INTRODUCTION
Continuous renal replacement therapy (CRRT) has revolutionized the management of critically ill patients with acute kidney injury (AKI) over the past decade. This review aims to provide a brief overview of the most salient developments, emerging trends, and key lessons learned in the field of critical care nephrology over the past years. By examining evolving evidence, we delve into the advancements in technology, optimization of CRRT deliverables, strategies to improve patient outcomes, and ongoing and future challenges in CRRT value-based care delivery. The insights gained from this review will contribute to enhancing the understanding of CRRT and guide future research endeavors in this vital area of critical care nephrology.
TECHNOLOGICAL ADVANCES
Since the original concept of CRRT, performed as arteriovenous hemofiltration in the 70 and 80s1,2 extracorporeal kidney support therapies have evolved considerably. Integration of blood, dialysate, and reinfusion pumps in a single machine was the major advance in CRRT in the 90s. This made CRRT possible with better safety, performance, and ability to accommodate different clearance modalities such as diffussion and convection. This was followed in the early 2000s by machines with user-friendly interfaces and pre-arranged, self-loading cartridges with filters and lines included, making CRRT more standardized and widely available which was reflected in the development of clinical trials that defined important aspects such as dosing, patient selection, and ideal clinical scenarios3. For the last decade, technology has been focused mainly on direct performance monitoring, programmatic and therapy data collection, improvement of systems functionality, and sequential multiorgan support therapy.
Direct monitoring
Online monitoring of clinical parameters during CRRT has been proposed since the late 90s mainly focusing on temperature, blood volume, and conductivity4. Modern machines have successfully added online monitoring of clinical parameters such as hematocrit and oxygen saturation by adding an optical sensor attached to a blood chamber with the objective of monitoring blood volume in real-time. Furthermore, the integration of blood temperature monitoring to the heating system has been added to some modern machines.
Data collection
The acute disease quality initiative (ADQI) recommended using information technology tools to enhance processes of patient care and clinical outcomes. Data acquisition, transformation, and processing are key to evaluate programmatic data such as filter life, time on machine/treatment loss, and complications related to accessing or clotting. Similarly, therapy data can be monitored and include prescribed versus delivered dose as well as fluid removal. One should recognize that data could be used to evaluate individual treatments for specific bedside decisions as well as systematically in aggregated fashion to evaluate key performance indicators of the CRRT program and design and implement quality interventions5. Development in data management procedures has been important in the last decade allowing connectivity between devices and electronic health records, facilitating the development of multimodal data registries6. Modern machines can achieve connectivity through chip-cards, cables, wireless connections, and cloud-based storage. These technological advancements provide opportunities for quality assurance and potentially improvement in patient outcomes7 (Fig. 1).
