INTRODUCTION
Hallucinations are vivid perceptual experiences involving all sensory systems that occur without the input of actual stimuli. Thus, hallucinogens are drugs that induce cognitive and perceptual distortion of reality. Several ethnic groups have traditionally used hallucinogens in ritual settings, but this practice is now common in many modern societies. The scientific interest in the effects of hallucinogens in controlled clinical settings has been documented since the late 1940s. In the 1950s, clinical research focused on studying LSD as a coadjuvant in psychotherapy and as a model for understanding psychosis and treating depression1. During the 1960s, there was a surge in hallucinogen use related to the social movements of the time. Later, the Controlled Substances Act of 1970 placed hallucinogens in Schedule I, the most restrictive category. In 1975, the U.S. National Institute of Mental Health withdrew the research funding for human studies on hallucinogens' effects. The primary reason was that clinical research (mainly pilot studies) conducted at that moment did not provide sufficient evidence of the safety or therapeutic efficacy of hallucinogen drugs for any psychiatric condition. After a pause of almost three decades, the scientific community resumed hallucinogen research in the 2000s with a particular interest in the therapeutic potential of these substances. As of today, only after marijuana, hallucinogens are the most used illicit drug among young adults in the U.S2.
Although hallucinating is a subjective experience known by humans through personal experience or eyewitnesses, the users' physiological alterations and the mechanism of action of hallucinogen drugs are a matter of extensive preclinical and clinical investigation. This paper aims to review the effects, mechanisms of action, risks, and therapeutic potential of main hallucinogens.
CLASSIFICATION OF HALLUCINOGEN DRUGS
Many compounds can produce hallucinations at toxic doses. However, the pharmacological criteria to consider a drug as a hallucinogen, as originally proposed by Humphry Osmond in the 1950s, are the production of perceptual alterations of reality at otherwise non-toxic doses. Although there are several classifications of hallucinogens, for this review, we will distinguish two main classes (a) serotonergic hallucinogens, represented by the lysergic acid diethylamide or LSD, and (b) dissociative anesthetics such as ketamine and phencyclidine (PCP). Classic hallucinogens include LSD, psilocin (in "magic" mushrooms), and N,N-dimethyltryptamine (DMT, in ayahuasca), among others3. These compounds have chemical structures similar to serotonin (5-hydroxy-tryptamine, or 5-HT) because they are tryptamines, characterized by the presence of an indole group (a six-member ring attached to a five-member ring containing nitrogen). Mescaline (in peyote) is also a serotonergic hallucinogen, but has a different chemical structure, because it is a phenethylamine, not a tyramine. Dissociative anesthetics are PCP and ketamine. Based on their chemical structure, these drugs are cyclohexanone derivatives, also called arylcyclohexylamine derivatives (Fig. 1)4.
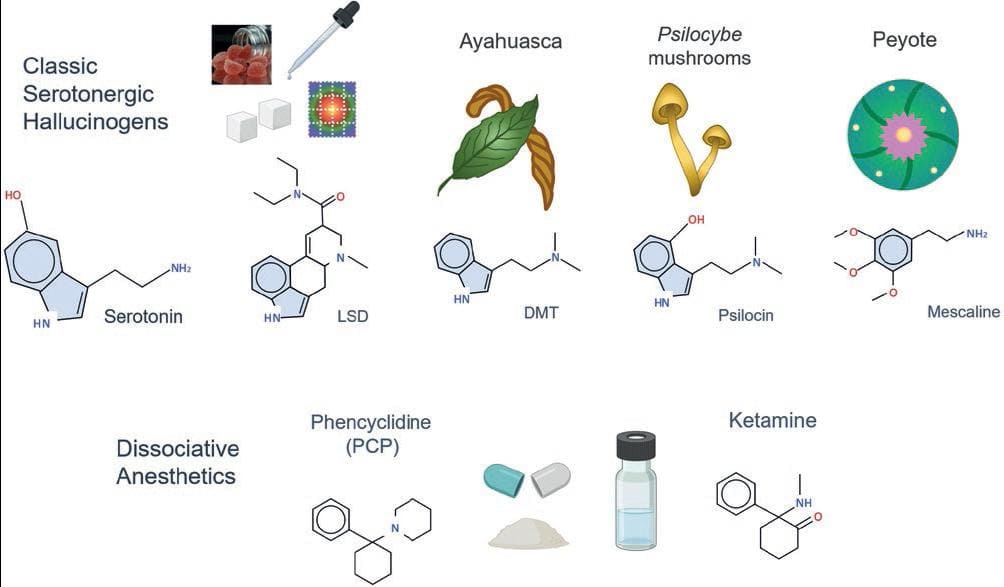
Figure 1. Classification of main hallucinogen drugs. Classic hallucinogens have chemical structures similar to serotonin. They include LSD (a synthetic drug), DMT (ayahuasca's psychoactive substance), psilocin (obtained from Psilocybe fungi), and mescaline (the active substance in peyote). Phencyclidine (PCP) and ketamine are synthetic dissociative anesthetics with hallucinogen effects.
