Duffy B, Nowak J, Clément C and Barka EA. 2005. Use of growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Applied and Environmental Microbiology. 71: 4951-4959. http://dx.doi.org/10.1128/AEM.71.9.4951-4959.2005 [ Links ]
De Meyer G and Hofte M. 1997. Salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 induces resistance to leaf infection by Botrytis cinerea on bean. Phytopathology 87: 588-593. http://dx.doi.org/10.1094/PHYTO.1997.87.6.588 [ Links ]
Essghaier B, Hedi A, Hajlaoui MR, Boudabous A, and Sadfi-Zouaoui N. 2012. In vivo and in vitro evaluation of antifungal activities from a halotolerant Bacillus subtilis strain J9. African Journal of Microbiology Research 6: 4073-4083. http://dx.doi.org/10.5897/AJMR11.403 [ Links ]
Guillén-Cruz R, Hernández-Castillo, FD, Gallegos-Morales G, Rodríguez-Herrera R, Aguilar-González CN, Padrón-Corral E y Reyes-Valdés MH. 2006. Bacillus spp. como biocontrol en un suelo infestado con Fusarium spp., Rhizoctonia solani Kühn y Phytophthora capsici Leonian y su efecto en el desarrollo y rendimiento del cultivo de chile (Capsicum annuum L.). Revista Mexicana de Fitopatología 24: 105-114. Disponible en: http://www.redalyc.org/articulo.oa?id=61224204 [ Links ]
Herrera-Parra E, Bacab-Pérez IM, Cristóbal-Alejo J, Tun-Suárez JM, y Ruiz-Sánchez E. 2011. Patogenicidad de Fusarium solani (Mart.) Sacc. y Alternaria alternata (Fries) Keissler en Thevetia peruviana (Pers.) K. Schum. y su control in vitro. Fitosanidad 15: 231-236. Disponible en línea: http://www.fitosanidad.cu/index.php/fitosanidad/article/view/139 [ Links ]
Kim PI, Ryu J, Kim YH and ChI YT. 2010. Production of biosurfactant lipopeptides iturin A, Fengycin, and Surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. Journal of Microbiology and Biotechnology 20: 138-145. http://dx.doi.org/10.4014/jmb.0905.05007 [ Links ]
Li L, Ma J, Li Y, Wang Z, Gao T and Wang Q. 2011. Screening and partial characterization of Bacillus with potential applications in biocontrol of cucumber Fusarium wilt. Crop Protection 35: 29-35. http://dx.doi.org/10.1016/j.cropro.2011.12.004 [ Links ]
Luna ML, Martínez PRA, Hernández IM, Arvizu MSM y Pacheco AJR. 2013. Caracterización de rizobacterias aisladas de tomate y su efecto en el crecimiento de tomate y pimiento. Revista Fitotecnia Mexicana 36: 63-69. Disponible en línea: http://www.redalyc.org/articulo.oa?id=61025678008 [ Links ]
Medina-Lara F, Echeverría-Machado I, Pacheco-Arjona R, Ruiz-Lau N, Guzmán-Antonio A and Martínez-Estévez M. 2008. Influence of nitrogen and potassium fertilization on fruiting and capsaicin content in habanero pepper (Capsicum chinense Jacq.). HortScience 43: 1549-1554. Disponible en: http://hortsci.ashspublications.org/content/43/5/1549.full [ Links ]
Mejía-Bautista MA, Reyes-Ramírez A y José Tun-Suárez JM. 2013. Actividad antagonista de cepas nativas de Bacillus spp. contra Fusarium sp. Memorias del XXXVI Congreso Nacional de Control Biológico. Oaxaca de Juárez, Oaxaca, México. Resumen, p. 26-29. [ Links ]
Martínez MA, Martínez MC, Bielza P, Tello J and Lacasa A. 2011. Effect of biofumigation with manure amendments and repeated biosolarization on Fusarium densities in pepper crops. Journal Indian Microbiology and Biotechnology 38: 3-11. http://dx.doi.org/10.1007/s10295-010-0826-2. [ Links ]
