HOME TREATMENTS
Peritoneal dialysis (PD) and hemodialysis (HD) programs performed at the patient's home have significant advantages compared to HD in the hospital or at an HD center. Various studies have provided evidence of the benefits of the two home modalities in important outcomes such as more prolonged survival, better quality of life, lower transportation costs, greater patient autonomy, and clinical benefits, including better control of blood pressure and phosphorus1-6.
Other significant advantages of home dialysis are the greater availability of time for school activities for children or young patients and more time for recreational activities and family and social life. It also avoids the inconvenience of traveling long distances and long times to attend HD centers. More importantly, home treatment costs are lower in-home therapies than in hospitals or HD centers7-9.
Despite these valuable advantages, home PD and HD have minimal use in developed countries8,10. The causes of underutilization vary; the reimbursement system is one of them since payment is generally higher for in-center HD11-13. Other frequent limitations include patients' or caregivers' fear regarding their ability or that of their caregivers to learn the use of dialysis devices because they require some computer skills and periodic retraining. The patient's perception of medical monitoring is less frequent and it could impact clinical outcomes and the belief that the therapy itself demand more self-care activities without immediate specialized supervision. The patient often perceives home care as a burden for the family because it is their responsibility to identify problems that require the intervention of the doctor, nurse, or service providers, which can reduce the time to carry out their work activities and decrease the economic income of the members who support the family economy14,15.
Physicians may underutilize home dialysis because of perceived technical complexity, concerns about complications, limited resources and infrastructure, lack of awareness, bias and tradition, and the concept of a lack of control of the patient's treatment. In addition, physicians may fear their inability to determine the patient's adherence to dialysis treatment.
THE HELP OF REMOTE MONITORING
With the advances in digital information technologies, wireless devices have been developed that allow automated PD (APD) devices to be connected with web based platforms and allowing a bi-directional connection with doctors' or nurses' computers or other devices to a clinical portal with a user-friendly interface. Here, they can review a treatment summary and quickly identify treatment deviations and, when needed, remotely adjust the prescription thorough device setting adjustments. APD-assisted remote monitoring (RM) (APD+RM) devices offer a new and compelling opportunity to recognize and solve clinical problems without moving the patient from home to the hospital15-19. Although there are few studies on telemedicine in the PD population, successes in other areas support its technical feasibility. The acceptance of the technology by the patient is very high and has also made it possible to improve clinical outcomes in the population with end-stage renal disease20,21. As examples in non-renal patients, several meta-analyses have shown the benefits of structured telephone support and the use of implantable electronic devices in the caring for patients with cardiac disease22,23. In addition, diabetes-related telemedicine programs allow timely changes in drug management and lower hemoglobin A1c percentage more effectively than standard-of-care monitoring24. However, there is still resistance and little confidence in using RM in home dialysis. Nephrology must be faster to accept telehealth technology in its daily practice, partly due to information security regulations. The need to guarantee the security of patient data is a relevant concern.
Current RM devices allow the recovery of information generated from APD devices; the patient does not need to collect data in a log as with conventional management. The RM device comprises a series of sensors, counters, and microprocessors that collect, analyze, and integrate information and present it as numerical indicators. The clinical administrator defines the configuration flags (red or yellow) according to the limits previously selected, at what moment the warning or danger signals or flags will light up and obtain a patient snapshot with information in graphs, prescribed device program, solutions used, UF and therapy details, in a daily basis or in periods of 7 or 30 days. The information stored is of significant volume, so it must be transmitted to a repository in the "cloud." From there, it can be consulted by the health team25.
This new technology requires the establishment of new clinical surveillance routines and levels of responsibility. In real life, the daily use of the APD+RM partly resembles "the clinical round" in a nephrology department following algorithms to review and manage detected deviations. It is possible to quickly consult the single image treatment dashboard the number of flags: preventive (yellow) or risk (red); the clinical administrator creates user accounts for the clinical staff assigning specific roles and levels of information visibility. If a patient with a recurrent deviation in the therapy is identified during daily dashboard assessment, the review of the treatment summary is recommended. With time, the clinical staff develop experience and its able to identify the patient's behavior patterns, and design effective and developing proactive intervention protocols to solve them or detect the need for call the patient to preemptively adopt solution strategies, which can be simple or require the intervention of the nephrologist, the nurse or the technician responsible for the equipment's operation. The patient can also be called for a clinic visit to solve more complex problems25. Table 1 shows some variables necessary for managing APD patients and the yellow and red flags limits the clinical health team set up, and Fig. 1 contains a suggested algorithm for routine review.
