Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.33 n.2 Texcoco 2015
Phytopatological notes
Epidemics caused by Mycosphaerella caryigena and their impact in defoliation pecan tree
1Centro de Investigación Regional Norte Centro-INIFAP, km 17 carretera Torreón-Matamoros, Coahuila. C. P. 27440 Tel: 871 82-30-81. México.
2Junta Local de Sanidad Vegetal de Zaragoza, del Estado de Coahuila Km 12 Carretera Zaragoza Cd. Acuña. Cp. 26450, Zaragoza, Coah. Tel. 01 (862) 621-25-11, 626-04-50. México.
3Unidad Regional Universitaria de Zonas Áridas de la Universidad Autónoma Chapingo. Carretera Gómez Palacio-Cd. Juárez, Chihuahua, km 38.5, C. P. 35230. Tel: (872) 77-60-190. Fax. (872) 77-600-43. Bermejillo, Durango. México.
In the pecan tree, the fungus Mycosphaerella caryigena is the causal agent of the pecan downy spot, which causes injury on foliage and defoliation. In the years 1998, 1999 and 2014, ten epidemiological models of lesion/leaf vs time were evaluated. In 1992, epidemiological models were tested for the capture of spores vs. time. In 1998, lesions/leaf defoliation and defoliation vs time was also evaluated. The epidemics of 1998, 1999 and 2014 fitted the models of Logistic Ln, Gompertz and Logistic Ln, respectively. Defoliation of pecan tree by M. caryigena began in August 1998 and ended with 95 % in September. The Gompertz and Log10 models were the best fit for defoliation vs time and lesions/leaf vs defoliation with R 2 0.924 and 0.937, respectively. The capture of spores of M. caryigena was >50 % from April to May; the monomolecular model was the best fit to the capture of spores (R 2 0.948). The lesions/leaf appeared in June and were >100 in September, just when the defoliation reached >90 %. The models lesions/leaf vs time, suggest prolonged periods to produce the inoculum and the incubation of the fungus on the leaf, which coincides with what is known of the disease cycle.
Key words: Fungi; foliage; diseases
En el nogal pecanero, el hongo Mycosphaerella caryigena es el agente causal de la mancha vellosa, el cual induce lesiones en el follaje hasta causar la defoliación. En los años 1998, 1999 y 2014, se evaluaron nueve modelos epidemiológicos de lesión/hoja vs tiempo. En 1992, se ensayaron modelos epidemiológicos para la captura de esporas vs tiempo. En 1998, también se evaluó lesiones/hoja vs defoliación y defoliación vs tiempo. Las epidemias de 1998, 1999 y 2014 ajustaron a los modelos Logístico Ln, Gompertz y Logístico Ln, respectivamente. La defoliación del nogal por M. caryigena en 1998 inició en agosto y culminó con 95 % en septiembre. Los modelos Gompertz y Log10 tuvieron el mejor ajuste para defoliación vs tiempo y lesiones/hoja vs defoliación con R 2 0.924 y 0.937, respectivamente. La captura de esporas de M. caryigena fue > 50 % de abril a mayo; el modelo monomolecular fue el que mejor ajustó a la captura de esporas (R 2 0.948). Las lesiones/hoja aparecieron en junio y fueron > 100 en septiembre justo cuando la defoliación alcanzó > 90 %. Los modelos lesiones/hoja vs tiempo, sugieren períodos prolongados para producir el inóculo y de incubación del hongo en la hoja, lo cual coincide con lo que se conoce del ciclo de la enfermedad.
Palabras clave: Hongos; follaje; enfermedades
The northern areas of the states of Coahuila and Nuevo León, Mexico, are humid and warm areas in which the pecan tree (Carya illinoinensis Wangenh. K.Koch) is grown in a surface of approximately 10,000 ha (SAGARPA, 2013). Both areas are favorable for foliar pathogens of the pecan tree, the most prominent being Fusicladium effusum G. Winter, causal agent of scabs; Cercospora fusca F.V. Rand, brown spot; Gnomonia dispora Demaree & Cole, stains in the veins of the leaf; Colletorichum spp., anthracnose; Microsphaera penicillata (Wallr.) Lév. and Trichothecium roseum (Pers.) Link, mildew; and the downy spot caused by Mycosphaerella caryigena Demaree & Cole, respectively (Aguilar-Pérez, 2014).
