Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.33 n.1 Texcoco 2015
Phytopatological notes
Etiology of the heart rot of pineapple ( Ananas comosus. L. Merril ) MD2 cultivar in Isla, Veracruz, México
1Recursos Genéticos y Productividad-Fruticultura. Colegio de Postgraduados Campus Montecillo. Montecillo, Texcoco, Edo. De México. México.
2Fitosanidad-Fitopatología. Colegio de Postgraduados Campus Montecillo. Km. 36.5 Carretera México-Texcoco, Montecillo Texcoco, Edo. de México C.P. 56230. México.
The heart rot of pineapple (Ananas comosus L. Merril) is a disease with a high incidence in the production areas of Veracruz, Mexico; the MD2 cultivar is the most susceptible. The objective of this study was to identify, morphologically and phylogenetically, the causative agent of the disease. Pineapple samples were collected between September 2011 and March 2012. Phytophthora was isolated from the basal leaves that came in contact with the heart that presented signs of rot. The morphological and molecular identification was done using the ITS6 and ITS4 initiators. Phytophthora nicotianae was identified in the morphological characterization. The molecular identification had a 100 % similarity with Phytophthora nicotianae. Pathogenicity tests were done on in vitro pineapple crowns in water and in vivo stems (roots) of 5 months in soil. Crown symptoms appeared on days 7 and 9 in 100% of the crowns and on days 16 to 20 in 95% of the inoculated stems. Based on the morphology, phlyogenetics and pathogenicity test are considered the first report of Phytophthora nicotianae in pineapples in Mexico.
Keywords: Heart rot; Ananas comosus ; Phytophthora nicotianae
La pudrición del cogollo de la piña (Ananas comosus L. Merril) es una enfermedad con alta incidencia en la regiones productoras de Veracruz, México, el cultivar MD2 es el más susceptible. El objetivo de este estudio fue identificar morfológica y filogenéticamente el agente causal de la enfermedad. Muestras de piña se colectaron entre septiembre de 2011 y marzo de 2012. De las hojas basales en contacto con el cogollo con pudrición se aisló a Phytophthora . Se realizó la identificación morfológica y molecular utilizando los iniciadores ITS6 e ITS4. En la caracterización morfológica se identificó a Phytophthora nicotianae. La identificación molecular tuvo 100% de similaridad con Phytophthora nicotianae. Se realizaron pruebas de patogenicidad en coronas de piña in vitro en agua y vástagos (gallos) de 5 meses in vivo en suelo. Los síntomas en corona se presentaron a los 7 y 9 días en 100 % de coronas y de 16-20 días en un 95 % de vástagos inoculados. En base a morfología, filogenia y prueba de patogenicidad se considera el primer reporte de Phytophthora nicotianae en piña en México.
Palabras clave: Pudrición del cogollo; Ananas comosus ; Phytophthora nicotianae
The pineapple (Ananas comosus L. Merril) is native to the tropical and subtropical regions of South America; it belongs to the Bromeliaceae, of the Bromeliales order and the Ananas comosus species.
The Food and Agriculture Organization (FAO) estimates that by 2014 the global production of tropical fruit will reach 82 million tons; 78 % corresponding to the "main fruits" (mango, pineapple, avocado and papaya) and 22 % to the "secondary fruits" (lychee, rambutan, guava, and passion fruit). Developing countries represent around 78 % of the total production, while the developed countries represent 80 % of the world trade (FAOSTAT, 2013).
The production of pineapple is financially relevant for the tropical and subtropical areas of the world; it is the second produced tropical fruit after the mango. The main pineapple producers are located in the Asia-Pacific (Thailand, India, Philippines, China, Vietnam and Malaysia) holding 47% of the global production. In Latin America the main producers are: Brazil and Costa Rica, ranking second and third place respectively on a global scale; Mexico ranks tenth. In 2012, the total area for pineapple cultivation was approximately 17 thousand hectares, with a total approximate production of 769 thousand tons, the main producers being the states of Veracruz, Oaxaca, Nayarit and Tabasco (FAOSTAT, 2013; SIAP, 2013).
