Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.33 n.2 Texcoco 2015
Scientific Articles
Yield loss caused by Candidatus Liberibacter asiaticus in Persian lime, in Yucatan Mexico
1Instituto de Fitosanidad. Colegio de Postgraduados. Campus Montecillo. Texcoco, Edo. de México, C.P. 56230, México.
2Centro de Investigación Regional Sureste, Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Mocochá, Yucatán. C.P. 97454, México.
3Campo Experimental General Terán, Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Nuevo León. C.P. 67413; México.
4SENASICA-DGSV. Coyoacán, D.F. CP 04100. México.
The aim of this study was to estimate yield losses induced by the agent associated with Huanglongbing, Candidatus Liberibacter asiaticus (CLas) in Persian lime (Citrus latifolia Tanaka) in Mexico, by evaluating morphological and organoleptic fruit variables. A four-year old Persian lime orchard in the state of Yucatán, Mexico, was selected; the trees were under same disease management and infection; this feature was verified by qPCR. The study was conducted under four severity levels (0=healthy, 1=25, 2=50, 3=75, 4=100, percentage of canopy with HLB symptoms). Eight morphological and organoleptic variables were evaluated in a restricted random block design with healthy and CLas infected trees. The values of weight, size, skin thickness, juice volume (JV) and pH were statistically higher in fruits from healthy trees as well as in asymptomatic branches of positive trees, in comparison with symptomatic branches (Tukey, P=0.05). CLas induced reduction on weight (17.3 %) and JV (18.6 %), with more damage in trees showing 100 % of canopy with HLB symptoms; the regression models were: Yweight = 217.2-4.2x+0.03x 2, R2= 0.86; Yvolume = 645.4-11.7x+0.09x 2, R2= 0.82. The weight reduction implicate a 2.4 tons/ha yield loss. The detrimental effect was associated with severity level and CLas concentration, we found that the intensity of the symptoms of HLB is a function of the bacterial concentration in the model Y [bacteria] = 1.174+0.8x-0.0067x 2, R2 = 0.91. This is the first quantitative study of CLas effects on production of Persian lime.
Key words: Severity; Huanglongbing; bacterial concentration; sour citrus
El objetivo de este estudio fue estimar pérdidas productivas inducidas por el agente asociado al Huanglongbing, Candidatus Liberibacter asiaticus (CLas) en limón persa (Citrus latifolia Tanaka) en México, mediante la evaluación de variables morfológicas y organolépticas en frutos. En el estado de Yucatán, México, se seleccionó una huerta de limón persa de cuatro años, con árboles bajo un mismo manejo e infección, esta característica se verificó por PCR cuantitativo. El estudio se condujo bajo cuatro niveles de severidad (0=sano, 1=25, 2=50, 3=75 y 4=100, porcentaje de síntomas de HLB en dosel). Se evaluaron ocho variables morfológicas y organolépticas en un diseño de bloques con tratamientos apareados. Los valores de peso, tamaño, grosor de cáscara, volumen de jugo (VJ) y pH fueron estadísticamente mayores en frutos de árboles sanos y en ramas asintomáticas de árboles positivos, en comparación con los frutos de ramas sintomáticas (Tukey, P=0.05). CLas indujo reducción en peso (17.3 %) y VJ (18.6%), con mayor daño en árboles con 100 % de síntomas de HLB en dosel; los modelos de regresión fueron: Ypeso =217.2-4.2x+0.03x2, R2=0.86; YVJ = 645.4-11.7x+0.09x2, R2=0.82. La reducción en peso implicó una pérdida en producción de 2.4 toneladas/ha. El efecto perjudicial se asoció al nivel de severidad y concentración de CLas, ya que se detectó que la intensidad de los síntomas de HLB están en función de la concentración bacteriana mediante el modelo Y[bacteria] =1.17+0.77x-0.0067x2, R2=0.91. El presente documento constituye el primer estudio cuantitativo del efecto de CLas en la producción de limón persa.
