Triatoma dimidiata (Latreille, 1811) is one of the main Chagas disease vectors in Mexico.1 Its control is difficult due to its mobility, flight range, and migration from wild habitats to human dwellings.2 In Mexico, the control of Chagas disease is based on the elimination of vectors through environment cleaning and insecticide applications indoors and outdoors.3 Constant exposure of Chagas vectors to insecticides and their residues can lead to development of insecticide resistance.4,5 Insecticide susceptibility studies should be undertaken in every state to elect the optimum insecticide for any vector control.3 Compared to traditional World Health Organization (WHO) bioassays for insecticide resistance determination, biochemical assays allow the detection of relatively lower frequencies of resistant individuals, so the use of both methods is encouraged,6 but they have not been currently used for T. dimidiata. Methods used for other triatomines are diverse, and one or two enzyme determination are sporadically used.7,8,9,10,11,12,13
We present results of lethal concentrations (LC50 and LC99) of three insecticides in T. dimidiata, as well as insecticide insensitive acethylcholinesterase (iAChE), and the levels of cytochrome P450, esterases and glutathione S-transferases (GSTs) of first instar nymphs of laboratory specimens. Our results indicate the feasibility of adapting routine WHO insecticide susceptibility tests using impregnated papers, and WHO biochemical assays recommended for other insects to monitor the insecticide susceptibility and resistance mechanisms of T. dimidiata.
Materials and methods
Triatoma dimidiata individuals were collected in Manacal (15° 06’14” N, 92° 12’ 53” W, 1 073 m) village, Chiapas, Mexico in 2006, with sporadical additions up to 2009. Insects were maintained in plastic containers at 27°C, 65% relative humidity and 12:12 h light-dark period in an insectary, and fed every week on rabbits (under accepted procedures by the Ethical Commission of Instituto Nacional de Salud Pública [INSP]). Individuals reared in the insectary during 7-8 generations were used in all assays from September 2012 to September 2014.
Insecticide susceptibility bioassays were conducted following WHO prescribed methods, with some modifications.14 For each insecticide a biological window of concentrations producing between 10 and 90% mortality was opened. Filter papers (Watman #1) were impregnated with three insecticides at six concentrations each: malathion (95.03% SNMA100401 Químix, Mexico) 18.56, 27.84, 37.12, 51.97, 59.30 and 74.20 µg/cm²; propoxur (97% Químix, Mexico) 1.56, 3.12, 3.67, 4.60, 7.65 and 10.62 µg/cm²; and deltamethrin (99.3% PMDN001274 Bayer, Mexico) 1.5, 3.12, 4.6, 6.25, 7.0 and 7.5 µg/cm². Olive oil (Sigma-Aldrich, Mexico, 0-1500) plus acetone (analytical grade JT Baker 9006-02) were used as solvents for malathion and propoxur, silicone oil (CTR 36OS101110) plus acetone for deltamethrin. For controls, papers were impregnated only with solvents for each bioassay.
Coprophagy was allowed to occur in first stage T. dimidiata nymphs.15 Five to seven day-old first stage unfed T. dimidiata nymphs were used to determine the LC50 and LC99 of the different insecticides and concentrations. For each insecticide concentration, ten nymphs were exposed to impregnated papers in Petri dishes covered with perforated plastic lids, at 27 °C and 65% relative humidity. Nymphs’ activity was observed every 10 min during 1 h of exposition. Posteriorly nymphs were moved onto clean containers covered with nylon netting.Malathion and propoxur bioassays mortalities were recorded at 48 h, and deltamethrin at 72 h.14 Nymphs were considered dead when they did not respond to mechanical stimulation. LC50 and LC99 were calculated using the EPA probit analysis program version 1.5.*
Frozen (-20ºC) first instar nymphs 5-7 days-old were individually placed in each well of a 96-well microplate (Corning Costar brand, México 3590). Microplates were kept always on a container with crushed ice to prevent degradation of enzyme activity. Each nymph was manually homogenized with a plastic pestle in a total of 200 µL of tri-distilled water and subjected to iAChE, cytochrome P450, esterases, GSTs and protein biochemical assays.16,17,18 For AChE assays, duplicates of 25 μl of each homogenate were transferred to flat bottom microplates. Microplates with the remaining homogenates were centrifuged (Thermo IEC Centra CL3R, USA at 4000 rpm during 25 min at 4 °C). Aliquots of 20 μl of centrifuged homogenates were placed in new microplates for cytochrome P450, and α-and β-esterases assays. For glutathione-S-transferases (GSTs), ρ-NPA esterases and protein assays, 10 μl of each supernatant were placed in three different new microplates, in duplicates. Tri-distilled water, instead of homogenate, was added in two wells as blanks in each plate. Three replicate microplates were assayed for each biochemical test with 47 nymph homogenates each assessed in duplicates. The total of nymphs were 141.