Table 1. Set-up limits for flag alerts
| Yellow flag | Red flag | ||
|---|---|---|---|
| Treatment duration | Lost treatment time | 15 min | 30 min |
| Treatment variations | Lost dwell time | 15 min | 30 min |
| Lost therapy volume | 5% | 10% | |
| Drain finished early | 2 | ||
| Initial drain variation | 50% | 100% | |
| High drain volume | X | ||
| Patient intervention | Bypass count in infusion or dwell | 2 | |
| System alerts | Events during treatment | 5 | 10 |

Figure 1. This graph is a suggested algorithm for daily routine evaluation of automated peritoneal dialysis+remote monitoring platform.
The use of APD+RM has the potential to closely monitor patients and obtain essential data without adding workload to the patient, such as the volumes infused, the total and effective duration of dialysis, the ultrafiltration obtained, and the presence of interruptions or changes in flow infusion and drain pattern of the solutions. The patient's information is valuable but frequently needs to be more detailed; it may contain involuntary forgetfulness, may be influenced by subjective aspects, or be frankly modified.
Treatment adherence
The APD+RM allows us to know the patient's treatment adherence, including daily connection and treatment time. With this information, it is possible to objectively analyze the patient's adherence to the indications, the omissions, the reduction of the volumes infused, or the reduction of the dwell time of the liquid in the cavity. Several studies have shown that surveillance with APD+RM makes it possible to detect connection omissions and take action to improve treatment compliance26-28. Non-adherence is a frequent problem. It has been reported in ranges of 2.6–53% for PD in general and 5%–20% for APD in particular28-30. Still, it is recognized to be most likely underestimated since these are self-reported data. Non-adherence to dialysis is a significant risk factor for mortality and hospitalizations29,31.
In a recent study, in which one of the monitoring systems available in our country (HomeChoice Claria® + Sharesource platform®, Baxter) was used, APD+RM reduced non-adherence from 10.5% to 4.9%. When a subgroup of known non-adherent patients was analyzed, the change was from 48.4% to 12.4%. Simultaneously, the serum Potassium and C-reactive protein serum concentrations were reduced32.
Much of the information on critical clinical outcomes using APD+RM such as mortality, hospitalizations, and technique failure comes from small studies involving few patients, and the conclusions may be underpowered. However, encouraging results were found in a recent systematic review with a moderate risk of deviations between the original studies33, which included seven clinical trials with patients receiving RM treatment. Five studies were cohorts and two were controlled clinical trials; six were in APD and one in HD. The cohorts included 9758 patients and in the RCTs, 217. Mortality was not reported in any study, and there was no uniformity in the monitoring time.
Hospitalizations
Despite the limitations, it was possible to establish that the number of days of hospitalization was lower in three of the studies in patients with APD+RM33-35. The number of hospitalizations for any cause was lower in the same group. Still, in one study, the results were the opposite35. When the specific causes of hospitalizations were considered, defined as the sum of PD-related infections + overhydration + access dysfunction35 or infections + fluid overload36, the difference tended to be less in RM. However, it did not reach statistical significance.
Technique failure
In five studies analyzed, technique failure as a cause of transfer to another dialysis modality was lower with RM34-38. When three studies containing adequate information were pooled, the reduction was significant in favor of RM in prevalent patients.
Quality of life
Quality of life was measured in two studies37,39. In one of them, the rating was equal in-patient satisfaction and commitment of the dialysis team37. Conversely, patient satisfaction was better in RM but the opposite in staff mood39.
Mortality
Mortality is an issue that needs to be adequately addressed. There are no studies, or they need to have the appropriate design. In a study carried out in Colombia, with a design to compare APD+RM versus APD patients matched with propensity score, it was found that the stay was 3.2 months longer in the APD+RM group compared with APD. However, the difference was dominated by less technique failure, as the difference in mortality did not reach statistical significance40.