Palabras clave: Severidad; Huanglongbing; concentración bacteriana; cítricos agrios
In México, Huanglongbing (HLB) has been associated with Candidatus Liberibacter asiaticus (CLas); the first report in the country of this devastating disease to citrus industries worldwide was made during july 2009, in Tizimin, Yucatan (Trujillo, 2010). This pathogen is transmitted and spreads by Diaphorina citri Kuwayama (Hemiptera: Liviidae) (Halbert and Manjunath, 2004; Hall et al., 2013). For February 2015, 23 of 25 citrus-producing states of the Mexican citriculture confirmed the presence of CLas in trees and mainly in vectors, defining two epidemic scenarios: 1) area of greatest intensity and prevalence in commercial orchards: Region of the Pacific, and 2) area of less intensity and prevalence in backyard: Yucatan Peninsula and Gulf of Mexico (Mora-Aguilera et al., 2013).
Unlike the effects of HLB in sweet orange (Citrus sinensis L. Osbeck) in Brazil, and Florida, U.S.A. (Bassanezi et al., 2011; Gottwald et al., 2007; Bové, 2006), in the country the highest severity of physiological and histological symptoms caused by HLB has been found in the Mexican lime (Citrus aurantifolia Swingle Christm.) and Persian lime (Citrus latifolia Tanaka) (Esquivel-Chávez et al., 2012). The symptoms in leaves of citrus trees include chlorotic spots, angular stains, corky and thickening veins, mottle and difuse chlorosis, as well as the generalized yellowing of the leaf and defoliation. Irregular ripening has only appeared in the Mexican lime, begining with a yellow color in the base, and eventually falling of fruits (Robles-González et al., 2013). Histologically, there is an increase in starch in the mesophyll of Mexican and Persian limes. In sweet orange, it concentrates in the palisade parenchyma. In all cases, there is hyperplasia, which causes a collapse in the phloem (Esquivel-Chávez et al., 2012).
Information about HLB's destructive impact with quantitative and epidemiological support is scarce; available studies estimated the impact based on the number of eradicated plants and costs of the vector control in sweet citrus plants (Brlansky et al., 2009). In Brazil, losses in production due to the effect of HLB were in the range of 41 and 100 % in sweet orange cv. Valencia (Bassanezi et al., 2009). Bassanezi et al. (2011) determined the relationship of production with the effect of the disease and indicated a damaging trend; nonetheless, they also found that the magnitude of the production impact depends on the agronomic management and tree age. In Mexico, there were estimated losses in production throughout the production chain of Mexican lime and Persian lime (17.6 %) and sweet orange (57.6 %) using multivariate methods and comparative epidemiology at 3 and 5 years since the beginning of the disease (Salcedo et al., 2010). Given the current dispersion of CLas in the citriculture of the Mexican Pacific and the Yucatan Peninsula and its prevalence in sour citrus for which mostly the effects of this pathogen was unknown (Mora-Aguilera et al., 2013), there is an urgent need to know the impact on production of this citrus group. Therefore, the objective of this study was to determine the CLas detrimental effect on the production of Persian lime, C. latifolia, by analyzing morphological and organoleptic variables on fruits.
Materials and Methods
Place of study. A 0.5 ha block was delimited in a commercial orchard made up of 30 ha of four-year old Persian lime trees located in Tizimin, Yucatan, attacked by the disease since 2010. Agronomic management included drip irrigation, fertilization, trimming, and chemical control of D. citri.
Evaluation of symptoms and severity. The evaluation of severity was carried out in 388 trees using an arithmetic scale, which considered the types 0=healthy, 1=25, 2=50, 3=75 and 4=100 % of canopy with HLB symptoms. The percentage of severity was calculated by dividing the canopy of the tree in four sections of 25% each. The total severity (Sevt) by tree was the sum of the percentage of each section (s) . Where s= 1-4.
Diagnostic and quantification of the concentration of CLas. The plant tissue was collected in March 2011, a favorable time for the multiplication of the bacteria and expression of symptoms. Each sample consisted of eight leaves; samples were separated by symptomatic and asymptomatic sections in diseased trees. Likewise, healthy trees were sampled as controls. Molecular analysis consisted in isolating DNA with DNeasy Plant Mini Kit (Qiagen (r)) from 100 mg of plant tissue following the manufacturer's protocol. Detection and quantification was carried out in a Biorad(r) CFX96 thermocycler with a TaqMan(r) probe and specific primers for CLas that amplify a portion of the gene 16S rDNA, an positive internal control based on cytochrome-oxidase (COX) was included primers for the as reaction (DGSV-CNRF, 2008; Li et al., 2006).