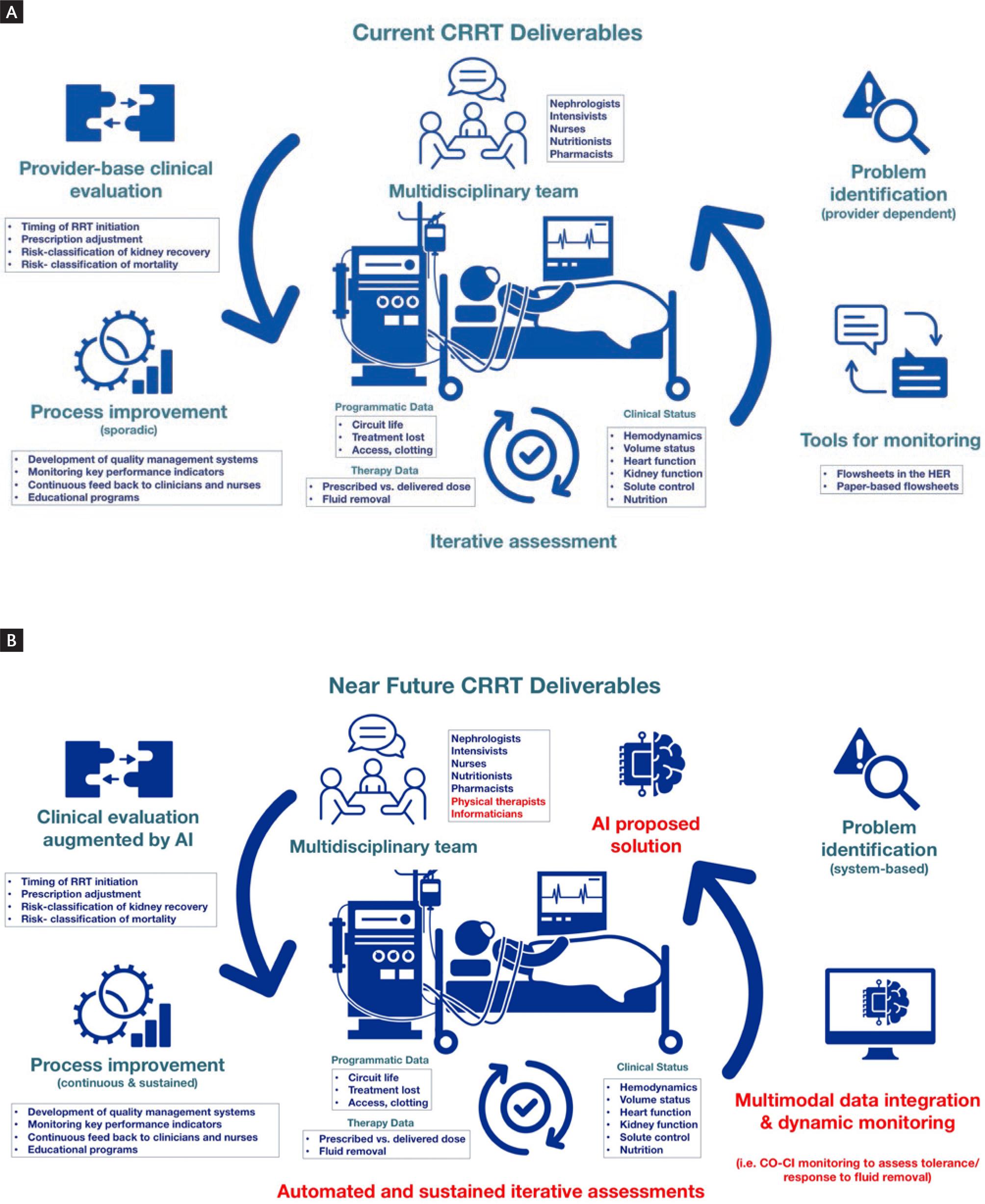
Figure 1. Current versus near future CRRT deliverables. (A) Current CRRT deliverables; provider based assessment, monitoring, problem identification and improvement of deliverables. (B) Near future CRRT deliverables; Machine learning and artificial intelligence tools can further enhance CRRT deliverables at multiple levels including automated and sustained assessment, data integration and problem solving.
Improved precise dose systems
There is a well-documented gap between the delivered and the prescribed dose of CRRT, which is caused mainly due to downtime (stopping pumps, bag changes, filter clotting, deficient vascular access, and patient disconnection)8-10. For this reason, the KDIGO guidelines suggest prescribing 10-20% over the desired delivery dose11. Further, the ADQI group determined as a quality metric the delivery of at least 80% of the prescribed dose.12,13. Technology has evolved in this aspect by including algorithms that can measure downtime and compensate ongoing delivery by automatically increasing the dose. Fourth-generation CRRT machines have successfully added these algorithms and proved to narrow the gap between prescribed and delivered dose14,15.
Multiorgan support therapy
CRRT devices have evolved from being exclusively for kidney support to integrating technologies for liver and lung support and therapeutic plasma exchange. Liver support has been added to CRRT machines in the form of coupled plasma filtration adsorption16 or the molecular adsorbent recirculating system17. Lung support using CRRT machines is now possible by adding extracorporeal CO2 Removal circuits18-20. CRRT devices can also accommodate plasma filters to perform membrane-based therapeutic plasma exchange without the need of a centrifuge-type separation. CRRT machines have also been adapted to integrate in tandem with high-volume devices such as extracorporeal membrane oxygenation for cardiopulmonary support21,22. Finally, CRRT machines have also become a platform to perform hemoperfusion with individual novel cartridges or in combination with standard ones23-25.
CLINICAL OUTCOMES AND PATIENT SELECTION
Since the development of new generation of CRRT machines with functional interfaces and preload cartridges, the therapy has become more standardized and widely available in developed countries albeit existing disparities in low and low-middle-income countries. The progressive increment in CRRT use has been reflected in the development of clinical trials and observational studies that have advanced evidence and value-based practice. Dosing, timing, and type of RRT have been widely studied (Table 1).
