Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Salud Pública de México
Print version ISSN 0036-3634
Salud pública Méx vol.55 n.3 Cuernavaca May./Jun. 2013
ENSAYO
Development and implementation of guidelines for quality assurance in breast cancer screening: The European experience
Desarrollo e implementación de guías para el aseguramiento de la calidad en el tamizaje de cáncer de mama: la experiencia europea.
Lawrence von Karsa, MDI; Silvina Arrossi, PhD.II
IQuality Assurance Group, Early Detection and Prevention Section, International Agency for Research on Cancer. Lyon, France
IIConsejo Nacional de Investigaciones Científicas y Técnicas, Centro de Estudios de Estado y Sociedad. Buenos Aires, Argentina
ABSTRACT
In Europe, as in many other regions of the world, breast cancer is a major cause of suffering and death. Early detection of breast cancer by systematic mammography screening can find lesions for which treatment is more effective and generally more favourable for quality of life. Comprehensive quality assurance guidelines for breast cancer screening based on mammography have been developed in the Europe Against Cancer programme with the aim of maximising screening benefits while minimising adverse effects, such as unnecessary examination or treatment resulting from false-positive screening tests. The present report provides an overview of the European experience in developing and implementing quality assurance guidelines for breast cancer screening. It highlights implications relevant to those regions of the world in which the burden of breast cancer in the coming years will make population-based screening an option for cancer control.
Keywords: systematic mammography screening; mammography; breast cancer; quality assurance
RESUMEN
En Europa, como en muchas otras regiones del mundo, el cáncer de mama es una causa importante de sufrimiento y muerte. La detección temprana del cáncer de mama a través de un programa de tamizaje organizado mediante la mamografía sirve para encontrar lesiones cuyo tratamiento es más efectivo y generalmente más favorable para la calidad de vida. En el programa Europa contra el Cáncer se han desarrollado guías integrales de garantía de calidad para el tamizaje del cáncer de mama basado en la mamografía, con el fin de maximizar los beneficios del tamizaje y minimizar sus efectos adversos, que pueden resultar en resultados falsos positivos. El presente trabajo ofrece una visión general de la experiencia europea en el desarrollo y aplicación de las guías de control de calidad para el tamizaje del cáncer de mama. En él se destacan las implicaciones relevantes para las regiones del mundo en las que la incidencia del cáncer de mama en los próximos años hará del tamizaje poblacional una opción para el control del cáncer.
Palabras clave: programa de tamizaje organizado; mamografía; cáncer de mama; control de calidad
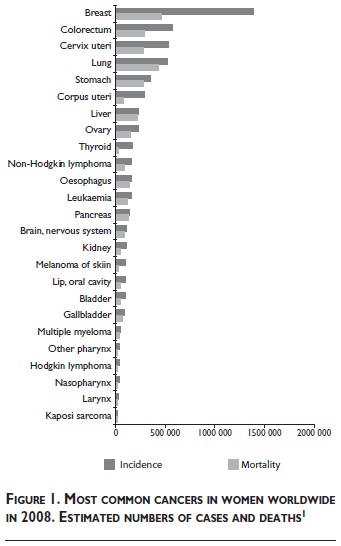
According to estimates based on the Globocan database,1 cancer of the breast is the most common cancer, and the most common cause of death from cancer in women worldwide (figure 1). Demographic trends indicate a continuing increase in this substantial public health problem, particularly in the world's less developed regions.1 Worldwide 1.38 million new cases (23% of all cancers in women) and 458 000 deaths due to breast cancer (12.7% of all cancer deaths in women) were estimated in 2008. The estimated incidence and mortality in Europe in 2008 was 424 755 cases and 128 770 deaths.
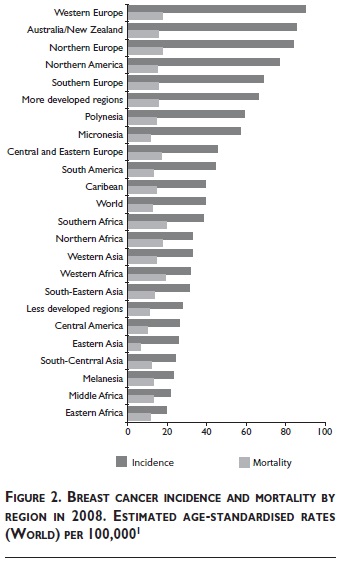
Figure 2 shows the range of estimated age -standardised incidence and mortality rates by region of the World in 2008. Western Europe and Eastern Africa are the regions with the highest and lowest incidence (89.9 and 19.3 cases per 100 000 women, respectively). South America and the Caribbean are in an intermediate range (44.3 and 39.1 cases per 100 000). Central America and most regions of Asia and Africa are in the moderate to low range of incidence (<40 cases per 100 000). All too frequently, however, regions with moderate to low incidence are saddled with mortality rates similar to or exceeding that of Western Europe (17.5 deaths per 100 000 women). This applies, for example to: South-ern, Western and Northern Africa (19.3, 18.9 and 17.8 deaths per 100 000, respectively). The age-standardised mortality rates in South America and the Caribbean are somewhat lower (13.2 and 14.2 deaths per 100 000). Common to all of these regions is a much less favorable relationship between incidence and mortality (approximately 2:1 in the above regions in Africa, and 3:1 in South America and the Caribbean) compared to 5:1 in Western Europe. This reflects substantially higher survival of breast cancer patients in Western Europe due to earlier diagnosis and more effective treatment.