EFFECTS OF CLASSIC HALLUCINOGENS
LSD is the prototypic classic hallucinogen. Its initial effects involve sympathomimetic responses, including increased blood pressure, accelerated heart rate and respiration, dry mouth, nausea, sweating, appetite loss, and pupil dilation. In general, within a relatively short time after the onset of these responses, individuals begin to experience illusions and visual hallucinations. These hallucinations are described as moving, bright, colorful figures, and geometric patterns. One common effect of hallucinogens is the crossover of senses, also called synesthesia, where smells may be heard, or colors may be tasted. Although less frequently, it is also possible to experience auditory hallucinations.
Hallucinogens also produce pronounced psychological changes described as an intense emotional insight, and transcendent or spiritual experiences. LSD subjective effects include mood swings (from a peaceful, hilarious state to an anxiety attack), attention impairment, misperception of time, and depersonalization/derealization. This last effect has been described as a sense of unreality and the sensation of being detached from the environment, body, thoughts, and feelings, which could be unpleasant, leading to severe anxiety. Ego dissolution, or a compromised sense of "self," also occurs, characterized by "the reduction in the self-referential awareness that defines normal waking consciousness, ultimately disrupting self-world boundaries and increasing feelings of unity with others' and one's surroundings"5,6. A common term used to summarize that someone is experiencing the effects of hallucinogens is "tripping," and an unpleasant experience is usually called a bad trip.
Although pharmacological factors (dose and tolerance) are determinants to experience the effects of hallucinogenic drugs, psychological factors also play a crucial role in their behavioral effects and subjective experience. The "set and setting" concept refers to the different response that a person can have to hallucinogens depending on mind state (e.g., relaxed, depressed, anxious) and the setting in which the person consumes the drug (e.g., alone or in groups, in familiar or unfamiliar situations, in a clinical setting or at a music festival)7.
Classic hallucinogens do not produce compulsive drug-seeking behavior, which is why they are not considered addictive. However, these substances can induce acute tolerance to their effects. Thus, a second dose within a short period after the first dose will not produce the same effects. This fast development of tolerance to a single dose lasts several days and limits the daily use of these substances. Classic hallucinogens differ in efficacy, potency (the amount of drug needed to produce a specific action), time to peak effects, and duration. The following sections review the effects and mechanisms of action of the main hallucinogens studied.
LSD
LSD is an ergot derivative. Ergot is a black fungus (Claviceps purpurea) that grows on rye. Conventionally, in the regions where this fungus grew, pregnant women drank water infused with ergot to facilitate labor work. Ergot was also responsible for San Anthony's fire or ergotism, a condition characterized by strong blood vessel constriction that could result in gangrene. Albert Hoffman synthesized LSD in 1938 while looking for ergot derivatives with therapeutic effects. However, he did not experience LSD's effects until 1943, when the formal research on hallucinogens began.
LSD is one of the most potent drugs known, with an effective oral dose of only 25-50 μg, although the doses available on the street market can reach up to 100-200 μg. The half-life of LSD (time needed to decrease 50% the drug concentration in bloodstream) is approximately 3-4 h, but its effects last longer, from 9 to 12 h, in most people8. LSD is available as tablets; however, due to its potency, it is usually diluted and applied to blotting papers, gummies, or sugar cubes that are dissolved in the mouth. Because LSD is a tryptamine, it has structural similarity to serotonin 5-HT and acts as a partial agonist of several 5-HT receptor subtypes (Table 1). Several pharmacological experiments indicate that the effects of LSD and other serotonergic hallucinogens are mainly mediated by 5HT2A receptors (see below). These receptors are highly expressed in the cortex, striatum, amygdala, hippocampus, and on layer V of cortical pyramidal neurons. Other molecular targets of LSD include dopamine D1 and D2 receptors9. At micromolar concentrations, LSD activates the trace-amine-associated receptor 1 (TAAR1), a relatively recently described receptor stimulated by the endogenous ligands tryptamine and β-phenylethylamine. Neurons and glial cells express mainly intracellular TAAR1 receptors, now recognized as molecular targets for many psychoactive substances10.