Table 1. Summary of key studies that have advanced practice
| Study | Design | Intervention | Eligibility | Primary outcome | Secondary outcomes | Limitations |
|---|---|---|---|---|---|---|
| Dose | ||||||
| ATN (Palevsky 2008)26 | MC- RCT, n = 1124 | Intensive (CVVHDF 35 ml/kg/h or 6 days a
week for IHD Versus less intensive (CVVHDF 20 ml/kg/h, or 3 days a week for IHD |
Critically ill patients with AKI and failure of other organ or sepsis | Death from any cause by day 60: 53.6% for
intensive strategy, 51.5% for less intensive; p =
0.47 Complete kidney recovery 28 days: 15.4% intensive, 18.4% less intensive; p = 0.24 Home discharge: 15.7% intensive, 16.4% less intensive; p = 0.75 |
In-hospital mortality: 51.2% for intensive, 48% less intensive; p = 0.27 | Timing of initiation was not standardized,
and wide variation was observed in practice among and within
participating institutions. Men were overrepresented. CKD patients were excluded from the study. |
| RENAL (Bellomo 2009)27 | MC- RCT, n = 1508 | Post dilution CVVHDF 40 ml/kg/hr Versus 25 mL/kg/h | Critically ill patients with AKI | Death within 90 days after randomization:
44.7% for each group. RRT dependence at 90 days: 6.8% high intensity, 4.4% low intensity; p = 0.14 |
RRT dependence at 28 days: 14.5% high intensity, 12.2% low intensity; p = 0.31 | Study personnel and staff were aware of
patients' treatment status. Timing of initiation was not standardized Data to assess the costs of the interventions were not gathered. |
| Timing | ||||||
| STARRT-AKI (Bagshaw 2020)31 | MN-RCT, n = 2927 | Accelerated strategy (within 12 h of randomization) versus standard strategy (Conventional indications or > 72 h of persistent AKI) | Critically ill patients with severe AKI (KDIGO 2 or 3) with clinical equipoise by intensivists and nephrologists for both strategies. | Death from any cause at 90 days: 43.9% accelerated strategy, 43.7% standard strategy; p = 0.97 | RRT dependence: 10.4% accelerated, 6%
standard; RR = 1.74 (1.24-2.43) No difference in the composite of death or dependence on RRT, death in the ICU at 28 days or length of hospitalization. No difference in number of ventilator and vasoactive free days at 28 days |
Equipoise is subjective and may be
modified by provider clinical bias. The standard strategy group had variable initiation times. The report of more adverse events (hypotension and hyphosphatemia) in the accelerated strategy may be attributed to prespecified focus on reporting of events. |
| AKIKI-2 (Gaudry 2021)32 | MC-RCT, n = 278 | Delayed strategy (after randomization) versus more delayed strategy (hyperkalemia, metabolic acidosis, pulmonary edema or BUN > 140 mg/dl | Critically ill patients with severe AKI (KDIGO 3), oliguria > 72 hours or BUN > 112 mg/dl | Number of days free of RRT at 28 days: 12 days delayed, 10 days more-delayed; p = 0.93 | Mortality at 28 days: 38% delayed, 4 5%
more-delayed; p = 0.26 Kidney function recovery at 60 days: 51% delayed, 69% more-delayed; p = 0.10 No difference in ventilator and vasopressor free days No difference in length of ICU stay and length of hospital stay |
The BUN criteria were arbitrary and not
based on evidence. The serum potassium that mandated RRT was similar to other RCTs, a higher value would be ideal for this question. |
| Contemporary Epidemiological Studies of CRRT | ||||||
| CRRnet (Rewa 2023)6 | MC –PLR, N analyzed = 1106 N Recruited = 1541 |
Observational Registry | Critically ill patients with AKI requiring CRRT from 12/2013 to 01/2021 | All cohort hospital mortality: 58.9%
HD-dependence at hospital discharge in survivors: 31.4% ICU days in survivors 15 (8-28) Hospital days in survivors 33 (18-58) |
Most common CRRT indications: Oligo/anuria
56.2%, Fluid overload 34.3% Most common condition predisposing to AKI: Sepsis 45.6% Most common modality: CVVHDF 80.5% Median prescribed dose: 31.3ml/kg/hr [25.6-40] |
The generalizability of these results to CRRT practices in non-academic centers or low-and middle-income countries is limited. |
MC: multicenter; MN: multinational; RCT: Randomized Control Trial; CVVHDF: continuous veno-venous hemodiafiltration; IHD: intermittent hemodialysis; AKI: cute Kidney Injury; CKD: chronic kidney disease; RRT: renal replacement therapy; PLR: prospective living registry.