APD+RM from the perspective of the clinical team
RM is generally well accepted, but it undoubtedly requires training from the nurse and the nephrologist. In a survey conducted among experienced and up-to-date nurses and physicians in the management of PD, both expressed the opinion (87% and 100%, respectively) that, in children, RM is a valuable tool to detect clinical problems with objective and reliable data. In addition, 80% considered that it is possible to act preemptively. Both nurses and physicians (67% and 59%) considered that nurses can address problems before physicians, and more than 75% believe that monitoring of patients is improved and time is saved. The nurses (73%) rated the RM as a proper nursing tool, but the physicians (41%) did not41.
The nurses thought that they knew more about RM than the doctors (73%), that treatment adherence is improved, and that it allows the participation of the patient with the PD team. Non-adherence can be more adequately documented in the daily connection and the early termination of the session, the shortening of the drainage time, or avoiding the alarms. The result is a more successful prescription41.
Regarding the disadvantages, half of the patients or their caregivers feared they could not handle APD+RM when starting the therapy, and 80% indicated that they could only talk to the nurse about the system. Among the most relevant advantages, patients or their caregivers considered that they had more satisfactory monitoring, giving them more security. Avoiding entries in a log was gratifying and improved the quality of sleep in patients and caregivers41.
In another study with patients, doctors, and nurses, it was found that APD+RM is perceived as a convenient and efficient option, which gives the patient security and, because they feel better monitored, they have greater adherence to treatment and more autonomy. The system needs to be more accurate regarding protecting their data and the reliability of the information collected by the device. With RM, the characteristics of the doctor-patient relationship change; the patient-initiated communication decreases, and the doctor has a more significant workload and responsibility. The doctor requires more training in technology and management and often makes more changes to the treatment prescription42.
Economic analysis
Despite the advantages described, the economic aspects of APD+RM still need to be explored. In a study with a crossover design, with 15 patients in consecutive randomized periods with and without RM, the primary outcome was patient satisfaction, and APD+RM had better scores in the perception of effectiveness and convenience. On the other hand, the secondary outcome, which was the consumption of resources, measured as consultations in the 12 weeks of observation, were significantly reduced as was the time of scheduled visits43.
In a simulation study, potential resource use scenarios of APD therapy in patients with and without RM in three European countries were developed with the help of 11 teams of nephrologists and nurses. Hospitalizations were reduced between one and two, visits to the Emergency Department between two and five, and visits between four and eight. The savings in all scenarios were USD 23,364 in the United States, USD 11,477 in Germany, and USD 7088 in Italy44.
In a pilot study with 21 patients monitored for 6 months in conventional APD and another period of 6 months with APD+MR, the savings for the health system were calculated at €335 (average per patient-month). The costs, represented by the loss of time of the patient and the caregiver, were calculated at €685 (average per patient-month)45.
Although the two studies mentioned are with a small number of patients and monitored in a short time, they are consistent with the results of the clinical outcomes described.
Why does RM have advantages in patient care?
RM has no direct effect on APD itself. It does not influence the composition of dialysis solutions, nor does it affect peritoneal permeability or modify the dialysis prescription. The advantage lies in the fast, reliable, and timely information it collects. The daily monitoring of the treatment provides information about how the patient is managing their therapy at home and adherence to the prescription. It allows to identify the number and types of alarm complications, such as catheter malfunction; with this, the clinical team can establish preemptive attention protocols addressing the potential complications.
When evaluating new technologies, it is essential to mention how they influence relevant variables of patient care from a biological point of view. Some of these variables are schematized on the left side of Fig. 2, and on the right side of Fig. 1, the specific RM applications that are related to them are indicated.