Table 1. Affinity (Ki; nM) of serotonergic hallucinogens for various receptors. Based on reviews10,18,22,23
| Hallucinogen | 5 HT1A | 5HT2A | 5HT2C | D1 | D2 | TAAR1 |
|---|---|---|---|---|---|---|
| LSD | 3 | 3-4 | 15 | 310 | 25 | 10,000 |
| Psilocin | 123 | 49 | 94 | >14,000 | 3700 | 1400 |
| DMT | 75-183 | 127-237 | 360-424 | 6000 | 3000 | 15,000 |
| Mescaline | 4100 | 6300 | 17,000 | >14,000 | >10,000 | 3000 |
TAAR1: trace-amine-associated receptor 1.
AYAHUASCA – DMT
Ayahuasca is a liquid concoction traditionally used in ceremonies by Amazonian groups and members of syncretic religious groups, such as Santo Daimo in Brazil. More recently, ayahuasca has emerged as a commonly used beverage in Westernized countries, often without clear regulations. Ayahuasca is prepared by boiling water with the leaves of a bush (Psychotria viridis, also known as "chacruna") and the stem or bark of the Banisteriosis caapi liana. The psychoactive substance contained in the leaves of P. viridis is DMT, another tryptamine similar to serotonin. In addition, B. caapi contains β-carbolines, also known as harmala alkaloids, which inhibit DMT metabolism, rendering it orally active11.
DMT is present in over 50 South American plants, including Diplopterys cabrerana and Desmanthus illionoensis. Small quantities are also present in humans. The concentration of DMT in P. viridis ranges between 0.1 and 0.66%. The amount of DMT in ayahuasca varies from 0.1 to 40 mg T12,13. DMT alone is inactive orally due to its rapid deactivation through mono amino oxidase A (MAO-A) enzymatic action. This enzyme breaks down serotonin, dopamine, adrenaline, noradrenaline, and DMT, reducing bioavailability. MAO-A is present in the liver, gastrointestinal tract, blood, kidney, and brain, rendering DMT inactive when ingested. If DMT is smoked or insufflated, it produces an almost immediate effect that lasts less than an hour. When combined with MAO inhibitors, DMT blood concentrations are high enough to enter the brain. The β-carbolines contained in B. caapi: harmine, harmaline, and tetra-hydroxy-harmine (THH) inhibit MAO-A and are weak selective serotonin reuptake inhibitors (SSRI). Harmine also inhibits dopamine transporters, increasing extracellular dopamine levels. Other compounds in ayahuasca have antioxidant and anti-inflammatory actions14.
Ayahuasca and LSD produce similar effects, but their time course is different. Ayahuasca-induced effects begin 30-60 min after intake, reach maximal intensity between 1 and 2 h, and last approximately 4 h. Nausea, diarrhea, and vomiting are very common after drinking ayahuasca, but in the ceremonial context, these effects are considered a purifying purge for the body and soul. There is risk of dehydration, especially if users are far from medical services. Like other classical hallucinogens, DMT produces its actions through 5-HT2A receptor activation but is also an agonist or partial agonist of other receptors including the 5-HT1A, 5-HT2C, 5-HT6, 5-HT7, sigma1, and TAAR1 receptors (Table 1).
MUSHROOMS – PSILOCYBIN AND PSILOCIN
Although hallucinogen drugs use is well documented in ancient cultures for religious and recreational purposes, the Florentine Codex is the earliest known written record of "magic mushrooms" use. This ethnographic manuscript was compiled between 1529 and 1579 in Mesoamerica, particularly in the Aztec culture of Mexico7. There are more than 100 species of the Psilocybe gender of mushrooms. They grow in humid environments in Mexico, several regions of the US, Europe, Asia, and other countries worldwide.