Dose
Dose is an aspect of CRRT delivery with the most solid evidence based on multicenter randomized clinical trials (RCTs). The ATN trial randomized 1124 patients to receive intensive strategy (CVVHDF 35 mL/kg/h or intermittent RRT modalities 6 days a week) versus less intensive strategy (CVVHDF 20 mL/kg/h, or intermittent RRT modalities 3 days a week), with no significant difference between the two groups in 60-day mortality (53.6% vs. 51.5%) or kidney recovery (15.4% vs. 18.4%)26. The RENAL trial randomized 1508 patients to 40 mL/kg/h versus 25 mL/kg/h with 90-day mortality of 44.7% for both groups27. Based on this evidence, the dose has an accepted guideline-based recommendation in the prescription (total effluent of 25-20 mL/kg/h) that has been widely adopted in the critical care nephrology community11. Therefore, dose constitutes a key performance indicator specifically for the benchmark of <20% gap between prescribed versus delivered dose13. Modern approaches to dose have also adapted to personalized dose delivery according to specific solute goals12.
Timing
A salient question that has been heavily addressed in the last decade is related to optimal timing of RRT in critically ill patients with AKI. The overarching hypothesis in some trials was that preemptively initiating RRT before severe complications arise could impact clinical outcomes.
The ELAIN trial randomized 231 patients with KDIGO 2 AKI and NGAL > 150 ng/mL to receive CRRT 8-12 h after randomization versus standard initiation based on solute/volume control. The study showed that 90-day mortality was 39% in the intensive arm versus 54% in the standard arm and almost all patients randomized to the standard arm received CRRT (91%)28. The AKIKI trial randomized 620 critically ill patients with need of mechanical ventilation and/or vasopressors and KDIGO 3 AKI to receive RRT after 6 h of randomization versus standard of care based on conventional indications (BUN > 112 mg/dL, oliguria > 72 h, volume overload, etc.). The study showed that 60-day mortality was 49% versus 50% in the intensive versus standard arm, respectively. Further, patients randomized to the intensive strategy had more vascular access complications and only 51% of patients randomized to the standard arm required RRT due to death or recovery29. The IDEAL-ICU randomized septic patients with severe AKI to receive RRT after 12 h of randomization versus 48 h if no kidney recovery and standard indications. In this study, 90-day mortality was 58% versus 54%, respectively, and only 62% of patients randomized to the standard strategy received RRT. Importantly, this study was stopped early due to futility30. The most recent multicenter, multinational STARRT-AKI trial randomized 2927 critically ill patients with KDIGO 2 or 3 AKI in whom there was clinical equipoise between intensivists and nephrologists regarding RRT initiation to an early start 12 h after randomization versus 72 h if no recovery or standard indications. This study showed that 90-day mortality was 44% for both groups and that only 62% of patients randomized to the standard strategy required RRT. Importantly, 90-day dependence of RRT was higher in the intensive versus standard group (10% vs. 6%)31. The AKIKI 2 trial randomized 278 critically ill patients with KDIGO 3 AKI and oliguria for more than 72 h and/or BUN > 112 mg/dL to receive RRT at 12 h of randomization or after urgent indications (K > 6 mg/dL, pH < 7.15, pulmonary edema or BUN > 140 mg/dL). RRT-free days were similar in both groups (12 vs. 10 days, respectively), and there was a higher risk of mortality in the late versus early group (HR 1.65, 95% CI 1.09-2.5, 32. After a decade of conducting the aforementioned trials, the main learning points are: Early/intensive strategies (before standard indications) do not provide additional benefits and could carry more complications such as RRT dependence. Similarly, waiting longer than standard indications does not confer additional benefits and could be harmful. In summary, the optimal approach to initiation of RRT conveys a careful dynamic monitoring of standard indications and precision in solute and fluid goals of therapy.
Types of renal replacement therapy
CRRT has become the preferred modality in critically ill patients due to its precision, safety, and flexibility, but RCTs available to date have not demonstrated a significant difference in mortality between CRRT and intermittent RRT (e.g., HD or PIRRT/SLED)33. These trials have faced methodological challenges, such as exclusion of hemodynamically unstable critically ill patients, small sample size, heterogeneous prescriptions and delivery of RRT, treatment crossovers, and withdrawal of life-sustaining therapy, which yield caution in the interpretation of results34. Further, one should note that these RCTs were conducted over 15 years ago, and therefore technological advancements in the field are not accounted for35. In contrast, recent observational studies have shown that CRRT is associated with better short- and long-term kidney recovery and independence from RRT compared to intermittent hemodialysis36-40. Importantly, CRRT is preferable to intermittent HD in specific clinical scenarios such as patients at risk of or with cerebral edema and elevated intracranial pressure, management of severe dysnatremias, and hemodynamic instability41-44. Another consideration is that critically ill patients can present with multiorgan dysfunction and CRRT can work as a platform to provide non-kidney organ support such as hemodynamic, respiratory, hepatic, and hematologic support. Furthermore, CRRT can be instrumental in the care of complex critically ill patients by enabling the personalization of RRT that meets the clinical demands of patients during the acute phases of critical illness34.