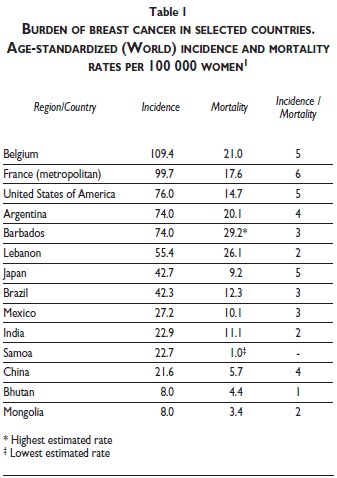
Table I shows the broader range of age-standardized incidence and mortality rates estimated on a national basis rather than by aggregating the numbers of cases and deaths for several countries at the level of world regions. The highest breast cancer incidence is estimated in Belgium and metropolitan France. The lowest incidence is estimated in Bhutan and Mongolia. The highest breast cancer mortality is estimated in Barbados and Lebanon, the lowest in Mongolia and Samoa. The estimated rates of a number of other countries and the ratio of the age-standardized incidence and mortality rates are shown for comparison. Except for Japan and the United States of America, the rates reveal substantial to moderate room for improvement in breast cancer survival in most of the countries outside Western Europe, as reflected in the lower ratios of incidence to mortality.
Mammography (X-ray examination of the breasts) can detect breast cancer before the tumour is palpable and before it causes symptoms.2 Tumours detected and treated at an early stage are associated with a better survival rate than those detected symptomatically. Early diagnosis may permit breast-conserving surgery (Stage I disease), reduce the need for adjuvant therapy and decrease complications related to intensive treatment and recurrence.3-5 A re-appraisal of the randomised controlled trials, conducted by a working group of experts convened by the International Agency for Research on Cancer in 2002, concluded that there is sufficient evidence for the efficacy of screening women aged 50-69 years by mammography as the sole screening modality in reducing mortality from breast cancer.6 A large randomized controlled trial in Shanghai did not find an impact of systematic training in self examination of the breast on breast cancer mortality.7 There is currently insufficient evidence from studies in high -resource countries to support the efficacy of clinical breast examination or the teaching of self-examination of the breast as a public health strategy to lower the number of breast cancer deaths in the population.2 These methods are being evaluated for screening in low-resource countries in which most patients currently present for treatment at very late stages.8 A study aiming to reduce the proportion of newly diagnosed advanced stage breast cancer from 80 to 60% using breast awareness, breast self-examination, clinical breast examination and centralised assessment of abnormalities is currently underway in India.2
Systematic early detection of breast cancer through mammography screening in countries and regions with sufficient resources, has the potential to lower current breast cancer mortality rates and to reduce the burden of the disease in the population.9 The potential harm caused by mammography includes the creation of unnecessary anxiety and morbidity, inappropriate economic cost and the use of ionizing radiation10-13 For these reasons, mammography screening can only be a viable option for cancer control when resources permit the strongest possible emphasis on quality assurance.11-13 Furthermore, the current or the projected future burden of disease must be high enough for screening of asymptomatic women to generate sufficient benefit to appropriately outweigh the cumulative harms of screening in the population.14
Numerous countries have adopted regulations and guidelines on quality assurance of mammography screening.15 In the United States, the Mammography Quality Standards Act (MQSA) has made certification of mammography facilities mandatory.16 Comprehensive multidisciplinary guidelines for quality assurance in breast cancer screening and diagnosis have been developed by experts and published by the European Commission.11,12,17 The present report outlines the methodology, scope and fundamental principles underpinning the standards and recommendations in the fourth edition of the European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis that were published by the European Commission in 2006.12 Furthermore, aspects of the European experience in implementing population-based breast cancer screening programmes are discussed below that appear to be relevant outside the EU. Due to the complexity of the screening process and the comprehensive scope of the European Guidelines, the present report, like any other synopsis (e.g. Perry et al.11) cannot substitute for consultation of the full guideline document.15
European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis
The EU guidelines have been prepared by over 200 authors and contributors participating in a multidisciplinary network involving all of the current 27 EU Member States, for exchange of experience and international collaboration in piloting, implementing and evaluating population-based breast cancer screening programmes.3 Persons active in the network projects, most of which have received cofunding from the Europe Against Cancer Programme and the EU Public Health programme, have included screening professionals and scientists (radiologists, radiographers, surgeons, gynaecologists, oncologists, radiotherapists, breast care nurses, psychologists, medical physicists, epidemiologists, programme planners and administrators and other screening staff) and interested health professionals, legislators and breast care advocates. Network participants also come from EU applicant countries as well as Iceland, Norway, Switzerland, Canada, Israel, and the United States. The involvement of the 12 new Member States that joined the EU in 2004 and 2006 and the additional EU applicant countries in development of the current Guideline edition was limited, but the 12 "new" EU Member States and the applicant countries have in the meantime been integrated into the continuous efforts to disseminate and to update the EU Guidelines. Although not all network members have provided written contributions, guideline drafts have been discussed, and final versions have been approved at network meetings attended by participants from all EU Member States.