Psilocybe mushrooms, also called magic or psychedelic mushrooms, were introduced to Western culture in the 1950s by R. Gordon Wasson, who published in a popular magazine his experience with psilocybin after participating in a Mazatecan ceremony in Mexico. In 1957, Albert Hoffman isolated psilocybin and psilocin from the mushroom species Psilocybe mexicanum, a sacred mushroom known as "teonanacatl" (meaning God's flesh) by the ancient Aztecs. A few years later, Sandoz Pharmaceuticals distributed Indocybin®, a pill containing 2 mg of psilocybin. Psychologists and psychiatrists used psilocybin in clinical practice and conducted research trials for approximately 10 years in the 1960s and 1970s. However, psilocybin was withdrawn from the pharmaceutical market in 1970 and placed in the Schedule I category, restricting its use in human research due to the lack of conclusive findings in the pioneer studies, the increasing use of psilocybin in non-controlled environments, and the crescent social negative perception7,15. The interest in psilocybin research re-emerged around the 2000s when several groups started testing it to treat psychiatric disorders, including anxiety, depression, and addiction. However, it was until 2020 that the legal status of psilocybin started changing in North America when Oregon became the first state in the US to legalize "magic mushrooms" consumption in supervised settings. These new regulations came into effect in January 2023. In 2022, Colorado enlisted as the second US state to legalize psilocybin mushrooms. When writing this review, California has a pending bill to legalize psilocybin and other hallucinogens, including LSD, mescaline, and DMT. In Mexico, psilocybin is illegal according to the "Ley General de Salud" (General Law of Health) but unenforced for indigenous groups.
Psilocybe mushrooms contain approximately 2-3 mg of psilocybin (4-phosphoryloxy-DMT) per gram of dry-weight mushrooms. The potency of psilocybin, relative to LSD, is approximately 1:100, the typical dose of psilocybin is 5-15 mg. According to its chemical structure, psilocybin is a tryptamine. When hydrolyzed, it produces psilocin (4-hydroxy-DMT), whose hallucinogenic properties are almost 2 times stronger than the parent compound. Psilocybin-induced effects become evident after 20-30 min of mushroom ingestion and reach peak effects within 2-3 h, which lines up with the maximum psilocin concentration in the bloodstream. The half-life of psilocin is approximately 1.5 h, and the mushroom-induced experience generally lasts 4-6 h. Psilocin effects are dose-dependent and include disorganized thought processes, emotional changes, synesthesia, euphoria, sensory illusions, and auditory and visual hallucinations. Since psilocin also alters time perception, users may undergo a non-ending experience, which could be unpleasant. After Psilocybe mushroom consumption, nausea, vomiting, and transient headaches are common15,16. Psilocin is a serotonin transporter inhibitor and a partial agonist of the 5-HT2A receptors but also has an affinity in the nanomolar or micromolar range for the 5-HT2C, 5-HT1A, 5-HT1B, D1, and D2 receptors (Table 1).
PEYOTE - MESCALINE
Peyote (Lophophora williamsii) is a small cactus with hallucinogen effects traditionally used as a sacred plant by native populations from the central and northern part of Mexico (huicholes and raramuries, respectively), native American populations from the United States and some First Nations in Canada. Mescaline (3,4,5-trimethoxyphenethylamine) is the psychoactive substance of peyote and produces effects similar to other classic hallucinogens, including the distortion of thinking processes, sense of time, and self-awareness. In addition, the sense of smell might be enhanced, and visual phenomena occur with closed and open eyes. A unique characteristic of mescaline is the tendency to perceive colorful, symmetrical, and geometric patterns/shapes as in a Cubist painting. Users also believe that they can trespass the limits of Earth, time, and space17.
Peyote tastes bitter, producing intense nausea and vomiting when swallowed. As it occurs with ayahuasca, these effects can be interpreted as a symbolic cleaning phase during the ritual use of the cactus. Peyote can be sun-dried and powdered in 30-150 mg doses. Another form of use is eating fresh buttons or diluting the powdered peyote to drink. The active oral doses of mescaline are between 150 and 700 mg, making it considerably less potent than LSD and psilocybin. In addition, mescaline has a slow onset of action, 2-4 h, and its effects last from 10 to 14 h. Because mescaline is a phenethylamine, it produces stimulant actions like amphetamine or MDMA, including increased heart rate, blood pressure, and pupil dilation. Mescaline is the less potent serotonergic hallucinogen with affinity, in the micromolar range, for the 5HT1A, 5HT2A, 5HT2C, TAAR1, D1, and D2 receptors, among others18 (Table 1).