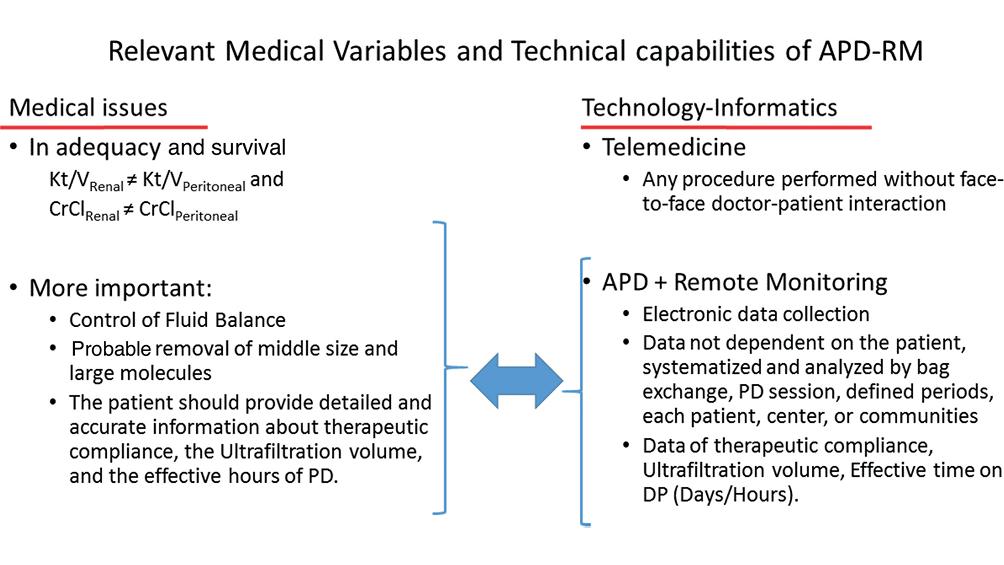
Figure 2. Schematic view of relevant medical variables in managing patients in automated peritoneal dialysis and the advantages of automated peritoneal dialysis+remote monitoring with potential impact on those variables.
The first lines on the left emphasize that peritoneal Kt/V and creatinine clearance do not have the same biological value as renal Kt/V and creatinine clearance. Other measurable variables in APD that have been considered or are candidates to be considered as indicators of adequacy of APD are also mentioned. One of them is the control of extracellular volume. Fluid overload is a frequent complication in PD patients and is a risk factor for all-cause mortality and all-cause and cardiovascular morbidity46,47.
RM is a valuable aid in the managing of fluid overload. The ultrafiltration volume is data that can be obtained for each replacement per day, total treatment, or in 7/30-day periods. With this information, timely adjustments can be made to the prescription to achieve UF needs. It can be used long-term to monitor loss of ultrafiltration capacity, a possible complication after years of PD treatment. Although the daily UF has wide variations of up to 20%, careful observation of alarm signs when values below the recommended values are obtained, can lead to an early suspicion of evolving peritonitis46. These advantages are noted on the right side of Fig. 2.
An objective feature of the APD+RM is the unbiased report of treatment adherence. The RM counts the number of days the patient connects and the effective time each session lasts. With this information, the dialysis team can encourage the patient to achieve greater therapeutic compliance and optimize the use of resources.
The most used parameter for the prescription or adequacy of the PD has been the Kt/V of urea; however, obtaining values of this indicator > 1.7 does not impact patient survival48. However, it should be emphasized that Kt/V only represents small, water-soluble, and non-protein-bound. The importance of obtaining an adequate clearance of solutes of molecular weight molecules between 12 and 50 kD in survival has not been analyzed in PD. In HD, the clearance of β2m improves the control of anemia and nutrition49. However, in HD, there is not enough information about the effect of the clearance of medium-sized molecules on survival50.
The clearance of medium- or large-sized molecules with PD depends on the hours of dialysis. It has been shown that long long dialysis effective time increase the clearance per hour of medium- and large-size molecular weight molecules51. Recording the effective hours of dialysis can provide indirect information about the clearance of medium-sized molecules, although this is only a guess.
APD+RM allows detailed recording of the flow of dialysis solutions' continuity and inflow and outflow rates. Graphic flow analysis provides valuable information to identify catheter obstruction, even partial or intermittent. The time of occurrence and the flow pattern allow us to suspect specific causes, such as migration52, kinking or compression during sleep. Two examples are shown in Figs. 3 and 4. The graphic information indicates the time of the event, if alarms were issued, what the patient's behavior was, and if they turned off the alarm or ended the session early due to the impossibility of resolving the problem.