The most cultivated crop in Mexico is the 'Smooth Cayenne' pineapple, occupying 80 % of the total cultivated surface (Rebolledo et al. 2006); in the last two decades, the main pineapple producers in the world, "Del Monte" and "Dole", released a new cultivar, the MD2 hybrid ('Gold Pineapplehoney), created in Hawaii. The MD2 cultivar produces fruit with yellower pulp, sweeter and less acidic than other cultivars. It possesses a total of 15 to 17° soluble solids, while the 'Smooth Cayenne' possesses 12 to 15°; it also has a higher vitamin A and C content, while additionally having a more cylindrical shape and not bottle-like, which facilitates its handling during its industrialization (FAOSTAT, 2013; Williams and Fleisch, 1992; Rebolledo et al., 1998; Rebolledo et al., 2011; Fold and Gough, 2008; Chan et al., 2002; Morgan and Thompson, 2000).
The main problem faced by the MD2 cultivar in the production regions of Veracruz is the heart rot of the pineapple. Coppens et al. (1997) have reported that this disease has caused severe financial losses in Australia, Hawaii, Philippines, South Africa and Thailand. Rebolledo et al. (2011) mention that it is caused by Erwinia chrysanthemi and Espinosa and Adam (1972), attribute this disease to Phytophthora nicotianae. The objective of this study is to identify the causative agent of the heart rot of the pineapple for the municipality of Isla, Veracruz, Mexico.
Materiales y métodos
Study site and sampling. The study area was located in Isla, Veracruz, Mexico at 18° 8' N y 95° 35' O, 60 m above sea level, in warm-humid weather, average temperature of 24.9 °C and average annual rainfall of 2,316 mm. From September 2011 to March 2012, 40 plants with heart rot characteristic symptoms were collected and processed in the laboratory of post-harvest fruit diseases at the Colegio de Postgraduados, Campus Montecillo, Texcoco, Estado de Mexico.
Isolation. The sick pineapple plants were washed under a stream of clean water to eliminate soil excess. 5 mm2 pieces between healthy and sick tissue were cut in basal leaves. They were disinfested through immersion in a solution of 3 % Sodium Hypochlorite for 4 min., rinsing 4 times with distilled water and dried with sterile paper towels. A total of 600 fragments were cultivated in three culture mediums: potato- dextrose- agar (PDA), nutrient agar, V8- agar (Natamycin 10 µg/L, ampicillin 292 µg/L, rifampicin 10 µg/L, pentachloronitrobenzene 0.10 g/L and hymexazol 0.25 µg/L), with a total of 150 cultivated boxes. All Petri dishes were incubated at 28 °C in white light as recommended by Jeffers and Martin, 1986 and Erwin and Ribeiro, 1996. The purification of isolated samples* was done through hyphal head, once purified they were transferred to Petri dishes with v8-agar culture mediums (acidified with lactic acid at 25 %) for their characterization.
Additionally, 20 basal leaves were cut from the rot diseased plants, they were disinfested as it was described for the basal leaves and they were placed in distilled water at 28°C in white light. After the second day, white colored mycelium growth was observed, and a segment of mycelium was transferred from each leaf in acidified vegetable juice V8 culture medium.
Preparation of the inoculum. From the Phytophthora isolates in PDA culture medium, fragments were taken and placed in distilled water for the formation of sporangiums and the release of zoospores. A suspension of zoospores was prepared with the Neubahuer camera, with a concentration of 108 zoospores ml-1 (Rodríguez et al. 2002).