Downy spots, along with scabs, are the most important foliar diseases of the pecan tree in Mexico and the United States (Aguilar-Pérez, 2014; Latham, 1982; Sparks, 1995).
The species of Mycosphaerella that attack the foliage of agricultural crops affect their physiology in a negative manner. In the case of the pecan tree, Mycosphaerella causes premature defoliation affecting the yield and quality of the nut throughout that year and the following one (Andersen et al., 1990; Pinkard y Mohammed, 2006; Rodríguez-Gaviria and Cayón, 2008).
In Northern Coahuila, it has been confirmed that the leaves of the pecan tree can have spots or lesions caused by M. caryigena that, when ripe, cause the leaves to fall (Aguilar-Pérez, 2014). The study of the epidemiology of the disease will contribute to improve its management, particularly the levels of inoculum and seasons in which the disease causes damage (defoliation) to the tree. The aims of this investigation lead us to the analysis of downy spot epidemics in the pecan tree (lesions/leaf), the capture of spores in time and the potential use of both to estimate the premature defoliation of the crop.
The study was carried out in the pecan tree orchards in SEZAR-INIFAP (1998), Santo Cristo (1999), and el Caracol (2014) located in the municipal areas of Zaragoza, Villa Unión, and Zaragoza, Coahuila, respectively. In each orchard, five trees were selected in the areas with downy spot backgrounds. The lesions caused by M. caryigena (spot/leaf) in each tree were counted in the leaves of 10 sprouts randomly chosen. For each year (1998, 1999, and 2014), the lesions were counted between April and late September. The data of the lesion count were processed to determine the epidemiological model (lesions vs time) with a better adjustment. The adjusted models were Monomolecular, Gompertz, Logistic, Exponential, log10, and in the first four the natural logarithm (Ln) (Campbell and Madden, 1990) was applied to the variable of time. Likewise, in the foliage of the trees in the orchard SEZAR-INIFAP, defoliation was quantified on a visual scale 1, 2, 3, 4, and 5, where the foliage in the tree was > 90, 90-50, < 50-25, < 25-10, and 0 %, respectively. The epidemiological models mentioned were run for the data on defoliation vs time and lesions. In 1992, in the orchard of Santo Cristo, the spores of M. Caryigena were captured. This capture was carried out using a vane-style spore trapper with a hygrothermograph cylinder, where the spores were counted for 24 h on a sheet of graph paper containing Vaseline. The base of the trapper contained an inverted fan for air suction, which entered the cylinder through an orifice located approximately 60 cm from the floor. This trapper worked with a car battery, and was placed in the middle of a tree's dripping area in the middle of the orchard; the 1992 captures were carried out between March and September and are expressed as spores/day. The data of the spores captured were adjusted to the models pointed out.
The dynamics of lesions/leaf throughout the plating cycle in the three orchards in the corresponding years are presented in Figure 1. The lesions are round chlorotic spots with an oily appearance and a diameter of 3 to 6 mm that turn silvery and then light brown. In all three orchards, defoliation after mid September was over 90 %. The lesions always appeared on the leaves in late June and over 100 lesions/leaf were counted towards mid-September (Table 1). When adjusting the data of Figure 1 to the epidemiological models, it turned out that for the epidemic of 1998, 1999, and 2014, the best-adjusted models were Logistic Ln, Gompertz, and Logistic Ln, respectively (Table 2).
Table 1 Starting dates of the first lesions/leaf caused by M. caryigena, and final date in which more than 100 lesions/leaf are reached in different years.

Table 2 Epidemiological models and their adjustment to the epifitias (lesions/leaf) in different years, numbers in bold with better adjustment (R2).
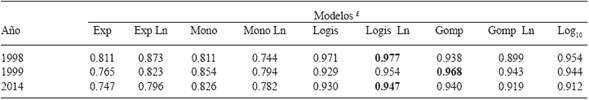
£ The models: Exp, Mono, Logis, Gomp, and Log10, correspond to Exponential, Monomolecular, Logistic and Gompertz, respectively. The first four models were applied a natural logarythm (Ln).