For the quantification of the number of genomic copies of CLas in the samples, an external calibration curve was generated. A fragment of the gene 16S rDNA was used, cloned in a plasmid PGEM-T (r) Promega(r)). The plasmid was purified and quantified using uv-spectrophotometry (Nanodrop 1000) and serial dilutions were carried out. The reaction were performed in triplicate per concentration and a logarithmic regression was used to estimate the CLas concentration (y), in which y = (-6.221) * LN(Ct) + 27.409.
Relationship of the severity of symptoms with the bacterial concentration was determined in a quadratic model fitted.
Evaluation of morphologic and organoleptic variables in fruits. The fruits were harvested, labeled and kept in refrigeration at 4 °C until their evaluation was performed 2 and 5 days after harvest. The morphological variables measured were weight per fruit (gr); equatorial diameter (ED), polar diameter (PD), skin thickness (ST) (mm); and volume of juice (VJ) (mL) of 10 fruits per treatment. The organoleptic variables were measured in the juice extracted from the fruit: we determinated mainly concentration of Brix degrees using a digital refractometer (Palette(r)), pH with a potentiometer (HACH(r)), and titratable acidity through the phenolphthalein method (Helrich, 1990).
Statistical analysis. The experiment consisted of a design in blocks with paired treatments: healthy trees (T1) adjacent to infected trees, in which branches were differentiated in asymptomatic (T2) and symptomatic (T3). Thirty-two trees were chosen, distributed in six blocks in incomplete sub repetitions with four, six, and eight trees. The blocks were selected in a directed way to disease outbreaks. This method is similar to that used by Cristóbal et al. (2006) to reduce the variability of the data by effects of intensity and infection periods, soil fertility, and agronomic management of the orchard. For the evaluation of morphological and organoleptic variables, in each tree 10 fruits were collected in T1, T2, and T3. The averages of each variable per tree were organized by treatments, severity, and block for an ANOVA and comparison of averages using Tukey test (P=0.05). Analyses were carried out using SAS V9.0. In addition, a correlative analysis was made of losses with 10 trees for each severity level (50 trees) for to increase the variability of the effect of production depending on the damage. Before the correlative analysis, outliers were detected and excluded. The values for each evaluated variable and the percentage of severity for each tree were independently adjusted at different correlative models.
Results and Discussion
Description of the symptoms of HLB in Persian lime. The Persian lime fruits evaluated in this study showed no symptoms typically attributed to HLB, such as the ones indicated earlier for sweet citrus fruits (Bassanezi et al., 2009) and for Mexican limes, like irregular ripening and fruit fall (Robles-González et al., 2013); the above was recorded even in fruits from trees with a severity of 100% HLB severity. In this study, HLB symptoms were found in Persian lime leaves, without a distribution pattern in the canopies of studied trees. The symptoms observed (Figure 1) begin with diffuse mottled (A) that become larger until they are clearly defined and formed internerval angular spots are formed (B), which become distorted in chlorotic spots that invade the whole leaf (C). Plants with severe infections present thickening and corky of the central rib (D) with a coriaceous appearance; finally, a semi-intense and generalized yellowing can be seen in the leaf (E) without reaching the abscission, as is the case in Mexican lime and whose symptoms occur faster (Robles-González et al., 2013). In sweet oranges these symptoms are less intense, furthermore, the generalized yellowing of the leaf occurs infrequently and no defoliation of the same is observed (Esquivel-Chávez et al., 2012). These authors also indicated this tendency in histological symptoms, suggesting that in Mexico, sour citrus fruits present symptoms at a greater speed, intensity, and generalization in the canopy than those registered in other countries (Bové, 2006; Brlansky et al., 2009).

Figure 1 Symptoms of HLB in Persian lime (C. latifolia) confirmed as positive by quantitative PCR. (A) Diffuse mottled; (B) Angular spots; (C) Angular and chlorotic spots; (D) Rib thickening and cork appearance; (E) Generalized yellowing of the leaf; (F) Fruits form symptomatic branch with yellowing associated with HLB; (G) Fruit from healthy branch. Tizimin, Yucatan, 2011.