Drafting and review of the guideline chapters and the overall document was coordinated by a multidisciplinary editorial board highly experienced in population -based breast cancer screening in Europe. Since most of the authors, contributors and reviewers were recruited from network projects, the guidelines rely significantly on knowledge and experience gained in piloting, implementing and evaluating population-based breast cancer screening programmes.
Unlike the recent preparation of the new European Guidelines for Quality Assurance in Colorectal Cancer Screening,19,20 a formal process of evaluating evidence was not adopted for preparation of the current, fourth edition of the EU breast screening guidelines. Instead, the editorial board determined the topics to be covered in the fourth edition, recruited the chief authors of each chapter and advised them on the recommended scope and key issues to be covered, and reviewed and edited each chapter. Chapter manuscripts were only accepted for publication if the editorial board concluded that current best practice in breast cancer screening and the relevant literature had been adequately taken into account. The editors were also conscious of the importance of raising and maintaining standards across the EU. While maintaining those standards that are of prime importance for mortality reduction, an equitable balance of best practice and performance indicators was sought that can be used across a wide spectrum of cultural and economic healthcare settings. Care was taken to avoid promotion of recent research findings before their putative benefit has been demonstrated in clinical practice.
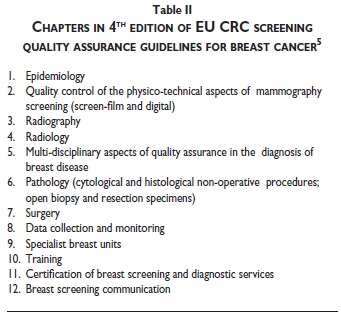
The current fourth edition of the multidisciplinary guidelines (figure 3) consists of approximately 400 pages of recommendations, standards and protocols divided into twelve chapters (table II).
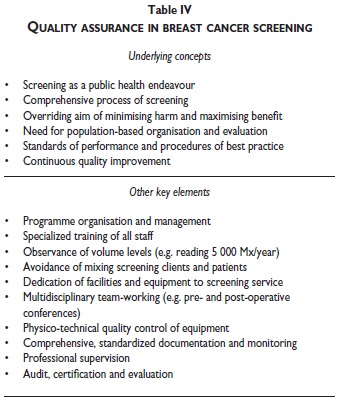
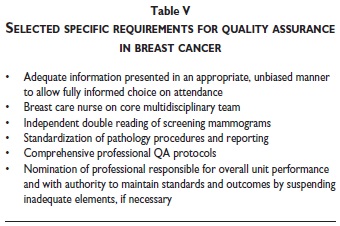
Given the length of the fourth edition, only a few aspects can be covered in the present article that illustrate the scope of the content of the EU Guidelines, and the core principles on which they are based (tables III-IV-V). More extensive summaries of the fundamental points and principles that should support any quality screening or diagnostic service have been prepared by the multidisciplinary editorial board of the fourth edition of the EU guidelines. These overviews include a Summary Table of Key Performance Indicators.11,12
Public health orientation
Breast cancer screening is a public health intervention that aims to lower the burden of the disease in the population. It has already been pointed out that breast cancer can only be detected in a very small proportion of women attending any given round of screening. There-fore only a relatively small number of women can have a direct health benefit from participation in screening. However, all of the participants are exposed to the risks of screening, even if only slight. It is therefore necessary to make every effort to minimize the cumulative risk of screening to the overall population while maximizing the benefit.2,11-13
Screening process
Achieving the potential benefit of cancer screening requires optimal quality at every step in the screening process (table III), beginning with information and invitation of the women eligible to attend screening, and extending from performance of the screening test to the diagnostic assessment of women with suspicious test results and, if necessary, treatment of women with screendetected lesions.2,11-13 In practice, the screening process is more complex than the schematic representation in table III. For example, the treatment phase includes not only clinical management of breast lesions but also rehabilitation of breast cancer patients and palliative care if necessary. The standards, protocols, procedures and other recommendations in the EU Guidelines therefore aim to optimize and continuously improve quality and performance at each step in this process, including for, example, not only taking and reading mammograms, but also all aspects of physico-technical quality control, and multidisciplinary team working in the diagnosis and management of breast lesions (tables II and IV).
