PACS: 78.67.-n; 78.67.Hc; 81.07.-b; 81.07.St
1. Introduction
Several reports show that photoluminescence and electroluminescence emission of CdSe nanoparticles can be tuned within the visible spectrum (from wavelengths 450 nm to 650 nm) controlling the nanocrystal size1-5. This versatility opens up a variety of potential applications for CdSe nanoparticles in photonic devices, such as: artificial lighting and displays6, color modifiers for light emitting diodes (LEDs)7, optical fiber amplifiers8, low threshold lasers9, self-assembled photonic sphere arrays10, polymer-based photovoltaic cells11, optical temperature probes12, chemical sensors13 and high-speed signal-processing filters14.
Colloidal semiconductor nanocrystals are relatively easy to synthesize without sophisticated facilities. To obtain good QDs properties, sufficient control of reaction parameters during synthesis is required, because the intrinsic properties of the nanoparticles are determined by different factors such as their size, shape, number and type of defects, impurities and crystallinity. The dependence of the optical properties on size arises from (1) changes in the surface-to-volume ratio with size and (2) quantum confinement effects. A number of different self-assembly techniques (bottom-up) have been used to synthesize semiconductor nanoparticles, and they may be broadly subdivided into vapor-phase and wet-chemical methods15. Wet-chemical methods are mainly conventional precipitation methods. They carefully control of the parameters for a single solution, or a mixture of solutions, and involve both nucleation and limited growth of nanoparticles. Some wet-chemical methods are: (i) sol-gel16-18, which allows obtaining homogeneity of QDs, but this method involves several steps and close monitoring is the main disadvantage. (ii) Co-precipitation, where the nanocrystal size and fluorescence intensity are changed by varying the pH of solution. In addition, it is not suitable for the precipitation of highly pure and accurate stoichiometric phase, as the control of the crystallinity size and morphology is also difficult. (iii) The competitive reaction chemistry is another method that has some disadvantages, such as the creation of lattice defects upon ion exchange; no clear indication of termination of reaction implicates that some of the initial ions may be present in the final product, which may degrade the optical and electrical properties in the nanocrystal. (iv) Methods based upon sonic waves and microwaves19 provide QDs between 1-5 nm. Acoustic cavitation generates a localized hotspot through adiabatic compression of the gas inside the collapsing bubble, enabling the reaction; however, this method implicates high-energy and high reaction rate. (v) Electrochemical methods20,21 allow to obtain nanocrystals with high degree of monodispersity and excellent photoluminescence emission properties, but the requirement is that substrate and nanoparticles have reasonable electrical conductivity and, there is the possibility of contamination of nanoparticles from the solution. Finally, (vi) the hot injection method is the most popular route for synthesizing CdSe nanoparticles, which consists in the simultaneous injection of organometallic precursors22-24 into a mixture of organic solvents heated at high temperatures. The fast injection of the precursors induces a high degree of supersaturation in the solution, resulting in an almost instantaneous nucleation of CdSe particles with an isotropic spherical morphology25. One of the substances in the hot mixture plays the role of a surfactant, which prevents aggregation among particles but, at higher temperatures, adsorbs and desorbs rapidly from the nanocrystal surface, enabling the addition of atoms to the nanoparticle in controlled growth26. This sequence of phenomena allows the control of the size distribution of particles. During the injection, the precursor concentration in the mixture decreases abruptly due to the particle formation, and the temperature decreases since the precursors are injected at room temperature. These two factors stop further nucleation events27. The use of surfactants with different binding affinities to the nanocrystal surface allows excellent control of the crystal size, size distribution and morphology28. The method works properly when the temperature of the organic solvent mixture is approximately 350°C, and thus high boiling point solvents are required. To avoid the oxidation of the reagents and/or nanoparticles, the reaction is usually conducted in a vacuum or in an inert atmosphere29. When the temperature of the solvent mixture is not as high, obtaining good results requires high pressures in the synthesis atmosphere. The working hypothesis of this research is that an air atmosphere could be used during the reaction when: a) the CdSe nanoparticles were sufficiently covered with surfactant molecules and b) adequate chose of solvents make the oxidation of the solvents irrelevant. In this way, the oxidation of CdSe would be avoided and the experimental setup could be greatly simplified. However, as will be shown below, the synthesis in air did have some influence on the particles.