Pathogenicity tests. In vitro pathogenicity tests were carried out in 60 crowns and 80 in vivo stems. Both materials were sun dried during 5 days and disinfected with 3 % sodium hypochlorite for 4 min. and were washed 4 times with distilled water. 4 lesions were inflicted in 60 crowns with a needle in the median stem of the crown, 30 of the crowns were placed in a recipient with 300 ml of the suspension of spores, 30 crowns were used as controls and were only added sterile water without zoospores. The treatment was placed under white light at 28°C until the appearance of symptoms. For the in vivo tests, 5 month old stems were used, cultivated in polyethylene bags in mixtures of soil and tezontle (3:2 v/v). In 40 stems, the soil was removed at about 8 cm around the heart of the pineapple, 4 lesions were inflicted on the median stem with the sterilized needle and 300 ml of inoculum were added along with mycelium fragments with sporangiums and was covered again with the removed soil. The same technique was applied to the 40 control samples, but only distilled water was used. Another polyethylene bag was placed on the other plants until the appearance of symptoms. For both treatments the damaged tissue was cultivated in acidified V8- agar culture.
Morphological characterization. It was characterized based on the mycelial growth, pigmentation and formation of reproduction structures of the colony growing in PDA and under perpetual white light at 28°C. The identification of the genus was done through the Erwin and Ribeiro (1996) key, and for the species with the ones described by Gallegly and Hong (2008).
Molecular Identification. The extraction of DNA was done from a 6 day growth cultivar in a V8 liquid medium at 28 °C, using the CTAB protocol (Doyle and Doyle 1990). The transcribed internal space of the ribosomal DNA was amplified using the ITS6 and ITS4 initiators (White et al. 1990). The sequences obtained were compared with the database in the National Center for Biotechnology Information NCBI (2012), USA (http://www.ncbi.nlm.nih.gov).
Results
Isolation of microorganisms. It was observed among the different mediums that in the V8 agar with antibiotic culture medium, Phytophthora sp. was isolated with more frequency, and less frequently in the nutrient and PDA agars. In the nutrient and PDA agars, Pythium was isolated with more frequency, the percentages are shown in Table 1.
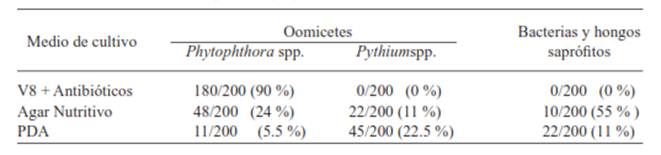
Table 1 Percentage of isolated microorganisms in pineapple plants (Ananascomosus L. Merril) MD2 cultivar with signs and symptoms of heart rot. Isla, Veracruz, Summer, 2011.
From the 20 leaves placed in flasks with distilled water, the growth of mycelium was observed after 2 days (Figure 1), and the fragments cultivated in PDA and acidified V8- agar developed colonies characteristic of Phytophthora sp.
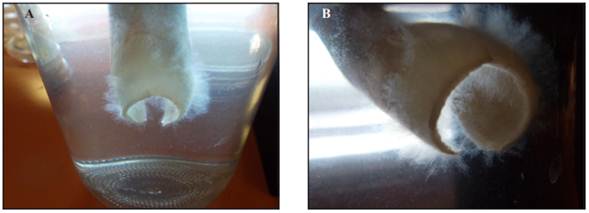
Figure 1 A) and B) Phytophthora sp. Mycelium grown in distilled water for two days, in basal leaves collected from the field.
Pathogenicity tests. In the in vitro tests, the symptoms presented a pale green color and necrosis on the tips of the leaves within 7 to 9 days. Furthermore, rotten middle leaves presented a light brown color in the middle of the rosette, and Phytophthora mycelium could also be observed (Figure 2). 100 % of the inoculated crowns got the disease. The control samples did not present any symptoms or signs and developed an abundant radical system. From the necrotic tissue, isolation was once again performed in acidified V8- Agar wherein Phytophthora nicotianae was isolated. For the in vivo tests, the symptoms and signs took 16 days to manifest, starting with a change in the color of the leaves to a lighter green, the tips of which were necrotic around the heart of the pineapple. Within 20 days, heart rot was evident, the center of the rosette was brown and mycelium was present, the base of the middle leaves had a putrid smell; 38 (95%) of the 40 inoculated plants showed symptoms of the sickness. The control samples did not present symptoms or signs of the disease. From the necrotic leaves isolations were performed in acidified V8- agar culture medium, where the presence of Phytophthora nicotianae was corroborated.