PRECISION CONTINUOUS RENAL REPLACEMENT THERAPY
Critically ill patients are complex and have multiorgan failure. Therefore, a single extracorporeal organ support intervention may be insufficient to impact patient outcomes if not coupled with other multiorgan support. Further, the complexity of critically ill patients makes the "one size fits all" approach of CRRT deliverables such as dose, modality, and timing to be also insufficient to positively impact important clinical and patient-centered outcomes. Precision CRRT is the use of individual patient and machine-level data to personalize the prescription, delivery, and monitoring of the therapy to accommodate specific solute and volume goals. Examples of data parameters that can assist in this process include fluid balance, solute control, residual kidney function, inflammatory markers, hemodynamics, comorbidities, medications, and CRRT key performance indicators. To this matter, the 17th ADQI consensus developed a series of concepts, summary of key performance indicators, and consensus benchmark recommendations focused on precision CRRT12,13:
Precision continuous renal replacement therapy on patient selection and timing
The timing of RRT should be considered when the metabolic and fluid demands exceed the total kidney capacity. The demand for kidney function is determined by non-kidney comorbidities, the severity of the acute illness, and the fluid burden, while the kidney capacity can be estimated with traditional static measures such as creatinine or urine volume, or with more dynamic and specific markers of kidney dysfunction, such as novel injury/functional biomarkers, kinetic GFR, or real-time GFR. Once the decision to start CRRT has been made, it should be started as soon as possible and the balance of metabolic demands and residual kidney/CRRT capacity should be evaluated regularly to adapt and personalize the therapy45. Although this concept has a theoretical clinical sense, it requires effective methods and tools to dynamically evaluate the demand/capacity ratio and be prospectively tested and implemented in prospective studies.
Precision renal replacement therapy on selecting and changing modality
The modality of RRT should be based on the availability of technology and the capability of the personnel to manage and monitor the therapy. As mentioned previously CRRT is preferred in situations where metabolic fluctuations and fluid shifts are not well tolerated such as hemodynamic instability and intracranial hypertension. Transition between modalities should be considered when the metabolic and fluid demands can be achieved with an alternative technique and a benefit can be obtained by changing modality. Potential benefits of changing from continuous to intermittent modalities when tolerated by the patient are: Overall less extracorporeal circuit contact, early mobilization/physical rehabilitation of the patient, early transfer outside the ICU, and health-care cost savings45.
Precision continuous renal replacement therapy on dosing, solute control, and quality indicators
Delivered dose and technological factors will have a direct impact on intended (selective) and non-intended (non-selective) clearance of solutes and acid-base balance. Factors such as loss of filter area due to clotting, loss of permeability due to clogging, clotting and concentration polarization, and intended and unintended treatment interruptions can considerably affect the delivered CRRT dose46. Therefore, dose delivery should be assessed at least every 24 h in the form of effective treatment hours (intensity), and dosing should be designed to target specific goals of selective solute control. Suggested key performance indicators are based on small molecule effluent saturation (> 80%), prescribed to delivered dose ratio (> 80%), prescribed to delivered net ultrafiltration rate and CRRT fluid removal (> 80%), effective treatment time (> 90%), and mechanical circuit indicators (0 alarm events, < 20% of catheters with dysfunction). One should note that these benchmarks are consensus-based and not yet validated in prospective studies13,47,48.
CONTEMPORARY EPIDEMIOLOGY OF CONTINUOUS RENAL REPLACEMENT THERAPY
Fifty years of CRRT have been characterized by technology, safety, availability, research, and efforts to standardize practice; however, still today, CRRT practice remains heterogeneous among centers and countries, particularly at non-academic centers and low and low-middle-income countries where the implementation of CRRT programs can be challenging.