Population-based approach
Comprehensive quality assurance is also required to maintain an appropriate balance between benefit and harm in the large numbers of women eligible to attend cancer screening programmes.11-13,21 Achieving and maintaining high quality at every step in the screening process requires an integrated, population-based approach to health service delivery, with personal invitation of each eligible person in the target population.11,12,21 Individual identification and invitation gives each eligible person an equal chance to benefit from screening and therefore reduces health inequalities.14,20,21
This approach is essential in order to maximize benefit, by making screening accessible to those in the population who are generally less likely to consume health resources of their own accord and in order to adequately monitor, evaluate and continuously improve performance.11,12,21
Programme organisation
Another fundamental aspect is the programme approach to implementation of cancer screening (table IV). In many countries around the world, breast cancer screening is delivered in a variety of ways, ranging from highly organized programmes to "opportunistic" activities that involve referral to mammography facilities by clinicians and self-referral by women themselves. Organized programmes are recommended because they include an administrative structure responsible for implementation, quality assurance and evaluation. The population-based, programme approach to implementation of screening services generally requires a high degree of organization in order to reliably identify and invite each eligible woman to attend screening. The population-based approach is also recommended because it provides an organisational framework conducive to effective management of performance, and continuous improvement of the screening process, such as through linkage with population and cancer registries for optimisation of invitation to screening and for evaluation of screening performance and impact.2,11-14,20,21
Programme management
Of special relevance to any discussion on implementation of screening programmes of appropriate quality is the importance of autonomy of programme management in the internal operation and administration of the screening programme.22 Any publically mandated health programme requires oversight and accountability in administrative, financial and clinical matters. But to effectively manage the quality of the screening service, senior management must be able to manage the way the available human and financial resources are used without undue external interference. Senior management also must be held accountable for overall quality and performance of the programme. It is therefore important to appoint professionals and staff responsible for continuously monitoring quality assurance procedures, protocols and standards and reporting problems at an early stage so that programme management can respond effectively to potential problems and can provide adequate support to develop new solutions, if needed. In essence, the organisation must be quality-driven, i.e., continuously striving to improve quality and performance by setting targets, auditing and revising policies and procedures, if necessary, based on scientific methods and principles of best practice.11-13,22,23
At the local or regional level, the professional head of a screening unit must also have the appropriate authority and means to maintain standards and outcomes. If necessary, suspension of inadequate elements of the screening service must be possible, until delivery of services of appropriate quality can be guaranteed.11,12,23
Further key requirements for effective quality assurance of breast cancer screening are mentioned in tables IV and V. All of these elements are of special importance. In the available space, the attention of the reader is drawn to the following aspects.
Communication
Women invited to attend screening must receive objective, balanced information that enables them to make an informed decision on whether or not to attend screening. This is an important aspect that has been recognized very early in the development of the EU Guidelines.10-13 Furthermore, women must receive sufficient and appropriate information to be able to make an informed decision to participate in each subsequent step in the screening process, and they must be able to withdraw consent to participate in screening at any point in the process, such as during diagnostic assessment of lesions detected in screening.
Specialization of staff and dedication of facilities
Another overriding element essential to achieving and maintaining high quality in implementation of breast cancer screening programmes is the need for specialization of staff and dedication of services (table III). Specialization need not prevent many of the professionals engaged in breast cancer screening from pursuing other professional activities outside of dedicated screening units. However, screening of appropriate quality requires substantial expertise, at every step in the process, for example in communication with women attending screening, or in reading screening films, or performing diagnostic work-up of lesions detected in screening. The requisite skills not only require specialized and continued training, but also high volumes of screening services performed by individual professionals in order to achieve sufficient throughput to maintain their special competence. Specialized training and dedicated facilities and organization are also of key importance in establishing adequate physico-technical quality control of equipment. Dedication of facilities also reduces un-necessary anxiety by avoiding intermingling of healthy screening clients with symptomatic patients.
In some cases, such as in diagnostic assessment of women with abnormalities detected in screening, it may not be cost-effective to reserve all available facilities in a diagnostic unit, for dealing with women who have attended the screening programme. In such cases, dedicated sessions can be scheduled in which a diagnostic unit is reserved for a team dealing entirely with assessment of women attending screening.