There are many reports on hot injection methods for synthesis of CdSe QDs. Early studies reported the use of pyrophoric metallic precursors, such as dimethyl cadmium14,20,30. Recently, these precursors have been substituted by precursors that are more stable in air, such as cadmium oxide31, cadmium acetate or cadmium chloride32. Some studies have researched into the injection of cold precursors into a solvent with a high boiling point33 at a high temperature (350°C). Synthesis with low-boiling point solvents requires high pressure34, an inert atmosphere35,36 and the use of different coordinating agents or surfactants such as phosphines oxides, phosphines, fatty acids, phosphonic acids (only in a mixture with trioctylphosphine oxide), amines and carboxylic acids20,37,38. Reports show that the alkyl chain length surfactant influences the diameter and size distribution of the nanoparticles because the steric hindrance of the surfactant prevents the growth and agglomeration of nanoparticles39. Other variant of this method is the synthesis of CdSe nanoparticles in which the solvent is a mixture of different species, including pure solvents and pure surfactants40. The rate of injection also influences the shape and morphology of nanoparticles41. In all of the above mentioned studies, CdSe particles were obtained by hot injection method or variant of it, however, inert atmosphere is used in each of the researches.
In this work, CdSe nanoparticles were synthesized by hot injection method using air in the reaction conditions. Results obtained showed that oxygen works as passivation agent in the reaction, covering defects of nanoparticles surface and increasing the reaction rate (in comparison with the inert atmosphere), which increases also the nanoparticles size. This behavior was corroborated with their optical properties.
2. Experimental
2.1. Materials
All of the following materials were purchased from Sigma-Aldrich: Cadmium acetate hydrate ((C2H3O2)2Cd·xH2O, 99.99%), Selenium (Se, 99.99%), tri-n-octylphosphine (C24H51P, 97.00%) (TOP), tri-n-octylphosphine oxide (C24H51PO, 90.00%) (TOPO) and 1-octadecene (C18H36, 95.00%)(ODE). Methanol (CH3OH, 99.8%) and chloroform (CHCl3, 99.8%) were purchased from J.T. Baker and were used directly without any further purification.
2.2. Synthesis of CdSe nanoparticles
Synthesis of the CdSe nanoparticles was accomplished by the hot injection method reported by Sugnan et. al. under two different atmospheres42. In the first case, the synthesis was prepared in an inert atmosphere, while the second synthesis was conducted in air. In both cases, a cadmium precursor was prepared by mixing 40 mg of hydrate cadmium acetate, 0.8 g of TOPO, 8 mL of TOP and 10 mL of octadecene in a three-necked flask. The mixture was heated at 350 °C and shaken vigorously for 90 min. The selenium precursor (TOPSe) was prepared mixing 26 mg of selenium powder and 3 mL of TOP and it was shaken vigorously at 200°C for 2 h. The CdSe nanoparticles solution was produced by injecting 1 mL of the selenium precursor at room temperature into the cadmium precursor whose temperature was 350°C. After this addition, temperature of the mixture decreased to 280°C quenching the nucleation and inducing the growth of the CdSe nuclei29. To monitor the growth of the nanoparticles, 2 mL aliquots of the solution were extracted at different reaction times. The first three samples were taken every 5 s, and the fourth sample was taken at 1 min. The next four samples were taken every 15 min, four samples more were taken every 25 min, and the last samples were taken every 30 min. In this way, it was possible to follow the change in the particle sizes as a function of the reaction time. At the end of the procedure, the CdSe nanoparticles were precipitated by repeated washing with a mixture of 1 mL of chloroform, 5 mL of acetone and 10 mL of methanol. The mixture was shaken vigorously at room temperature. The final precipitate was re-dispersed in 3 mL of chloroform. It should be noted, that the amounts of the Cd and Se precursors do not correspond to the stoichiometric ratio in the particles. An excess of the Cd precursor was used to ensure the complete reaction of Se precursor.
2.3. UV-vis analysis
The ultraviolet-visible (UV-vis) spectra were obtained in a 1 cm quartz cell using a Perkin Elmer Lambda 25 UV-vis spectrometer from 200 to 900 nm at room temperature. Samples were prepared with 0.5 mL of the unwashed CdSe nanoparticles solution and 3 mL of chloroform.
2.4. Photoluminescence analysis
The photoluminescence (PL) spectra were obtained in a 1 cm quartz cell using a Hamamatsu R943-02 GaAs photomultiplier tube with a 50 mW. Measurements were made with a 325 nm He-Cd laser in the wavelength range from 400 to 800 nm in an oxygen atmosphere at room temperature. Samples were prepared with 0.5 mL of washed CdSe nanoparticles in 3 mL of chloroform.