Diagnostic and quantification of the concentration of CLas in Persian lime. The sanitary condition of the selected trees was confirmed by PCR in real time as either healthy or infected. The latter displayed different concentrations of CLas; the intensity of the symptoms induced were preliminarily found to depend on the bacterial concentration. The relation between the concentration of CLas and the severity was shown to fit a quadratic correlation model y= 1.1736+0.774x-0.0057x2, R2= 0.91 (Figure 2).

Figure 2 Correlation of the severity of HLB in Persian lime vs concentration of CLas (number of copies of the gene 16S rDNA estimated by quantitative PCR in healthy and infected trees with different degrees of severity of HLB severity). The line and the model represent the adjustment using a quadratic regression.
The relation between bacterial concentration and the severity of HLB suggests that there is a direct relashionship between expression of symptoms and the increase of bacterial inoculum of the bacteria in the plant, which explains why high HLB severity leads to a greater negative effect on the production of Persian lime (Figure 4). This confirms the results obtained with a standard PCR in which detection is successful only in samples with evident symptoms of the disease (Teixeira et al., 2008); also, it explains the difficulty of carrying out these detections in latent infections, when bacterial concentration is very low (Li et al., 2008).
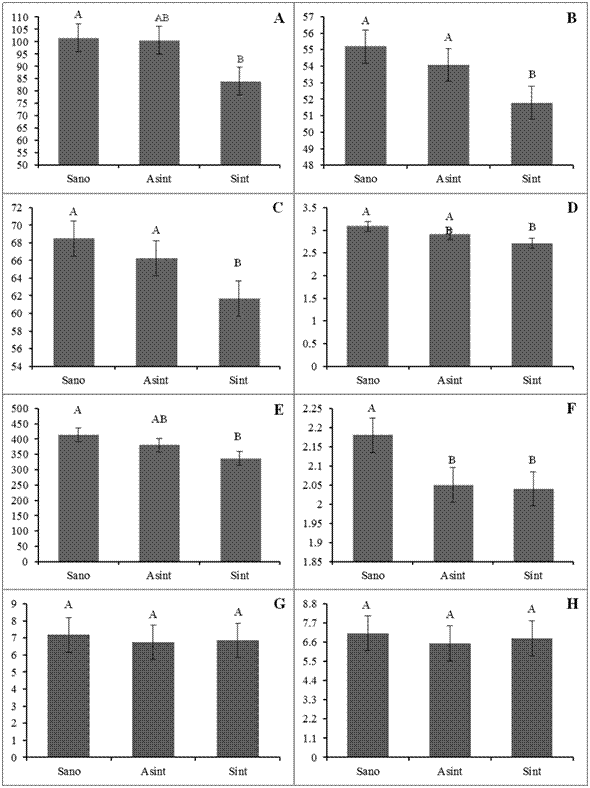
Figure 3 Comparison of average of weight (A), equatorial diameter (B), polar diameter (C), thickness of skin (D), volume of juice (E), pH (F), Brix degrees (G), and titratable acidity (H) evaluated in Persian lime fruits (C. latifolia) from healthy trees and symptomatic and asymptomatic branches of trees infected by CLas. For each variable, bars with at least one letter in common are statistically equal, Tukey (P= 0.05). The lines on the bars represent the standard error.

Figure 4 Correlation between severity of HLB and morphological variables: weight (A), equatorial diameter (B), polar diameter (C), thickness of skin (D), and volume of juice (E); Organoleptic: pH (F), Brix degrees (G) and tirtatable acidity (H), in Persian lime fruits (C. latifolia). The line and the model represent the adjustment using a quadratic regression.
Estimation of losses with an analysis of variance. The variables of weight, equatorial and polar diameters, thickness of skin, volume of juice and pH were statistically higher in healthy trees (T1) than in fruits from HLB symptomatic branches (T3) (Tukey, P=0.05) (Figure 3). These results show that bacterial concentration, even in a recent infection (15 months), has a negative influence on the morphological and organoleptic evaluated variables. This effect is influenced by the productive pattern of the Persian lime, since in the trees the fruits are concentrated in branches with secondary growth in the external part of the canopy, even with active growth (Agustí et al., 1995; Davies and Albrigo, 1994).