Multidisciplinary diagnosis and clinical management of breast lesions
Effective screening programmes require integration into a routine health care infrastructure capable of delivering high quality diagnosis and treatment. The scope of the fourth edition of the EU guidelines therefore also covers multidisciplinary diagnosis of symptomatic lesions, and requirements for specialist breast units for multidisciplinary management of both screen-detected and symptomatic breast cancers.
Performance parameters
Given the complexity of the screening process, a large number of organisational, professional and technical performance parameters must be continuously monitored in order to recognize and respond to unfavourable trends before they can lead to inappropriate effects for women attending screening. For correct interpretation, data from various steps in the screening process should be monitored jointly, taking into account potential effects of changes in performance at one step in the process, on performance at other steps or in other sub-processes. In a Summary Table, 50 key parameters for monitoring 39 items have been collated from the individual chapters of the EU guidelines.11,12 Monitoring, though essential, cannot substitute for the long-term evaluation of the impact of a screening programme on the burden of disease in the population.
Discussion
Substantial experience in implementation of cancer screening programmes for large segments of the adult female population currently exists in Europe due, to a large part, to the coordinated efforts in the Europe Against Cancer Programme that began in the late 1980s and continued for fifteen years. Those efforts included provision of technical and scientific support for planning and piloting and for exchanging information and experience in population-based breast and cervical cancer screening programmes in the EU Member States. The need to explain the fundamental standards and principles of best practice that a screening programme of appropriate quality must achieve became evident early in these efforts. For this purpose, the European Guidelines for quality assurance in breast and cervical cancer screening were developed. The EU breast screening Guidelines quickly became an internationally recognized reference for best practice in breast cancer screening and diagnosis and later also in multidisciplinary management of breast cancer; the guidelines have been repeatedly updated and expanded since the publication of the first edition by the European Commission in 1993.17 The scope and the principles of quality assurance and best practice anchored in the current edition of the EU breast cancer screening guidelines have served as a model for the new EU Guidelines for quality assurance in colorectal cancer screening and diagnosis.19,20
Gold standard for breast cancer screening
The availability of the regularly expanded and updated European quality assurance guidelines since 1993 has created a "gold standard" for breast cancer screening programmes, even though the European Commission does not have a legal mandate to enforce their implementation. It is currently unlikely, for example, that mammography equipment that does not fulfill the standards recommended in the European Guidelines can be profitably marketed in Europe. So great is the recognition and acceptance of the EU Guidelines that manufacturers now attempt to outperform each other in compliance with the guidelines.
The existence of a gold standard has substantially facilitated the process of commissioning national and regional breast cancer screening programmes. In principle a government agency or a legislative body seeking to establish a breast cancer screening programme no longer has to describe in detail a programme of appropriate quality prior to officially mandating its implementation. Only key parameters such as the target population and age group, the test and the screening interval need to be specified in the official decision to establish a programme, provided the respective directive, law or parliament resolution also specifies that the standards and recommendations in the European Guidelines must be fulfilled.
The experience in Europe shows that not only the quality of the process of screening, but also the quality of the process by which screening programmes are implemented can be assured.22 Determinants of successful implementation of population-based cancer screening programmes have been developed that also apply to breast cancer screening.23
Council Recommendation on Cancer Screening
In 2003, based on the positive experience in the Europe Against Cancer Programme, the Council of the European Union, that is the highest governing body in the EU, recommended implementation of population-based breast, cervical and colorectal cancer screening programmes, using evidence-based methods and following the EU Guidelines for quality assurance in breast and cervical cancer screening (colorectal cancer screening guidelines were not yet developed at the time). The unanimous adoption of the Council Recommendation on Cancer Screening of 2 December 200324 by the Health Ministers of the EU underlined the importance of the principles and procedures of best practice in the EU quality assurance guidelines for cancer screening and substantially facilitated their implementation by the EU Member States. By the end of 2007 population-based programmes for breast cancer screening were running or being established in 22 of the 27 EU Member States. In 11 Member States rollout of the population-based breast screening programme across the entire country was already complete. In the other 11 Member states establishing population-based breast cancer screening, rollout had already begun in seven Member States; four other Member States were still in the publically mandated planning and/or piloting phase.21,25
Translational phase of programme implementation