3. Results and Discussion
As per the hot injection method, the cold selenium precursor (TOPSe) was injected into a hot mixture (350°C) containing the cadmium precursor. Once the TOPSe was injected, the reaction temperature decreased to 280°C and the formation of the nanoparticles was detected with the naked eye because the color of the mixture changed from light yellow to brown. This change in color also indicated the onset of the growth stage43. To verify the composition of the particles, EDS was performed on the samples with a scanning electron microscope (SEM). The analysis showed that the concentration of Cd was close 1.64 times the concentration of Se, which was expected since an excess of Cd was deliberately added to ensure the formation of CdSe. As it will be shown below, the optical results agree with the results found in the literature for CdSe nanoparticles, which supported the claim that the chemical composition of the particles corresponds to CdSe. In addition, samples were taken at different reaction times and observed under UV lamp irradiation (365 nm). Figure 1 shows a photograph of all the samples synthesized in nitrogen. A similar photograph for the samples synthesized in air is presented in Fig. 2. As observed in Fig. 1, the emission changes from blue to red; that is, the wavelength increased as the reaction time increased. Particle size depends on the reaction time, so Fig. 1 suggests a relationship between the particle size and the fluorescence of the dispersions. Similar characteristics are observed in Fig. 2. However, the samples synthesized in air had redshift emission compared with the the nitrogen-synthesize at the same reaction time. This fact suggests that the growth rate was higher during the synthesis in air than during the synthesis in nitrogen. It is also apparent that each sample emitted several different colors, which could be an indication of a multimodal distribution in the particle size.

Figure 1 Emission colors of the colloidal CdSe QDs synthesized under nitrogen atmosphere conditions.
The UV-vis absorption spectra of QDs synthesized in nitrogen and air atmospheres are shown in Figs. 3A and 3B, respectively, at different reaction times. The onset wavelengths and the maxima of the lowest energy peaks are listed in Table I. Some trends are evident: the onset point and the first emission peak occurred at higher wavelengths in the samples synthesized in air than in the samples synthesized in nitrogen at the same reaction times. This trend suggests that the particles of the samples synthesized in air are larger than the particles of samples synthesized in nitrogen44. As it is shown in Fig. 3, the peaks appeared at lower wavelengths for every sample. These additional peaks could be the result of the excitations of discrete energy levels other than the HOMO and LUMO levels that defined the band gap. However, UV fluorescence was observed in the monodispersed CdSe nanoparticles where only one defined peak appeared in the UV wavelength range45. Hence, the emission curves in Fig. 3 suggest some polydispersity in the particle size distribution.
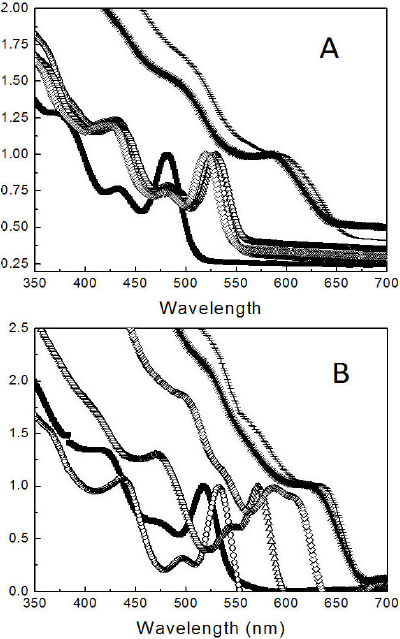
Figure 3 Normalized absorption spectra showing the excitation wavelength for CdSe QDs synthesized under (A) nitrogen and (B) air. The samples were taken from the reaction mixture at different times: 10 s(■), 20 s (○), 1 min (∆), 16 min (◊), 61 min (*) and 136 min (-).