The variables of Brix degrees and titratable acidity were not statistically different between T1 and T3. No differences were observed between fruits from symptomatic and asymptomatic branches (T2 and T3), except in the variable of diameter, probably because the physiological effect does not depend only on the hosting of CLas in the phloem of the analyzed tissue, but also on the interference of the mobilization of photoassimilates due to the hosting of CLas in primary branches (Esquivel-Chávez et al., 2012). Between T1 and T3 the greatest reduction was in weight (17.31 %) and volume of juice (18.62 %). In skin thickness, reduction was 12.2 %, in polar diameter 10 % and in equatorial diameter 6.2 %. In the case of organoleptic variables, there was only a drop in pH observed, with 6.4 % ( Table1 ).
Table 1 Effect of Candidatus Liberibacter asiaticus on morphological and organoleptic variables of Persian lime fruits (C. latifolia)

ℓ Values with at least one letter in common in the columns are statistically equal (Tukey, p≤ 0.05).
ℓℓ Factor T1 (healthy), T2 (asymptomatic), and T3 (symptomatic) were confirmed by real time PCR.
ℓℓℓ The percentage was calculated with the difference of averages between fruits from healthy trees and symptomatic branches. *ED= Equatorial Diameter, PD= Polar diameter, ST= Skin Thickness and VJ= Volume of Juice.
The reduction in variables of weight and juice is explained by the direct physiological relation between them, since a greater weight of the fruit implies more juice (Davies and Albrigo, 1994). The effect of CLas in T3 in comparison to T1 (Table 1) translates into production losses of 17.3 % per productive year, more than what Salcedo et al. (2010) estimated in the absence of a disease, reporting losses of 17.6 % in sour citrus fruits and 52.7 % in sweet oranges, 5 years after CLas entered Mexico. In addition, these researchers explained that the highest productive impact of HLB would be on sweet oranges; this observation possibly was due to lack of information on epidemic scenarios in sour citrus, and that data on sweet orange epidemic were used for comparative purpose.
In Brazil, Bassanezi et al. (2009) observed weight losses of 17.5 % (early Valencia) to 42.3 % (late Valencia) due to Candidatus Liberibacter spp. In this region the disease is endemic, implying a prolonged exposure of trees to the bacterial infection, since it was detected in 2004. In our study, the losses found were similar to early Valencia (17.3 and 17.5 %) even though the evaluated plantation presented only a period of 6-15 months of exposure to the bacterial infection; therefore, expected losses in periods of disease exposure and disease development similar to those of Brazil may be higher in Persian lime in Mexico, especially when the severity of visual and histological symptoms are more intense in Persian lime than those reports for sweet orange (Esquivel-Chávez et al., 2012). In addition, the highest frequency of vegetative sprouting of the sour citrus fruits allows prolonged periods of infection and increase of the bacteria, due to the association with frequent infestations of infective vectors (Grafton-Cardwell et al., 2013; Hall et al., 2013; Robles-González et al., 2013).
Estimation of losses with correlative models. The association of the severity of HLB with morphological and organoleptic variables was confirmed with the fitting of correlative quadratic models (Figure 4). Despite the paired selection of healthy and infected trees to avoid variability effects in soil fertility and agronomic management, it was necessary to eliminate outliers, which were related with low-vigor trees (12/50); however, a number of trees remained under observation even superior to that used in a similar study of Bassanezi et al. (2009) where 14 and 21 trees were studied for each planting period and variety.
The variables that showed the highest correlation with severity were the morphological variables, since they fitted to the quadratic models. The models obtained in this study were as follow: Yweight =217.21-4.1958x+0.0288x2, (R2=0.86); YED =106.75-1.92x+0.014x2, (R2=0.87); YPD =131.7-2.30x+0.0169x2, (R2=0.87); YsT =6.56-0.12x+0.0008x2, (R2=0.85); YVJ =645.4-11.7x+0.09x2, (R2=0.82). In the organoleptic variables, only pH fitted the model YpH =2.17-0.0117+9E-05x2, (R2=0.83). Brix degrees and titratable acidity did not associate significantly with severity and there were no clear effects found of the bacteria on such variables. This possibly was due to the physiology of Persian lime fruits that tend to accumulate low concentrations of sugar, and to the nature of the physiological disorders induced by CLas; thus, a more prolonged infection period may be required to induce a detectable alteration in these variables (Davies and Albrigo, 1994). The variability of the production data due to the vigor of trees by multifactor effects of management was observed in a similar study with sweet oranges carried out in Brazil, where the dispersion of data in the correlation of weights of fruits with a severity of HLB was high, with low determination coefficients (R2=0.4) (Bassanezi et al., 2011); however, in that study, harvests from different years were combined (2004-2007) with different periods of endemicity (periods of infection); the above it was avoided in our study where a substantial improvement is obtained in the determination coefficients of the generated models.