In 2008, the European Commission published the first report on implementation of the Council Recommendation on Cancer Screening.21 A key conclusion of the report was that a longterm translational phase is essential to successfully plan, pilot and rollout population-based cancer screening programmes across an entire country, and particularly also across several countries. The time frame depends, to a large extent, on the professional and organisational capacity that must be developed to successfully perform, monitor and evaluate high quality services integrating all steps in the screening process. This activity not only entails coordination of complex communication and training, but also integration of multidisciplinary teams into the diagnosis and treatment of screen-detected lesions, and integration of cancer registration and cancer registries into the monitoring and evaluation of programme performance. Even in countries with relatively small target populations, the magnitude of the task can be substantial, compared to initially available resources. Successful preparation and completion of the nationwide implementation process may require ten years or more.21,22
Process of quality-assured programme development
The experience in Europe also demonstrates the importance of international collaboration during the translational phase of establishing a population-based cancer screening programme. International collaboration can compensate for the relative shortage of expertise in any given country embarking on this complex and protracted journey. Collaboration can not only improve results, it can also avoid unnecessary delays in establishing fully functional screening programmes.21-23
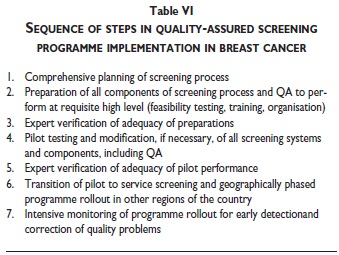
Table VI provides an overview of the key steps in the process of quality-assured implementation of a population-based screening programme.22,23 Professionals experienced in establishing population-based programmes and providing high quality screening, diagnostic and therapeutic services are not only needed to check the adequacy of preparations prior to piloting (phase 3) or before transition of a pilot to service screening (phase 5). The accumulated experience in Europe shows that international collaboration in specialized training of staff, in the procurement, installation and technical quality assurance of equipment, as well as in development and continuous improvement of documentation and monitoring systems and particularly also in coaching the professionals managing and providing the screening services can avoid common pitfalls and can substantially improve the quality and accelerate the pace in every phase of programme implementation.21-23
Impact of implementation of a population-based breast cancer screening programme on symptomatic (usual) care
Another key conclusion in the first report on implementation of the Council Recommendation on Cancer Screening21 deals with the potential impact of establishing population-based cancer screening programmes on the quality and effectiveness of symptomatic, i.e., usual care. Implementation of screening programmes of high quality has the potential to not only lower the burden of disease in the people attending screening.
That is the primary goal and benchmark of success of any screening programme.11-13 However, it should be kept in mind that the protracted process of establishing a population-based breast cancer screening programme of high quality also has the potential to substantially improve the overall level of breast cancer care in a country, because large numbers of professionals undertake additional training to meet the high screening standards. Furthermore, in regions and communities all over the country, the same professionals are also involved in diagnosis and treatment of cancer detected outside of the screening programme. Moreover, new or improved multidisciplinary standards and procedures of diagnosis are developed and tested in the early phases of programme planning, feasibility testing and piloting. Thus, the same high standards of diagnosis and treatment that are developed, piloted and rolled out across a country when a nationwide population-based screening programme is established, will be widely used in provision of symptomatic care. The resulting widespread improvements in the quality and effectiveness of symptomatic breast care contribute substantially to the positive impact of population-based screening programmes on the overall control of breast cancer. This positive development applies also to screening for other chronic disease, such as cervical and colorectal cancer.14,21,22
Accreditation of specialist breast units
In 2008, the Council of the EU adopted conclusions on reducing the burden of cancer in the EU in which the experience in implementation of the Council Recommendation on Cancer Screening was taken into account.26 Among other things, the Council encouraged the European Commission to ensure medium- and long-term scientific and professional support to Member States in implementation of the Council Recommendation on Cancer Screening. It also encouraged the European Commission to explore the potential of implementing another recommendation in the above report, namely, the development of a European pilot accreditation scheme for breast cancer screening and follow-up based on the European guidelines for quality assurance in breast cancer screening and diagnosis. The project has been included in the 2010 work plan of the
EU Health Programme with the aims of developing a tool that will: 1) enable women to recognize that breast units meet the European quality assurance standards, 2) assist the Member States in ensuring that quality standards are met, and 3) encourage continuous quality improvement in breast cancer care throughout the EU. The project will concentrate initially on accreditation of multidisciplinary diagnostic and therapeutic services in specialist breast units. A key focus in piloting the accreditation protocol will be on providing appropriate support to breast centers in Member States interested in learning how to achieve the accreditation standards.
Outlook
Supplements to the fourth edition of the European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis have been developed in recent years. They include updates on quality assurance in pathology and an updated European protocol on physico-technical aspects of quality control, as well as a protocol for type testing of digital mammography equipment. Preparations are also underway for development of a completely revised 5th edition of the European Guidelines. The new 5th edition will include additional new chapters, such as one on breast care nursing.