AChE assay: 145 μl of 1% Triton 0.1 M phosphate buffer pH 7.8 and 10 μl of 0.01 M dithiobis 2-nitrobenzoic acid solution in 0.1 M phosphate buffer pH 7.0 were added to each homogenate replicate. Twenty-five μl of the substrate acetylthiocholine iodide (0.01 M ASCHI) were added to one replicate to initiate the reaction, and 25 μl of ASCHI containing 0.2% of propoxur (0.1 M) as inhibitor were added to the second replicate. The enzyme reaction was monitored continuously at 405 nm for 5 min in a Multiskan Spectrum microplate reader (Thermo Labsystems, USA). The percentages of propoxur inhibition of AChE activity in the test compared to the inhibited well were calculated. Individuals without an iAChE had >60% inhibition of activity.12
Cytochrome P 450 assay: 80 μl of potassium phosphate buffer (0.0625 M, pH 7.2) were added to each well, followed by 200 μl of a tetramethylbenzidine solution (TMB 0.01g in 5 ml methanol mixed with 15 ml of 0.25 M sodium acetate buffer, pH 5.0), and 25 μl of 3% H2O2 as substrate. The reaction was incubated for 1 h at room temperature and readings were done at 650 nm, optical densities were compared against a standard curve obtained from known concentrations of rabbit cytochrome P450 2B4 (Sigma, México). Results were reported in pmol of cytochrome P450/mg protein.
Esterase assays: 200 μl of 0.3 mM α-naphthyl sodium acetate in 0.02 M Na2HPO4 (pH 7.2), and 200 μl of 0.3 mM β-naphtyl acetate in 0.0 2M Na2HPO4 (pH 7.2) were added to alternative well rows of the microplate containing the homogenates. After 30 min at room temperature 50 μl of a fast-blue solution (23 mg fast-blue + 2.25 ml tri distilled H20 + 5.25 ml 5% SDS) were added to stop the reaction, and enzyme activity readings undertaken at 570 nm. Optical densities were compared against a standard curve obtained from known concentrations of α- and β-naphthol acetate (Sigma), and results reported in nmol of α- or β- naphthol acetate produced/min/mg protein. For the ρ-NPA-esterase assay, 200 μl of substrate ρ-NPA (20 ml phosphate buffer 50 mM Na2HPO4, pH 7.4 + 100 μl of ρ-nitrophenyl acetate 100 mM 0.1 M in a ratio of 1:100) was added to each well. Enzyme kinetics activity was measured at 405 nm during 2 min. Esterase activity per individual was calculated using the extinction coefficient 6.53 mM-1 cm-1 corrected for the path length of the solution in the microplate well and reported as µmol of the formed product/min/mg protein.
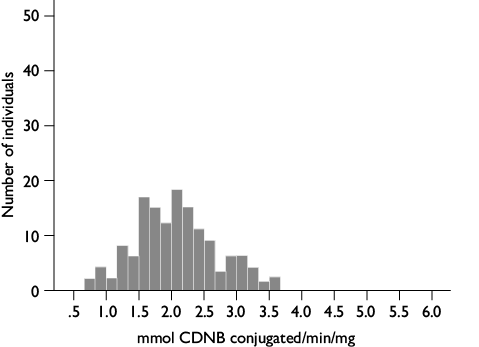
Gluthatione S-transferase assays: 200 μl of the GSH / CDNB solution (65 mg GSH in 20 ml of 0.1 M Na2HPO4 buffer pH 6.5 + 13 mg CDNB in 1 ml of methanol) was added to each well, and a kinetic measured at 340 nm for 5 min. The GST activity per individual was calculated using the extinction coefficient (9.6 mM-1 cm-1),19 corrected for the path length of the solution in the microplate well and reported as mmol of conjugated CDNB/min/mg protein.
Protein determinations.20 The reaction was run according to BioRad manual in a total amount of 300 μl of BioRad solution/well. The reaction stood at room temperature during 5 min and read at 570 nm on a fixed point. Protein values were calculated in mg/ml for individual insects from a standard curve of known concentrations of bovine serum albumin.