Figure 2 Síntomas y signos de la pudrición del cogollo de la piña. A) Pruebas in vitro de la pudrición del cogollo de la piña. B) Signos y síntomas en coronas inoculadas. C) Pudrición del cogollo. D) Hoja de corona con pudrición. E) Corona de piña 9 ddi. F) Signos del oomiceto en plantas inoculadas. G) Signos y síntomas en plantas inoculadas en invernadero. H) Cogollo podrido de la planta de piña. I) Cogollo y raíz de planta testigo de prueba in vivo. J) Cogollo y raíz de planta inoculada. K) Planta de piña 16 ddi.
Morphological characterization. The growth of mycelial hyaline was carried out in a V8- agar culture medium, non-defunct, spherical and/or limoniform sporangiums with a prominent papilla, the average size of 100 sporangiums was 44.6 x 36.8 µm; spherical, non-papillate chlamydospores were present, terminal and intercalated hyalines of 30.2 µm (average of 100 chlamydospores) (Figure 3). The formation of sporangiums in distilled water appeared within 48 hrs. under perpetual white light. The characteristics coincide with those reported by Erwin and Ribeiro (1996) and Gallegly and Hong (2008) for Phytophthora nicotianae.
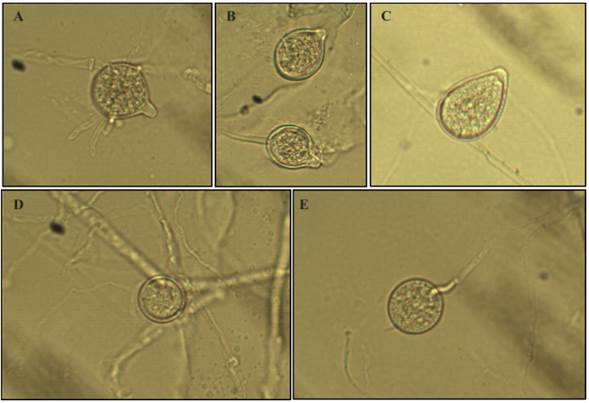
Figure 3 Asexual structures of Phytophthoranicotianae. A) Spherical Sporangium. B) Limoniform Sporangium. C) Ovoid Sporangium. D) Interspersed Chlamydospore. E) Terminal Chlamydospore.
Molecular identification. The product of amplification that was obtained with the ITS4 and ITS6 initiators was of 850 bp (Fig. 3). The comparison of the nucleotide sequence of the isolate showed 100% similarity with the Phytophthora nicotianae sequence.
Discussion
The in vivo and in vitro heart rot results similar to those observed in the cultivation fields of Isla, Veracruz, that start with aqueous lesions at the base of the leaves (tissue without chlorophyll) and the heart of the fruit, the leaves turn a lighter green, the tips become necrotic and develop a characteristic putrid smell, all caused by Phytophthora nicotianae, coinciding with the description by Espinosa and Adam (1972) in Mexico and by Joy and Sindhu (2012) in India. These symptoms are probably present due to the physical obstruction of the vascular system caused by the growth of hyphae and the penetration of other secondary organisms (fungi and bacteria), increasing the formation of gums that originate due to oxidation and the accumulation of cellular degradation residues, as happens with ornamental plants (Arévalo-Galarza, 2012; Agrios, 2005). Subsequent to inoculation and the formation of zoospores, these are attracted to the elongation zone and differentiation of the root, where they form cysts, and even invade the secondary roots and not the tips (Galiana et al., 2005; Attard et al., 2010). Most cases of heart rot in pineapples in the sampling areas were observed during the rainy season, with temperatures that oscillated between 24-26 °C and a relative humidity of 90-100 %; the high relative humidity induces the development of the diseases caused by Phytophthora sp. in pineapple plantations (Duniway, 1983).