Conclusions
Implementation of breast cancer screening programmes is a public health endeavor that aims to lower the burden of the disease in the population. To achieve the potential benefit of breast cancer screening, quality must be optimal at every step in the screening process that begins with information and invitation of the eligible target population and includes performance of the screening test, diagnostic assessment of abnormalities detected in screening and, if necessary, treatment and aftercare. This requires significant sustainable resources for quality assurance.
In a collaborative international network focused on the Member States of the European Union, comprehensive guidelines for quality assurance in breast cancer screening have been developed and continuously updated over the past two decades. The existence of this gold standard has facilitated the commissioning of programmes of appropriate quality in over 20 European countries.
Implementation of breast cancer screening in the organizational framework of population-based programmes is recommended because organized programmes include an administrative structure responsible for implementation, quality assurance and evaluation. The population-based approach generally requires a high degree of organization in order to reliably identify and invite each eligible woman to attend screening. This alleviates health inequalities and provides an organisational framework conducive to effective management of performance, evaluation, and continuous improvement of the screening process.
The experience in Europe also demonstrates that the quality of the lengthy process of establishing population-based breast cancer screening programmes can also be assured. International collaboration and exchange of experience during the translational phase of planning, piloting and rolling out a population-based screening programme can avoid common pitfalls and can improve the pace and the outcome of programme implementation.
During this period, specialized training of personnel and development and testing of improved diagnostic and treatment protocols has the potential to also improve the quality and effectiveness of symptomatic breast cancer care, and to thereby also improve control of breast cancer detected outside of the screening programme.
The potential positive impact of population-based breast cancer screening on symptomatic care should be taken into account in the evaluation of any breast cancer screening programme.
The European experience of promoting implementation of population-based breast cancer screening programmes through international collaboration in development and implementation of common standards, principles and protocols of best practice may be a model for other countries and regions in which the burden of breast cancer in the coming years will make population-based screening an option for improving control of the disease.
Acknowledgements
The preparation of this report and the activities described therein have received financial support from the European Commission [grant agreement. No 2004309, European Cancer Network; No. 2004114 (ECN), European Network for Information on Cancer (EUNICE), and No. 2006322, European Cooperation on Development and Implementation of Cancer Screening and Prevention Guidelines (ECCG)].
The present article is a slightly modified version of the original manuscript submitted in August 2010 for publication in a special issue of SPM.
References
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM, GLOBO-CAN 2008. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer, 2010. [ Links ]
2. Boyle P, Levin B (eds.). World Cancer Report. Acting for Prevention - Screening for Breast Cancer, in World Cancer Report 2008. Lyon, France: International Agency for Research on Cancer, 2008: 296-301. [ Links ]
3. Chen HH, Duffy SW, Tabar L, Day NE. Markov chain models for progression of breast cancer Part I: tumour attributes and the preclinical screen-detectable phase. J Epidemiol Biostat 1997; 2 (1): 9-23. [ Links ]
4. Fletcher SW, Elmore JG. Clinical practice. Mammographic screening for breast cancer. N Engl J Med 2003;348(17):1672-1680. [ Links ]
5.Tabar L, Fagerberg G, Chen HH, Duffy SW, Gad A. Tumour development, histology and grade of breast cancers: prognosis and progression. Int J Cancer 1996;66(4): 413-419. [ Links ]
6. IARC. IARC handbooks of cancer prevention, Breast cancer screening. Lyon, France: International Agency for Research on Cancer, 2002, vol. 7. [ Links ]
7. Thomas DB, Gao DL, Ray RM, Wang WW, Allison CJ, Chen FL et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002; 94(19):1445-1457. [ Links ]
8. Anderson BO, Shyyan R, Eniu A, Smith RA, Yip CH, Bese NS et al. Breast cancer in limited-resource countries: an overview of the Breast Health Global Initiative 2005 guidelines. Breast J 2006; 12 Suppl 1:S3-S15. [ Links ]
9. Swedish Organised Service Screening Evaluation Group. Reduction in breast cancer mortality from organized service screening with mammography: 1. Further confirmation with extended data. Cancer Epidemiol Biomarkers Prev 2006;15(1): 45-51. [ Links ]
10.Paci E, the EUROSCREEN Working Group. Summary of the evidence of breast cancer service screening outcomes in Europe and first estimate of the benefit and harm balance sheet. J Med Screen 2012;19 Suppl. 1: 5-13. [ Links ]