SPSS version 21.0* software was used to plot enzyme activity in histograms, and to calculate means and standard deviations.
Results
Insecticide susceptibility bioassays
During one hour of insecticide exposure, nymphs were active at any concentration during the first 40 and 20 min with malathion and propoxur respectively; after that, activity was poor or not observed. However at any deltamethrin concentration, nymphs were highly active during the whole exposition hour. Control nymphs calmly walked or rested. After 48 h incubation, LC50 and LC99 was 43.83 and 114.38 µg/cm2 for malathion, and 4.71 and 19.29 µg/cm2 for propoxur, respectively. After 72 h incubation, LC50 and LC99 was 5.80 and 40.46 µg/cm2 for deltamethrin, respectively (table I). Chi2 for the three insecticides showed that observed values were not different than expected (p=0.05).
Table I: Estimated LC PROBIT for laboratory 1st stage 5-7 days-old nymphs of Triatoma dimidiata following exposure for 1 h to malathion, propoxur and deltamethrin, with mortalities recorded within 48, 48 and 72 h of incubation, respectively
| Insecticide | n | slope ± SE | Point LC | µg/cm² | 95% confidence limits Lower - Upper | Calculated Chi² | Table Chi² (P=0.05) | |||||||
| Malathion | 215 | 5.58 ± 0.90 | 1.00 | 16.80 | 10.03 - 22.12 | 3.18 | 9.49 | |||||||
| 10.00 | 25.84 | 18.66 - 30.96 | ||||||||||||
| 50.00 | 43.83 | 38.47 - 48.63 | ||||||||||||
| 90.00 | 74.35 | 64.94 - 93.30 | ||||||||||||
| 99.00 | 114.38 | 91.61 - 172.33 | ||||||||||||
| Propoxur | 220 | 3.80 ± 0.59 | 1.00 | 1.15 | 0.58 - 1.67 | 3.21 | 9.49 | |||||||
| 10.00 | 2.17 | 1.43 - 2.75 | ||||||||||||
| 50.00 | 4.71 | 4.00 - 5.45 | ||||||||||||
| 90.00 | 10.24 | 8.32 - 14.58 | ||||||||||||
| 99.00 | 19.29 | 13.77 - 35.61 | ||||||||||||
| Deltamethrin | 186 | 2.75 ± 0.51 | 1.00 | 0.83 | 0.30 - 1.37 | 5.19 | 9.49 | |||||||
| 10.00 | 1.99 | 1.14 - 2.64 | ||||||||||||
| 50.00 | 5.80 | 4.93 - 7.14 | ||||||||||||
| 90.00 | 16.92 | 11.91 - 34.46 | ||||||||||||
| 99.00 | 40.46 | 22.89 - 132.84 |
Biochemical assays
AChE assay. Inhibition percentages of AChE activity by propoxur ranged from 0 to 100%. A frequency of the iAChE allele of 0.30 was calculated based on the number of susceptible individuals identified in the population (figure 1).
Cytochrome P450 assays. A mean value of 0.0007 ± 1.4x10-4 with ranges between 0.0001 and 0.0049 pmol of cytochrome P450/mg protein was obtained (figure 2). Only 13% of individuals had higher enzyme content compared with the rest of the population.
Esterases assays. Mean values of 0.00020 ± 5.2 x 10-6 (ranges: 0.00005-0.00050) nmol α-naphthol acetate /min /mg protein, and 0.00120 ± 4 x 10-5 (ranges: 0.00025-0.00200) nmol β-naphthol acetate/min/mg protein (figures 3 and 4) were obtained. β-esterases showed higher activities than α-esterases. Only 3% of individuals overexpressed β-esterases compared with the rest of the population. Esterases determinations using ρ-NPA as substrate had a mean activity of 1.11 ± 2.3x10-2 µmols of the formed product/min /mg protein (figure 5). Activity values in the analyzed nymphs were distributed in a normal pattern with a range of 0.25 to 2.2 µmols of the formed product/min/mg protein.
Glutathione S-transferase assays. The GST activity values followed a normal distribution with a range of 0.70 to 3.60 and an overall mean value of 2.06 ± 0.05 mmol of conjugated CDNB/min/mg protein (figure 6).