Table I Wavelength (nm) of the onset point and the first peak (lowest energy peak) of the luminescence of all the samples (Fig. 3).
| Reaction | Nitrogen atmosphere | Air atmosphere | ||
| time | Onset wavelength |
1st peak wavelength |
Onset wavelength |
1st peak wavelength |
| 10 sec | 510 | 481 | 548 | 518 |
| 20 sec | 553 | 527 | 553 | 532 |
| 1 min | 556 | 532 | 597 | 573 |
| 16 min | 548 | 520 | 633 | 608 |
| 61 min | 638 | 592 | 667 | 625 |
| 136 min | 658 | 600 | 667 | 637 |
The PL spectra of the CdSe nanoparticles synthesized in the two different atmospheres for 10 s, 20 s, 1 min, 16 min, 61 min and 136 min are shown in Fig. 4. The intensity of the emission was normalized. The wavelengths of the emission peaks showed a similar trend to that of the one described for UV spectra. Shoulders can be noticed in the emission peaks of the samples synthesized in nitrogen for 10 s and 16 min. These shoulders are the result of the overlapping of additional emission peaks, suggesting polydispersity in the samples. Emission peaks in the samples synthesized in air are not completely symmetric but are well defined, encouraging their use in possible optical applications.
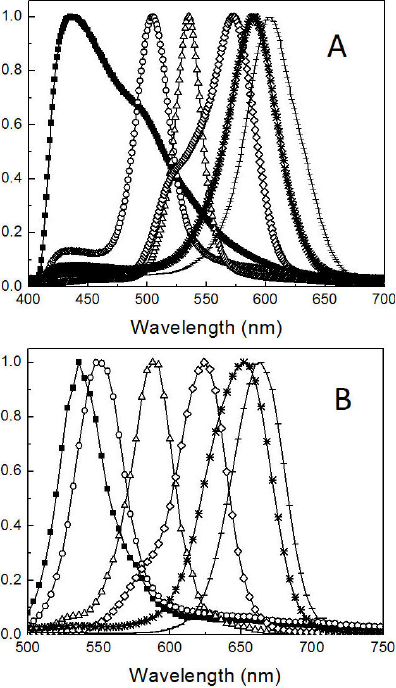
Figure 4 Normalized emission spectra for CdSe QDs synthesized under (A) nitrogen and (B) air. The samples were taken from the reaction mixture at different times: 10 s (■), 20 s (○), 1 min (∆), 16 min (◊), 61 min (*) and 136 min (-).
According to other studies, optical properties of CdSe nanoparticles obtained in this work are similar to those synthesized under inert atmosphere with the same synthesis method, or when a different technique of synthesis was used. For example, Gaeeni et. al.46 obtained CdSe nanoparticles by sol-gel method with 1 h of reaction time, using an inert atmosphere. Their results showed the formation of cubic cadmium selenide structure with average diameter of 3 nm and UV fluorescence onset wavelength at 550 nm. Similar results are observed in Fig. 4B where nanoparticles synthesized under air presented a similar size and onset wavelength at 20 s of reaction time. Gao et. al.47 synthesized CdSe nanocrystals using aqueous precipitation method at lower temperatures (50-90°C) under inert atmosphere. Optical results showed broad emission spectra, this means bigger size dispersity than the one obtained in this work under proposed air conditions. In addition, Jagtap et. al.48 reported the CdTe nanoparticles synthesis by hydrothermal method also under nitrogen atmosphere. This work showed that nanoparticles had lower emission intensity than those CdSe synthesized in this study under air conditions. Since it is well established that the UV-vis and photoluminescence depend on nanoparticle size, the average diameter of each sample was estimated with an empirical equation reported by W. William et. al. (Eq. (1))49:
In this equation, the average nanoparticle diameter (D) is a function of the excitation wavelength (λ (nm)). This wave-length is being, the wavelength of the first exiton absorption peak at each reaction time48.
Figure 5 shows the size of the CdSe nanoparticles synthesized in air and nitrogen as a function of the reaction time. It is noticed from this figure that the growth rate is higher in samples synthesized in air, than those synthesized in nitrogen.
In order to corroborate the results of particle size, HRSEM photographs were taken (see Fig. 6). It is possible to observe that nanoparticles synthesized under air conditions at 15 s of reaction (see Fig. 6A) show a diameter close to 5 nm while nanoparticles synthesized under nitrogen conditions, (see Fig. 6B) have a size close to 3 nm. In the same way CdSe, at 111 s of reaction time presents a particle size near 10 nm and 5 nm when are synthesized by air and nitrogen conditions respectively. Additionally, a spherical morphology is observed in both cases and it is attributed to a fast injection of precursors and use of technical grade TOPO. It has been reported that the use of technical grade concentration, which contains additional components50, apparently decreases growth and allows obtaining spherical shapes. However, particles with rods morphology are also obtained by synthesis using pure TOPO26.