Peña-Yam LP, Ruız-Sánchez E, Barboza-Corona JE and Reyes-Ramírez A. 2016. Isolation of Mexican Bacillus Species and Their Effects in Promoting Growth of Chili Pepper (Capsicum annuum L. cv Jalapeño). Indian Journal of Microbiology. http://dx.doi.org/10.1007/s12088-0160582-8. [ Links ]
Pleban S, Chernin L and Chet I. 1997. Chitinolytic activity of an endophytic strain of Bacillus cereus. Letters in Applied Microbiology 25: 284-288. http://dx.doi.org/10.1046/j.1472-765X.1997.00224.x [ Links ]
Ragazzo-Sánchez JA, Robles-Cabrera A, Lomelí-González L, Luna-Solano G y Calderón-Santoyo ML. 2011. Selección de cepas de Bacillus spp. productoras de antibióticos aisladas de frutos tropicales. Revista Chapingo serie Horticultura. 17: 5-11. Disponible en: http://www.redalyc.org/articulo.oa?id=60920104001 [ Links ]
Ramarathnam R, Bo S, Chen Y, Fernando WG, Xuewen G and De Kievit T. 2007. Molecular and biochemical detection of fengycin-and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of canola and wheat. Canadian Journal of Microbiology 53: 901-911. http://dx.doi.org/10.1139/W07-049 [ Links ]
Rios-Velasco C, Caro-Cisneros JN, Berlanga-Reyes DI, Ruíz-Cisneros MF, Ornelas-Paz JJ, Salas-Marina MA, Villalobos-Pérez E y Guerrero-Prieto VM. 2016. Identification and antagonistic activity in vitro of Bacillus spp. and Trichoderma spp. isolates against common phytopathogenic fungi. Revista Mexicana de Fitopatología 34: 84-99. http://dx.doi.org/10.18781/R.MEX.FIT.1507-1 [ Links ]
Ruiz-Sanchez E, Mejia-Bautista MA, Serrato-Diaz M, Reyes-Ramirez A, Estrada-Giron Y y Valencia-Botin AJ. 2016. Antifungal activity and molecular identification of native strains of Bacillus subtilis. Agrociencia 50: 133-148. Disponible en línea: http://www.colpos.mx/agrocien/Bimestral/2016/feb-mar/art-1.pdf [ Links ]
Saravanakumara D, Vijayakumarc C, Kumarb N and Samiyappan R. 2007. PGPR-induced defense responses in the tea plant against blister blight disease. Crop Protection 26: 556-565. http://dx.doi.org/10.1016/j.cropro.2006.05.007 [ Links ]
SAS Institute. 2010. User’s Guide: Statistics, version 9.3. SAS Inst. Inc., Cary, North Caroline, USA. [ Links ]
Servicio de Información Agroalimentaria y Pesquera (SIAP). 2013. Anuarios estadísticos. Secretaría de agricultura, ganadería, desarrollo rural, pesca y alimentación. http:/www.siap.gob.mx/ [ Links ]
Soria FM, Tun SJ, Trejo RA y Terán SR. 2002. Paquete tecnológico para la producción de chile habanero (Capsicum chinense Jacq.). SEP. DGTA. ITA-2 Conkal, Yuc, México. 75 p. [ Links ]
Sosa-Pech M, Ruiz-Sánchez E., Mejía-Bautista M, Reyes-Ramírez A., Cristóbal-Alejo J, Valencia-Botín A y GutiérrezAlonzo O. 2012. Actividad antagónista in vitro de aislados de la clase Bacilli de la península de yucatán contra cuatro hongos fitopatógenos. Universidad y Ciencia 28:279-284. Disponible en http://132.248.10.25/era/index.php/rera/article/view/16 [ Links ]
Yu X, Ai C and Zhou G. 2011. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. European Journal of Soil Biology 47: 138-145. http://dx.doi.org/10.1016/j.ejsobi.2010.11.001. [ Links ]
Received: March 17, 2016; Accepted: June 15, 2016











 text in
text in