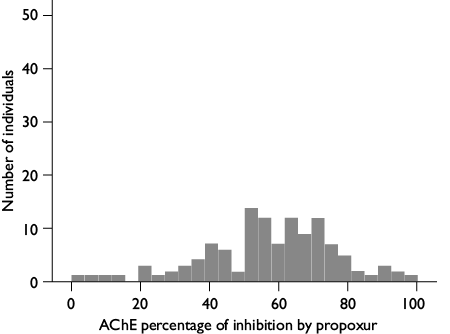
Figure 1. Ranges of acetylcholinesterase inhibited by propoxur in nymphs of the 1st stage of Triatoma dimidiata 3-5 days old, originally collected in Manacal, Chiapas, Mexico from 2006-2009 and maintained for 7-8 generations in the laboratory
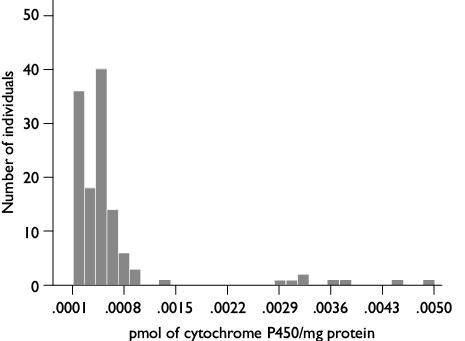
Figure 2. Ranges of cytochrome P450 content in nymphs of the 1st stage of Triatoma dimidiata 3-5 days old, originally collected in Manacal, Chiapas, Mexico from 2006-2009 and maintained for 7-8 generations in the laboratory

Figure 3. Ranges of α-esterase activity in nymphs of the 1st stage of Triatoma dimidiata 3-5 days old, originally collected in Manacal, Chiapas, Mexico from 2006-2009 and maintained for 7-8 generations in the laboratory

Figure 4. Ranges of β-esterase activity in nymphs of the 1st stage of Triatoma dimidiata 3-5 days old, originally collected in Manacal, Chiapas, Mexico from 2006-2009 and maintained for 7-8 generations in the laboratory
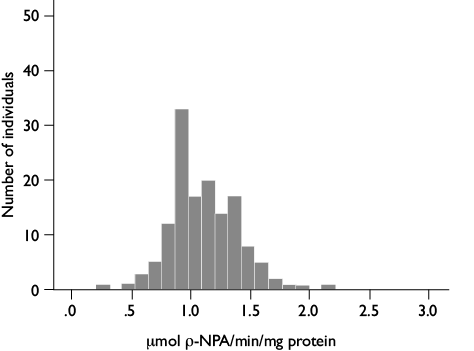
Figure 5. Ranges of ρ-NPA-esterase activity in nymphs of the 1st stage of Triatoma dimidiata 3-5 days old, originally collected in Manacal, Chiapas, Mexico from 2006-2009 and maintained for 7-8 generations in the laboratory
Discussion
Resistance to most used chemicals has been documented in the most important triatomine vectors in Latin America.9,10,11,12,13,21,22,23,24 However, the overall assessment and comparisons are difficult because of the diversity and lack of standardization of the used methods,5 the lack of reference strains for each species, the shortage of single insect stages that could be collected in the field, and the length of their life cycle, which precludes the use of adults and late nymph stages.
Insecticide susceptibility tests using topical application for lethal doses (LD) determination are difficult in early nymph stages, thus exposure by tarsal contact for LC determination could be advantageous, but improvements in the time of exposure and follow up are still required, since the method we followed was recommended for older nymphs.14 These results indicate the feasibility of standardizing WHO tests for LC and enzyme determination using insecticide impregnated papers and biochemical assays routinely applied in other insects.14,16,24,25
The LC50 and LC99 of malathion, propoxur and deltamethrin obtained for first stage nymphs cannot be interpreted to define susceptibility levels, as there is not yet T. dimidiata reference strain for comparison. Previous reports of resistance to pyrethroids in some species of Triatoma, comparing field results versus those of a susceptible reference strain7,9,23,24,26 took into account a discriminating dose of 5 ng a.i./insect for deltamethrin; or used a final dose ranged from 0.02 to 6 ng of deltamethrin to calculate LD50; or used four different concentrations of pyrethroids to estimate each EC50 (half maximal effective concentration) value; or used four levels of dose recording 10 to 90% mortality. LC50 and LC99 for deltamethrin in Manacal T. dimidiata strain fell between 5.80 and 40.46 µg/cm2, respectively (table I), while LD50 and LD99 of 0.44 and 2.22 ng/insect respectively, was reported for field 1st nymph T. dimidiata.23
In previous tests using insecticide-impregnated papers, bugs were exposed during 24 h, and survival registered 24 h later.7 One hour exposure represents with more fidelity the field conditions, as insects move away shortly after detecting the insecticide, as was observed in our bioassays. Our results, with only one-hour exposure indicated its suitability for future resistance diagnosis following WHO recommendations.25
There is not a standard method for enzyme activity determinations in triatomines. Triatomine stages and enzyme substrates varied in previous insecticide metabolic enzymes evaluations.7,9,10,11,12,13 Thus, extreme caution is required when comparisons are attempted. The concentration of propoxur should inhibit at least 70% of the total AChE activity in the susceptible insect,16 near a third of the Manacal T. dimidiata strain had its AChE less sensible to propoxur than the rest of the population. Whether a loss of resistance took place in this strain, which was maintained under laboratory conditions during five years without insecticide pressure, cannot be determined, since this evaluation was not estimated at the time of field collection. Other studies to corroborate different allele frequencies based on the identification of mutations27 are required, since no previous studies to identify mutations in the Ace gene involved in insecticide resistance exist for T. dimidiata.