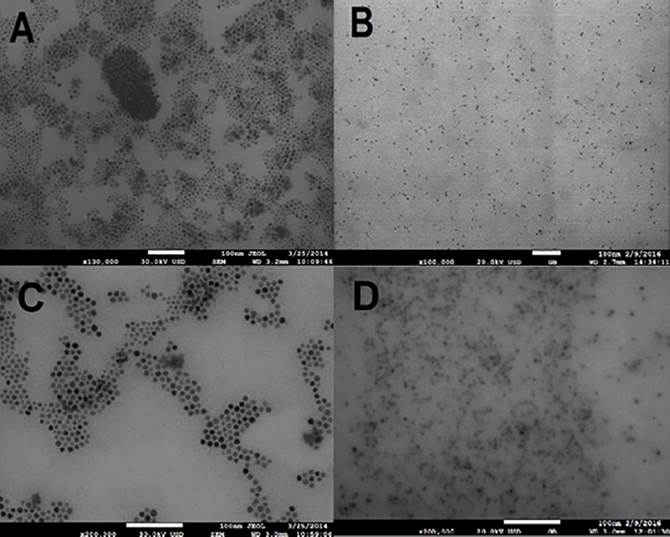
Figure 6 HRSEM images of CdSe QDs taken after 15 s of reaction time and synthesized under (A) air and (B) nitrogen. Photographs (C) and (D) correspond to the samples reacted for 111min in air and nitrogen, respectively.
Figure 7 shows a plot of the peaks calculated from the absorption spectra as a function of the average particle size at different reaction times and for both atmospheres. The magnitude of this energy (∆E) is related to the particle size by Eq. (2) 51.
where Eg is the macroscopic band gap,
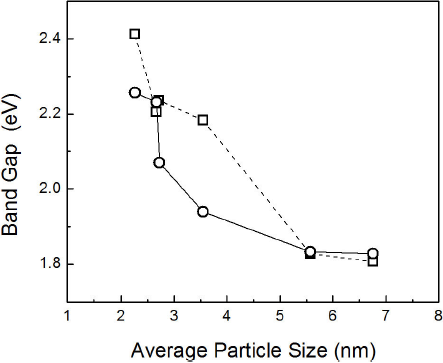
Figure 7 ∆E as a function of the average particles size at several reaction time for nanoparticles synthesized in (□) nitrogen and (○) oxygen.
It has been reported that during the particle ripening in presence of oxygen, oxygen atoms help to mend the point defects in the particle, allowing growth to occur on the surface of a more perfect (or less distorted) crystalline lattice52. This probably occurs because oxygen has polar characteristics and can be absorbed into the surface of nanoparticles to eliminate the polar surface defects formed by dangling Se/Cd bonds53,54 during synthesis. Fewer crystalline defects enhance the fluorescence of the nanoparticles55. However, Nima E. Gorji56 studied oxygen incorporation into CdS/CdTe thin film solar cells and his results showed that grain size is greatly affected by the oxygen content. Films with higher oxygen content had larger grain sizes than those grown in nitrogen.
To obtain more information on the differences between the synthetic conditions, IR spectroscopy was conducted. The spectra are presented in Fig. 8.
The FT-IR spectra of nanoparticles synthesized in nitrogen and oxygen showed the characteristic Cd-Se stretching mode at 746 cm-1. The - CH2 - stretching modes of the three-alkyl chains of surfactant appeared at 2850 cm -1 and 2937 cm-1, while the stretching of the C-H bond was found at 1477 cm-1. Additionally, the signal at 1214 cm-1 corresponded to the P=O bond and the interaction between P-O and the nanoparticles surface. As shown in Fig. 8, both spectra were similar, which suggests that there is no difference in the composition of the CdSe QDs synthesized in air and inert atmospheres.
4. Conclusions
CdSe nanoparticles were successfully synthesized by hot injection with air as the reaction atmosphere. To avoid particle oxidation, organic solvents and surfactants such as TOP and TOPO were used. However, the presence of air in the reaction atmosphere increased the growth rate of the particles compared to those particles grown in an inert atmosphere. This effect can be explained in terms of oxygen ability to improve the crystalline quality of the nuclei allowing better growth. To ensure the complete reaction of Se precursor, an excess of Cd precursor was used. The particles had emissions in the visible range whose wavelength could be tailored to the particle size, as expected. Hence, optical applications for these particles are viable.











 text new page (beta)
text new page (beta)