Premature defoliation caused by M. caryigena in pecan trees during 1998 in the orchard of SEZAR-INIFAP began in mid-August and reached a maximum of 95 % towards the end of September. The models Gompertz and model Log10 had the best adjustments for defoliation vs time and lesions/leaf vs defoliation with R 2 0.924 y 0.937, respectively.
In the orchard of Santo Cristo, the capture of M. caryigena spores, from mid-April to mid-May accounted for over 50 % of the total of spores captured (Figure 2); the monomolecular model was the best adjusted to the capture of spores vs time, with a R 2 0.948, suggesting that the source of inoculum comes from the discharge of spores from one point.
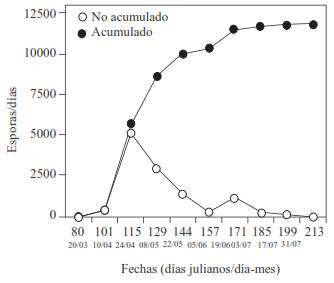
Figure 2 Capture of M. caryigena spores in 1992 in the Santo Cristo orchard, in the municipal area of Villa Unión Zaragoza, Coahuila. Accumulated, or amount of spores captured; not accumulated, spores captured in the date of sampling.
The data in Table 1 indicate that the disease initially manifested itself in June, and that after reaching 100 or more spot/leaf in August, the disease will cause a defoliation of at least 90 %.
The prediction models for the diseases caused by Mycosphaerella spp. are useful. The model developed by Salam et al. (2009) determined that when capturing more than 40 % of Mycosphaerella pinodes ascospores, cause significant damage to pea (Pisum sativum L) plantations. The data on Figure 2 suggest that around 50 % of the M. caryigena spores are produced in April-May and cause significant damage (defoliation) three months later. When measuring the effects of the downy spot on the pecan tree, foliage discoloration, its necrosis, and defoliation could also be taken into account in a better damage model, similar to the one developed for Eucalyptus spp. in Australia (Stone et al., 2003).
Both models, Logistic Ln and Gompertz, are variants of the logistic model; in the first one, the exponential part of the epidemic comes early or late in time compared to the logistic model. In this investigation, the two models that predominated in lesions/leaf vs time, along with the periods of latency, weeks long, allocated for M. caryigena (Goff et al., 1987) suggest that the appearance of the symptoms is delayed in time, if we consider that the spores in the environment are abundant between March and May (Figure 2). The inoculum of M. caryigena is known to be produced in the leaves that fall to the ground and hibernate (months), where pseudothecia form, grow, and finally release ascospores (Goff et al., 1987).
Downy spots have a wide variability, both in the manifestation of lesions/leaf and in defoliation. For example, in the years 2000 and 2005, a variation of 50 to more than 300 lesions/lea was recorded, respectively (data not shown).
Downy spots are currently handled using preventive, curative, and unconventional (plant extract) fungicides, as well as with the use of pecan trees that are resistant to the disease (Aguilar-Pérez, 2014).
The use of fungicides in Mycosphaerella spp. has caused a resistance to the fungus (Aguilar-Barragán et al., 2014); to avoid a possible resistance of M. caryigena, plant extracts was evaluated for their use on the pecan tree (Aguilar-Pérez, 2008; Patiño et al., 2007).
Being able to record weather factors such as temperature, humidity, and wind inside the orchards, related to downy spots in the pecan tree, will undoubtedly help to optimize and predict the disease-environment-time models. Another unresolved theme is the taxonomic part of Mycosphaerella in the pecan tree, since there is no study on this in Mexico. The Mycosphaerella species are constantly being taxonomically relocated, since the genus has a polyphyletic origin, anamorphic states and species encrypted, all in relation to its pathogenesis in susceptible agricultural crops (Crous, 2009; Crous et al., 2004 and 2007).
In short, the M. caryigena epidemics in pecan trees become evident as of June. If the lesions/leaf are 100 or more by September, defoliation will be at least 90 %.