Additionally, when cultivating in slope areas, cultural labors (fertilization, weed control, soil removal, etc.) and lesions caused by other organisms (nematodes and rodents) to the radical system, facilitate the dispersion and the infection risk by Phytophthora sp. as mentioned by Erwin and Ribeiro, 1996, Elliot, 1989, Jung and Blaschke, 2004 and; Galiana et al., 2005.
REFERENCES
Agrios, G. N. 2005. Plant Pathology. 5a Ed. Elsevier Academic Press. New York. 922 p. [ Links ]
Arévalo-Galarza, L., García-Osorio, C. y Rosas-Saíto, G.H. 2012. Factores que afectan la vida de florero en flores de corte. Agroproductividad 5 (3):28-35. [ Links ]
Attard, A., Gourgues, M., Callemeyn-Torre N., and Keller, H. 2010. The immediate activation of defense responses in Arabidopsis roots is not sufficient to prevent Phytophthora parasitica infection. New Phytologist 187 (2): 449-460 [ Links ]
Chan, Y. K., Coppens, d'Eeckenbrugge G, Sanewski, G. M. 2002. Breeding and variety improvement. P. 33-35 In: Bartholomew, D.P., Paull,R.E., Rohrbach.K.G. (eds.) The pineapple, botany, production and uses. CABI Publishing, New York. [ Links ]
Coppens, G., Leal, F., Duval, M. F. Germplasm resources of pineapple. 1997. Hort.Rev., 21:133-175. [ Links ]
Doyle, J. J. and Doyle J. L. 1990. A rapid total DNA preparation procedure for fresh plant tissue. Focus 12:13-15. [ Links ]
Duniway, J. M. 1983. Role of physical factors in the development of Phytophthora diseases. p. 175-187 In: Phytophthora: Its Biology, Taxonomy, Ecology and Pathology. D. C. Erwin, S. Bartnicki-Garcia, and P. H. Tsao (eds.) American Phytopathological Society, St. Paul, MN. [ Links ]
Elliott, C. G. 1989. Some aspects or nitrogen nutrition and reproduction in Phytophthora. Mycol. Res. 92 (1): 34-44 [ Links ]
Erwin, D and Ribeiro, O. 1996. Phytophthora diseases worldwide. American Phytopathol. Soc., MN., p. 9-14 [ Links ]
Espinosa, R. G. and Adam, A. V. 1972. Major diseases of pineaple in Oaxaca, México, and their control.FAO Plant Protect. Bull. 20 (4): 79-87 [ Links ]
FAOSTAT, 2013. Food and Agriculture Organization of the United Nations. Consultado Enero 2013. http://faostat.fao.org [ Links ]
Fold N. and Gough K. V. 2008. From smallholders to transnationals: The impact of changing consumer preferences in the EU on Ghana's pineapple sector. Geoforum. 39 (5): 1687-1697 [ Links ]
Galiana, E., Rivière, M.-P., Pagnotta, S., Baudouin, E., Panabières, F., Gounon, P., and Boudier, L. 2005. Plant-induced cell death in the oomycete pathogen Phytophthora parasitica. Cellular Microbiol. 7 (9), 1365-1378 [ Links ]
Gallegly, E. M. and Hong, C. 2008. Identifying species by morphology and DNA fingerprints. The American Phytopathol. Soc. St. Paul MN. U.S.A. 108 p. [ Links ]
Hassan, A., Othman, Z., and Siriphanich, J. 2011. Pineapple (Ananas comosus L. Merr.) p. 194-212 In: Postharvest biology and technology of tropical and subtropical fruits. Vol. 4 Mangosteen to white sopote. Elhadi M. Yahia (ed.) Woodhead Publishing.UK. [ Links ]
Jeffers, S. N. and Martin, S. B. 1986. Comparison of two media selective for Phytophthora and Pythium species. Plant Dis. 70:1038-1043. [ Links ]