In a situation of systemic or chronic infection like the induced by CLas, the estiomated losses in Persian lime indicated in our study are expected to increase with time due to the gradual weakening and productive tree death (Da Graca, 1991; Gottwald et al., 2007; Bassanezi et al., 2011). Yield loss in Persian lime was estimated at an amount of 17.3% after 15 months of the beginning of HLB; it is predictable that in the absence of effective measures to control the pathogen, tree impairment would increase annually to probably overcome loss record indicated for sweet orange (Bassanezi et al., 2011) furthermore, reach tree death. On the other hand, an endemic process of HLB requires various infection cycles to achieve an incidence of 100 % of the orchards; therefore models to estimate losses must integrate this consideration to avoid overestimating the impacts of HLB. This principle has already been applied in the study (Salcedo et al., 2010).
Conclusions
Fifteeen months after the detection of Candidatus Liberibacter asiaticus in an orchard of Persian lime (C. latifolia) in the region of Tizimin, Yucatan, Mexico, it was found that HLB had a detrimental effect on the size, thickness of skin, pH, volume of juice and weight of the fruit. In the two latter variables, the greatest reduction was found with 18.62 and 17.31 % in fruit trees with HLB in comparison with fruits from healthy trees, in a productive cycle. The weight reduction implicate a 2.4 tons/ha yield loss, which is related to the severity of HLB in the canopy tree (R2=0.8-R2=0.87), and on the bacterial concentration, which was estimated using quantitative PCR. This study constitutes the first quantitative evidence of the effect of Candidatus Liberibacter asiaticus on Persian limes, under the condition of recent invasion of Mexico.
Acknowledgements
For the scholarship and financing granted by FONSEC SAGARPA-CONACYT 2009-108591, and CONACYT; to the State Committees of Plant Health of the Yucatan Peninsula, for the logistical support provided; to Edwin Hernández Chan for his support on the field and in the lab. To the members of the GIIIC for their support in planning, and Aurelia Herrera López for measuring titratable acidity.
REFERENCES
Agustí M, Zaragoza S, Bleiholder H, Buhr L, Hack H, Klose R y Staub R, 1995. Escala BBCH para la Descripción de los Estadios Fenológicos del Desarrollo de los Agrios (Gén. Citrus). Levante Agrícola 332, 189-199. [ Links ]
Bassanezi RB, Montesino LH and Stuchi ES. 2009. Effects of Huanglongbing on Fruit Quality of Sweet Orange Cultivars in Brazil. European Journal of Plant Pathology 125:565-572. [ Links ]
Bassanezi RB, Montesino LH, Godoy GMC, Filho AB and Amorin L. 2011. Yield Loss Caused by Huanglongbing in Different Sweet Orange Cultivars in Sao Paulo, Brazil. European Journal of Plant Pathology 130:577-586. [ Links ]
Bové MJ. 2006. Huanglongbing: a Destructive, Newly-Emerging, Century-Old Disease of Citrus. Journal of Plant Pathology 88:7-37. [ Links ]
Brlansky HR, Dewdney EMM, Rogers M and Chung RK. 2009. Florida Citrus Pest Management Guide: Huanglongbing (Citrus Greening). Plant Pathology Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. USA. 3p. [ Links ]
Cristóbal AJ, Mora AG, Manzanilla LRH, Marbán MN, Sánchez GP, Cid del Prado VI and Evans K. 2006. Epidemiology and integrated control of Nacobbus aberrans on tomato in Mexico. Nematology 8:727-737. [ Links ]
Da Graca JV. 1991. Citrus greening disease. Annu. Rev. Phytopathol 29: 109-136. [ Links ]