Acknowledgements
The authors wish to thank the SAGARPA-COANCYT Fund for funding investigation and publication of this work, which was performed through the project code 2011-13-175247.
REFERENCES
Aguilar-Barragán, A., García-Torres, A. E., Odriozola-Casas, O., Macedo-Raygoza, M., Tetsuya Ogura., Manzo-Sánchez, G., James, C. A., Islas-Flores, I. and Beltrán-García, M. I. 2014. Chemical management in fungicide sensivity of Mycosphaerella fijiensis collected from banana fields in México. Brazilian Journal of Microbiology 45: 359-364. [ Links ]
Aguilar-Pérez, H. 2008. Fungicidas orgánicos para el control de la mancha vellosa del nogal. Ficha tecnológica por sistema producto. Páginas 75-76. http://biblioteca.inifap.gob.mx:8080/xmlui/bitstream/handle/123456789/815/289.pdf?sequence=1. [ Links ]
Aguilar-Pérez, H. 2014. Manual para el Manejo Orgánico del Nogal Pecanero. Palibrio. Estados Unidos. 274 p. [ Links ]
Andersen PC, Aldrich JH and Gould AB. 1990. Impact of pecan leaf blotch on gas exchange of pecan leaves. Plant Disease 74: 203-207. [ Links ]
Campbell CL and Madden LD. 1990. Introduction to Plant Disease Epidemiology. John Wiley & Son. 532 p. [ Links ]
Crous PW. 2009. Taxonomy and phylogeny of the genus Mycosphaerella and its anamorphs. Fungal Diversity 38:1-24. [ Links ]
Crous PW, Groenewald JZ, Pongpanich K, Himaman W, Arzanlou M and Wingfield MJ. 2004. Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Studies in Mycology 50: 457-469. [ Links ]
Crous PW, Braun U. and Groenewald, J. Z. 2007. Mycosphaerella is polyphyletic. Studies in Mycology 58: 1-32. [ Links ]
Goff WD, Drye CE and Miller RW. 1987. Ecology and epidemiology of pecan downy spot. Phytopathology 77: 491-496. [ Links ]
Latham AJ. 1982. Effects of some weather factors and Fusicladium effusum conidia dispersal on pecan scab occurrence [Carya illinoensis]. Phytopathology 72: 1339-1345. [ Links ]
Patiño LF, Bustamante E y Salazar LM. 2007. Efecto de Sustratos Foliares Sobre la Sigatoka Negra (Mycosphaerella fijiensis Morelet) en Banano (Musa × paradisiaca L.) y Plátano (Musa acuminata Colla). Agricultura Técnica 67: 437-445. [ Links ]
Pinkard EA and Mohammed CL. 2006. Photosynthesis of Eucalyptus globulus with Mycosphaerella leaf disease. New Phytologist 170: 119-127. [ Links ]
Rodríguez-Gaviria PA y Cayón G. 2008. Efecto de Mycosphaerella fijiensis sobre la fisiología de la hoja de banano. Agronomía Colombiana 262: 256-265. [ Links ]
SAGARPA. Sistema de Información Agropecuaria de Consulta, 1980-2012. México, DF, 2013. [ Links ]
Salam MU, Galloway J, MacLeod WJ, Seymour M, Pritchard I, Barbetti MJ and Maling T. 2009. Translating research into the field: meta-analysis of field pea blackspot severity and yield loss to extend model application for disease management in Western Australia. In APPS 2009: Plant Health Management: An Integrated Approach: Abstracts of the 17th Australasian Plant Pathology Conference (pp. 74-74). APPS. [ Links ]
Sparks D. 1995. A climatic approach to pecan scab control. Hort-Technology 5: 225-230. [ Links ]
Stone C, Parsons M, Matsuki M and Carnegie AJ. 2003. Pest and disease assessment in young eucalypt plantations: Field Manual for using the Crown Damage Index. Bureau of Rural Sciences. Australia. 30 p. [ Links ]
Received: May 30, 2015; Accepted: June 29, 2015











 text in
text in 