Biochemical assays for metabolism-based resistance mechanisms detection are useful for the management of insecticide resistance in insects,17 as they detect the overexpression of enzymes that metabolize insecticides. In Manacal T. dimidiata strain, β-esterase enzymes could be identified as overexpressed, because were higher than those of α-esterases, contrary to that of Aedes aegypti resistant to pyrethroids,28 and An. albimanus,17 but similar to that of T. infestans and malathion-tolerant R. prolixus.7 The esterase gene expression is indicative of the main pathway to metabolize insecticides; our results warrant direct studies of organophosphorus and pyrethroids esterase metabolism in the Manacal strain, in order to elucidate the differential participation of α-and β-esterases.
Glutathione S-transferase activity mean of 5 to 7 days-old first instar Manacal T. dimidiata strain was about one hundred times higher than that reported for 12-14 days-old adults of T. infestans8 and more than a million times that of the reported for first instar T. infestans.12 These differences could be attributed to the basal expression between the species, and some of that to the different insecticide pressure received in the field. Increased levels of GSTs have been found in other insects resistant to DDT, organophosphorus and pyrethroids.29,30,31,32 Maintaining high expression levels of GSTs has no important fitness cost,32 which may explain our findings in the Manacal strain maintained without contact with insecticides during five years (2009-2014). Although loss of resistance during five captivity years cannot be determined as explained above, metabolic tests are necessary to identify which insecticides are being metabolized by these enzymes. Such results could help us to elucidate if LC obtained represent resistance.
The use of F1 first stage nymphs for field samples in the susceptibility tests is recommended,14 since it provides the benefit of excluding previous exposure to insecticides, and a handy size of the specimens and the possibility to conduct the required replicates at a considerable short period. However, for the enzyme activity assays, it is important to define a shorter age range for its evaluation even when working with a single stage. Age-dependent responses to insecticides and detoxifying enzyme activity have been reported for larvae and adult stages of other species.33,34 Additionally, it is recommended that insects should be tested at about the same time of the day, since gene expression response to the insecticides differs along the day.35,36
Although a Susceptibility Reference Lineage (SRL) strain should have more than five generations in the laboratory without insecticide contact, and no inclusion of external material,37 the Manacal strain should be considered a strain in process of regression to the susceptibility, since biochemical tests still revealed individuals with cytochromes P450 and β esterases out of the normal distribution of a population, as well as a wide range of individuals with variable AChE activity inhibited by propoxur. Such individuals may be slightly increasing the LC levels in the bioassays, guiding us to think that in this population could be present resistance to organophosphates and carbamates.16 Pessoa and colleagues (2015)5 found lower resistance levels than the SRL in diverse field triatomine population which, along with our results with the Manacal strain, support the proposal that the concept of a SRL should be revised.
Insecticide susceptibility and characterization of the potential resistance mechanisms are strongly recommended in vector control interventions. Using WHO methods with impregnated papers and the biochemical assays standardized for other insects, insecticide susceptibility in T. dimidiata could be compared in different geographic sites to monitor the efficacy and pertinence of insecticides for its control. Standardized methodologies would make comparable triatomine resistance studies by other scientists and vector control programs.
This is the first study aimed at introducing monitoring insecticide resistance in T. dimidiata using the routine WHO methodology for LC determination with impregnated papers, including enzyme activity. Our results demonstrated the viability of the methodology in this species and feasibility of using the same tools already in use for monitoring other insects’ insecticide resistance for T. dimidiata control.











 text new page (beta)
text new page (beta)