In this context, the contemporary epidemiological evaluation of CRRT practice could further improve the standardization and delivery of CRRT. To this matter, the recently published multicenter CRRnet study covered the last decade of practice of CRRT among five academic centers in Canada and the United States including a total of 1106 critically ill patients with AKI. Heterogeneity in patient characteristics and CRRT delivery among centers was confirmed in this study, although some aspects such as CRRT dose are more standardized currently. Overall hospital mortality was 59%, and survivors were younger and had less comorbidity and less acuity of illness compared to non-survivors. More than half of patients started CRRT due to to oligo/anuria (56.2%) and fluid overload (34.3%), and sepsis was the most common condition predisposing to AKI (45.6%). The median prescribed CRRT dose was 31.3 mL/kg/h (25.6-40) and the most common modality utilized was CVVHDF (80.5% of patients). The preferred anticoagulation strategy was regional citrate anticoagulation (45.8%) and no anticoagulation was preferred in 47.7% of patients. The authors concluded that these data highlight the need for establishing benchmarks of CRRT delivery performance and patient outcomes. CRRTnet shows a contemporary view of CRRT prescription and delivery and therefore the results reflect the evolution of therapy and patients that receive it. While technology has evolved, patient complexity has also increased and therefore, there is a lot to be done to improve the practice of CRRT6.
WHO SHOULD PRESCRIBE CONTINUOUS RENAL REPLACEMENT THERAPY AND PRIMARILY MONITOR THE TREATMENT?
In the last decade and with the growing accessibility and utilization of CRRT in the ICUs, the controversy of who should prescribe and monitor it in critically ill patients has arised. Intensivists are more available to initiate and adjust the prescription in the ICU and have comprehensive training in multiple organ support49. On the other hand, the nephrologists possess the expertise and experience required for prescribing and managing all forms of RRT and can better serve patients throughout the AKI continuum from diagnosis to recovery50. Both approaches acknowledge the importance of collaboration between nephrologists and intensivists, as well as other health-care professionals in the ICU, while delivering CRRT. Finding a common ground and ensuring the patient's well-being should be the central focus of this controversy. It is crucial to strike a balance between the expertise of nephrologists in kidney care and the comprehensive knowledge of intensivists in managing critically ill patients51.
TOWARD THE NEXT 10 YEARS
Artificial intelligence
The use of artificial intelligence (AI) may further advance CRRT delivery. Proposed clinical applications for AI in CRRT are: Risk-classification to accurately predict mortality and kidney recovery and improve bedside decisions, personalized patient selection and initiation of CRRT, precision in dose delivery, anticoagulation management, prolongation of circuit patency, and clinical subphenotyping that could promote precision CRRT52. Research efforts in this aspect will evolve in the coming years.
Novel blood purification and sequential multiorgan support therapies
The concept of blood purification in the critically ill patient has been present for several years, in the form of therapeutic plasma exchange, adsorptive membranes, plasma perfusion, and hemoperfusion, but there has been hesitation to its use given the lack of mortality benefit. In recent years, blood purification has become commercially and widely available, especially with hemoperfusion columns that focus on patient selection (e.g., specific patient populations and windows of intervention in the course of septic shock). Research and development in this area will focus on personalized medicine and endpoints other than mortality that could prove value-based care25,53,54.
Challenges and limitations for the next 10 years
Research and innovation in the coming years should focus on aspects that could improve and standardize the practice of CRRT through implementation science, including the validation of the proposed key performance indicators. Multinational and multicenter data repositories that are sustainable and represent diverse large patient populations are key to capture aspects of CRRT care that need additional research and create strategies for making CRRT more equitable to patients in low-resource settings. With the growing popularity, safety, and ease of use technology, CRRT is now more available in non-academic centers and low-middle-income countries. Therefore, education, training, and dissemination of best practices should be prioritized using accredited educational programs focused on CRRT, and inclusion of advanced training in CRRT in nephrology and critical care residency programs. Resource allocation and cost-effectiveness research should be promoted especially in low and low-middle-income countries55 (Fig. 1).
CONCLUSIONS
This review encompasses a snapshot of the progress made in CRRT over the past 10 years, highlighting salient technological advancements, established and evolving evidence, and future challenges in clinical research and implementation science. The accumulation of knowledge and experience gained over the past years has significantly advanced the field of critical care nephrology, paving the way for improved care processes and outcomes for critically ill patients with AKI, albeit heterogeneity, and inequity in CRRT practice exist. As we continue to explore new avenues and confront ongoing challenges, this review serves as a valuable resource for trainees, health-care professionals, researchers, and policymakers working toward optimizing CRRT utilization and deliverables.











 text new page (beta)
text new page (beta)