Davies FS and Albrigo LG. 1994. Citrus. Wallingford: CAB International. 254p. [ Links ]
DGSV-CNRF. (Dirección General de Sanidad Vegetal-Centro Nacional de Referencia Fitosanitaria). 2008. Protocolo de Diagnóstico de Candidatus Liberibacter spp. mediante la Técnica Reacción en Cadena de la Polimerasa (PCR) en Tiempo Real. DGSV. 17p. [ Links ]
Esquivel-Chávez F, Valdovinos-Ponce G, Mora-Aguilera G, Gómez-Jaimes R, Velázquez-Monreal JJ, Manzanilla-Ramírez MA, Flores-Sánchez JL y López-Arroyo JI. 2012. Análisis Histológico Foliar de Cítricos Agrios y Naranja Dulce con Síntomas Ocasionados por Candidatus Liberibacter asiaticus. Agrociencia 46(8):769-782. [ Links ]
Gottwald TR, Da Graça JV and. Bassanezi RB 2007. CitrusHuanglongbing: The pathogen and its impact. Online. Plant Health Progress doi:10.1094/PHP-2007-0906-01-RV. [ Links ]
Grafton-Cardwell EE, Stelinski LL, Stansly PA. 2013. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Ann. Rev. Entomol 58:413-432. [ Links ]
Halbert SE and Manjunath KL. 2004. Asian citrus psyllid (Sternorryncha: Psyllidae) and greening disease of citrus: a literature review and assessment of risk in Florida. Florida Entomologist 87: 330-53. [ Links ]
Hall DG, Richardson ML, Ammar E-D and Halbert SE. 2013. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomol. Exp. App. 146: 207-223. [ Links ]
Helrich K. 1990. Official Methods of Analysis of the Association of Official Analytical Chemists: 2. Food composition; additives; natural contaminants. 15th Edition. AOAC: Arlington. 865-1298 pp. [ Links ]
Li W, Hartung JS and Levy L. 2006. Quantitative Real-Time PCR for Detection and Identification of Candidatus Liberibacter Species Associated with CitrusHuanglongbing. Journal of Microbiological Methods 66:104-115. [ Links ]
Li W, Li D, Twieg E, Hartung JS and Levy L. 2008. Optimized Quantification of Unculturable Candidatus Liberibacter spp. In Host Plants Using Real-Time PCR. Plant Disease 92:854-861. [ Links ]
Mora-Aguilera G, Robles-González P, López-Arroyo JI, Velázquez-Monreal JJ, Flores-Sánchez JL, Acevedo-Sánchez G, Domínguez-Monge S y González-Gómez R. 2013. Situación Actual y Perspectivas de Manejo del HLB de los Cítricos. Revista Mexicana de Fitopatología, 31(Suplemento) S6-S12. [ Links ]
Robles-González MM, Velázquez-Monreal JJ, Manzanilla-Ramírez MA, Orozco-Santos M, Medina-Urrutia V, López-Arroyo JIy Flores-Virgen R. 2013. Síntomas del Huanglongbing (HLB) en Limón Mexicano (Citrusaurantifolia) y su Dispersión en el Estado de Colima, México. Revista Chapingo Serie Horticultura 19: 15-31. [ Links ]
Salcedo BDR, Hinojosa G, Mora AG, Covarrubias GI, DePaolis F, Cíntora GC y Mora FS. 2010. Evaluación del Impacto Económico de Huanglongbing (HLB) en la Cadena Citrícola Mexicana. Instituto Interamericano de Cooperación para la Agricultura (IICA). México. 141 p. [ Links ]
Teixeira DC, Saillard CC, Couture EC, Martins NA, Wulff S, Jagoueix E, Yamamoto APT, Ayres J and Bové MJ. 2008. Distribution and Quantification of Candidatus Liberibacter americanus, Agent of Huanglongbing Disease of Citrusin São Paulo State, Brasil, in Leaves of an Affected Sweet Orange Tree as Determined by PCR. Molecular and Cellular Probes 22:139-150. [ Links ]
Trujillo AJ. 2010. Situación Actual, Regulación y Manejo del HLB en México. Memorias del 2° Taller Internacional del Huanglongbing y el Psílido Asiático de los cítricos. Mérida, Yucatan, México p. 141-149. [ Links ]
Received: June 22, 2015; Accepted: June 29, 2015











 text in
text in 