Joy P. P. and Sindhu G. 2012. Diseases of pineapple (Ananas comosus): Pathogen, symptoms, infection, spread & management. Consultado Agosto 2013. http://www.kau.edu/prsvkm/Docs/DiseasesofPineapple.pdf [ Links ]
Jung, T. and Blaschke, M. 2004. Phytophthora root and collar of alders in Bavaria: distribution, modes of spread and possible management strategies. Plan Pathol. 53: 193-208 [ Links ]
Kaneshiro, W. S., Burger, M., Vine, B., de Silva, A. S. and Alvarez, M. 2008. Characterization of Erwinia chrysanthemi from a bacterial heart rot of pineapple outbreak in Hawaii. 2008., Plant Dis. 92 (10): 1444-1450. [ Links ]
Mircetich S.M., and Browne G.T. 1987. Phytophthora root and crown rot of deciduous fruit tres: Progress and problems in etiology, epidemiology and control. Commemorative Symp. 111 p. [ Links ]
Morgan, T. and Thompson, T. 2000. Del Monte mixes and matches Costa Rican products. Americafruit 3: 45-47 [ Links ]
NCBI. 2012. National Center for Biotechnology Information. Gen Bank. Consultado Julio 2012. http://www.ncbi.nlm.nih.gov/ [ Links ]
Ochse, J. J., Soule, M. J., JR., Dijkman, M. J., y Wehlburg, C.. 1965. Cultivo y mejoramiento de plantas tropicales y subtropicales. Editorial Limusa - Willey S. A., México, D. F. pp. 639-643 [ Links ]
Peckham G.D., Kaneshiro, W.S., Luu V., , Berestecky J.M. and Alvarez A.M.. 2010. Specificity of monoclonal antibodies to strains of dickeya sp. that cause bacterial heart rot of pineapple. Hybridoma 29(5): 383-389. [ Links ]
Py, C. and Tisseau, M.. 1969. La piña tropical. Ed. Blumé. Barcelona, España. 278 p [ Links ]
Rebolledo, M. A., Del Ángel P. A.L., Rebolledo, M. L. y Becerril, R. A. E., Uriza, A. D. 2006. Rendimiento y calidad de fruto de cultivares de piña en densidades de plantación. Rev. Fitotec. Mex. Vol. 29 (1): 55-62 [ Links ]
Rebolledo, M. A., Uriza, A.D.E., Pérez, A. del A., Rebolledo, L. M. y Zetina, L. R.. 2011. La piña y su cultivo en México: Cayena Lisa y MD2. Campo Experimental Cotaxtla, Veracruz, México. Libro Técnico No. 27 306 p. [ Links ]
Rebolledo, M. L., Uriza, A.D.E., Rodríguez, J. G. y Rebolledo, M. A. 1998. Tecnología para la producción de piña en México. Libro técnico No. 20. SAGARPA. INIFAP. CIRGOC. Campo Exptl. Papaloapan. Veracruz, México 159 p. [ Links ]
Rodríguez, Y., Mosqueda, M., Companioni, B., Arzola, M., Borras, O., Perez, M. C., Lorenzo, J. C., and Santos, R. 2002. Bioassay for in vitro differentiation of pineapple cultivar resistence levels to heart rot disease. In vitro Cell. Dev. Boil.-Plant 38:613-616. [ Links ]
SIAP, 2013. Servicio de Información Agroalimentario y Pesquero. Consultado Enero 2013. http://www.siap.gob.mx [ Links ]
White, T. J., Lee, B. S. and Taylor, J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. p. 315 - 322. In: Innis, M. A., Gelfand, D. A., Sninsky, J. J., White, T. J. (eds.). PCR Protocols: A Guide to Methods an Applications, Academic Press, CA, U.S.A. [ Links ]
Williams, D. D. F., and Fleisch H. 1992. Historical review of pineapple breeding in Hawaii. Acta Horticulturae. 334: 67-76. [ Links ]
Received: July 19, 2014; Accepted: December 28, 2014











 text in
text in 