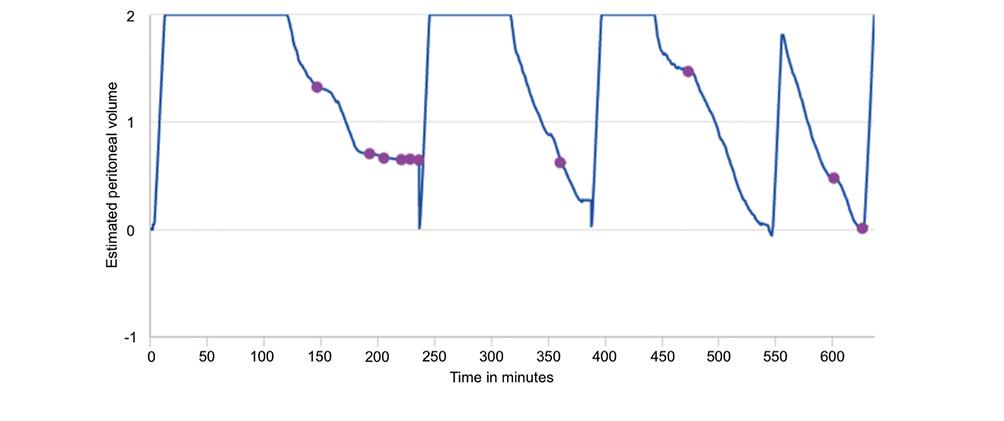
Figure 3. The graph shows the behavior of the dialysis solution flows. The dots indicate the occurrence of problems and alarms in the drains. Besides, the shortening of the effective dwell time is appreciated.
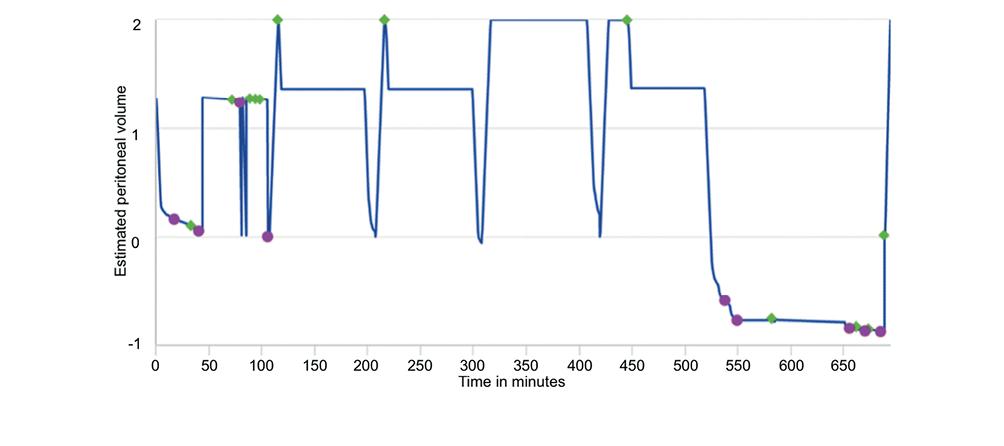
Figure 4. The graph shows the behavior of the dialysis solution flows. The dots indicate the occurrence of problems and alarms in the drains. The diamonds indicate the interventions of the patient to turn off alarms, reduce the dwell time of the solutions in the peritoneal cavity, and terminate the therapy early.
THE FUTURE OF APD+RM
The application of home dialysis therapies, particularly APD+RM, depends on several factors. One of the most important is the nephrologist's conviction of this modality's advantages in the management and well-being of the patient. The confidence of the nephrologist depends, in turn, on their experience and the access to the network, and the access of the patient to a phone line. Another determining factor is the lifestyle of the patient, their skill and safety in handling the devices, and the support they receive from the health team, as well as their confidence in protecting their data.
CONCLUSIONS
It must be highlighted the need for controlled clinical trials that demonstrate on time the benefits of APD+RM in the most important outcomes, such as patient and technique survival, morbidity, quality of life, and cost-benefit analyses. The latter is essential for decision-makers to ponder adopting new technology based on optimizing available human and financial resources. The available data are promising; they will indeed be confirmed.











 text new page (beta)
text new page (beta)