11.Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. 4th edition, summary document. Ann Oncol 2008;19(4): 614-622. [ Links ]
12.Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, von Karsa L (eds.). European guidelines for quality assurance in breast cancer screening and diagnosis. 4th ed. Luxembourg: European Commission, Office for Official Publications of the European Communities, 2006. [ Links ]
13. von Karsa L. Mammographie Screening - umfassendes, populationsbe-zogenes Qualitätsmanagement ist hier gefragt! Mammography screening - comprehensive, population-based quality assurance is required! Z Allgemeinmed 1995; 71:1863-1867. [ Links ]
14.von Karsa L, Lignini TA, Patnick J, Lambert R, Sauvaget C. The dimensions of the CRC problem. Best Pract Res Clin Gastroenterol 2010; 24: 4: 381-396. [ Links ]
15. Yankaskas BC, Klabunde CN, Ancelle-Park R, Renner G, Wang H, Fracheboud J et al. International comparison of performance measures for screening mammography: can it be done? J Med Screen 2004; 11(4):187-193. [ Links ]
16.US Food and Drug Administration. Radiation emitting products. Mammography Quality Standards Act and Program. 2013. [Accessed 14 April 2013]. Available from: http://www.fda.gov/Radiation-EmittingPro-ducts/MammographyQualityStandardsActandProgram. [ Links ]
17. Kirkpatrick A, Törnberg S, Thijssen M (eds.). European guidelines for quality assurance in mammography screening. Luxembourg: European Commission, Office for Official Publications of the European Communities, 1993. [ Links ]
18. de Waard F, Kirkpatrick A, Perry NM, Törnberg S, Tubiana M, Wolf C. Breast cancer screening in the framework of the Europe against Cancer programme. Eur J Cancer Prev 1994; 3 Suppl. 1: 3-5. [ Links ]
19. Segnan N, Patnick J, von Karsa L (eds.). European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis. Luxembourg: European Commission, Publications Office of the European Union, 2010. [ Links ]
20. von Karsa L, the European Colorectal Cancer Screening Guidelines Working Group. European guidelines for quality assurance in colorectal cancer screening and diagnosis: Overview and introduction to the full Supplement publication. Endoscopy 2013;45(1):51-59. [ Links ]
21. von Karsa L, Anttila A, Ronco G, Ponti A, Malila N, Arbyn M et al. Cancer screening in the European Union - Report on the implementation of the Council Recommendation on cancer screening, First Report. Luxembourg: European Commission, 2008. [ Links ]
22. von Karsa L, Suonio E, Lignini T, Ducarroz S, Anttila A (eds.). Current Status and Future Directions of Breast and Cervical Cancer Prevention and Early Detection in Belarus. Cancer Control Assessment and Advice Requested by the Belarus Ministry of Health. Report of Expert Mission to Minsk, Belarus, 15-18 February 2011. Lyon, France: IARC/WHO, 2012. [Accessed 14 April 2013]. Available from http://www.iarc.fr/en/publica-tions/pdfs-online/wrk/wrk6/Belarus_Report.pdf [ Links ]
23. Lynge E, Törnberg S, von Karsa L, Segnan N, van Delden JJ. Determinants of successful implementation of population-based cancer screening programmes. Eur J Cancer 2012; 48(5): 743-748. [ Links ]
24. Council of the European Union. Council Recommendation of 2 December 2003 on cancer screening (2003/878/EC). Off J Eur Union no. L 327 2003: 34-38. [ Links ]
25. Commission of the European Communities. Report from the Commission to the Council, the European Parliament, the European Economic and Social committee and the Committee of the Regions - Implementation of the Council Recommendation of 2 December 2003 on cancer screening (2003/878/EC) Brussels, COM (2008) 882 final. Brussels: Commission of the European Communities, 2008. [ Links ]
26. Council of the European Union. Council Conclusions on reducing the burden of cancer. 2876th Employment, Social Policy, Health and Consumer Affairs Council meeting, Luxembourg, 10 June 2008. Press Office of the Council of the European Union, Brussels, Belgium. [Accessed 14 April 2013]. Available from: http://consilium.europa.eu/Newsroom [ Links ]
 Corresponding author:
Corresponding author:
Lawrence von Karsa
Quality Assurance Group, Early Detection and Prevention Section
International Agency for Research on Cancer. 150 cours Albert Thomas
69372 Lyon, Cedex 08, France
E-mail: KarsaL@iarc.Fr
Accepted on: January 7, 2013
1 In the Globocan data base,1 "more developed" regions are defined as: all regions of Europe plus Northern America, Australia/ New Zealand and Japan. "Less developed" regions are defined as: all regions of Africa, Asia (excluding Japan), Latin America and the Caribbean, Melanesia, Micronesia and Polynesia. The definitions are intended for statistical convenience and do not necessarily express a judgment about the stage reached by a particular country or area in the development process (source United Nations).
2 R. Sankaranarayanan, personal communication, 4 August 2010.
3 The European Breast Cancer Screening Network was initially established in 1988 as a network of breast cancer screening pilot projects receiving financial support under the first Action plan of the Europe Against Cancer programme.18 The network expanded over the years and in 2004 was consolidated with the other European Cancer Screening Networks in the European Cancer Network for screening and primary prevention.