MECHANISM OF ACTION OF CLASSIC HALLUCINOGENS
As mentioned, classic hallucinogens generally activate the serotonergic system, binding to several receptors, including 5-HT1, 5-HT2A/B/C, 5-HT5, 5-HT6, and 5-HT7, but it is mainly the 5-HT2A receptor activation what mediates the hallucinatory experience. In humans, the selective blockage of 5-HT2A receptors with ketanserin abolishes perceptual and subjective effects induced by psilocybin19. Similarly, ketanserin prevents the neurochemical and behavioral changes produced by classic hallucinogens in animal models. Furthermore, transgenic mice lacking 5-HT2A receptors do not express the typical head-shake behavior produced by hallucinogens; however, this behavior reappears after restoring the expression of 5-HT2A receptors20. It is worth mentioning that not all 5-HT2A receptor agonists have hallucinogenic properties. Classic hallucinogens and non-hallucinogenic 5-HT2A receptor agonists activate different intracellular pathways in cortical pyramidal neurons. In addition to the serotonergic receptors' activation produced by LSD, it also shows intrinsic activity on dopamine D1, D2, and α-adrenergic receptors (Fig. 2). It is believed that the initial effects of LSD are directly related to the activation of the serotonergic system. However, there is a delayed activation of the dopaminergic system21 that might be related to the so-called "bad trips" in humans since they generally occur several hours after LSD intake.

Figure 2. Representation of classic hallucinogens' pharmacological profile, depicting their affinity (Ki values) to different receptors (based on data from Table 1).
EFFECTS OF THE DISSOCIATIVE ANESTHETIC KETAMINE
The first dissociative anesthetic was PCP. Developed initially as an analgesic, PCP was in use until 1967 in the United States, when it was discontinued due to severe adverse effects in some patients, including dysphoria and psychosis. Ketamine was synthesized in 1962 and marketed as a human anesthetic in 1970. Today, ketamine is still used for patients in several countries and as a veterinary anesthetic worldwide. In addition to the effects common to classic serotonergic hallucinogens, ketamine can produce out-of-the-body experiences (feeling detached from the environment and oneself), confusion, vivid dreams, and near-death experiences. It is misused at subanesthetic doses, snorted, and sometimes injected (i.m.) or swallowed4. Ketamine is a mixture of two enantiomers (S)-ketamine and (R)-ketamine. (S)-ketamine produces more analgesia, less salivation, and less delirium than (R)-ketamine. Under control settings and at the proper doses, ketamine and (S)-ketamine (esketamine) are effective novel antidepressant drugs, especially for people with suicidal thoughts and in combination with other traditional antidepressants24.
MECHANISM OF ACTION OF KETAMINE
Ketamine has a complex mechanism of action. It is a non-competitive antagonist of the glutamate N-methyl-D-aspartate (NMDA) receptor (NMDAR). NMDARs are ionic channels of different subunit compositions: GluN1, GluN2, and GluN3. NMDARs typically have two GluN1 subunits and GluN2 or GluN3 subunits. Ketamine has a high affinity for NMDAR with GluN2D subunits, which are abundant in GABAergic neurons and indirectly activates 5-HT2A receptors25. According to the glutamatergic disinhibition hypothesis, glutamate release increases in cortical and subcortical brain regions when ketamine or other NMDA receptor antagonists block the NMDARs expressed in GABAergic neurons. Glutamate activates pyramidal neurons in the prefrontal cortex (PFC) that send their projections to the Raphe Nucleus and stimulate serotonergic neurons, which, in turn, send projections back to the PFC where serotonin directly activates 5-HT2A receptors26. Ketamine also has other mechanisms of action independent of NMDAR inhibition. Among them, ketamine activates the mTORC1 pathway. mTORC1 stands for mechanistic target of rapamycin complex 1. Mechanistic TOR (formerly called "mammalian" TOR) is a protein kinase inhibited by rapamycin, an antibiotic with immunosuppressant effects. mTORC1 is a protein complex that regulates cell metabolism and growth by promoting protein and lipid synthesis, suppressing autophagy, and modulating lysosomal biogenesis27. In addition, mTORC1 regulates neural development. Other NMDAR-independent actions of ketamine include increased brain-derived neurotrophic factor (BDNF) plasma levels28. BDNF promotes neuronal survival and facilitates long-term potentiation. It has been associated with plastic changes in learning and memory and is essential to cognitive function. Dysregulation of BDNF and other neurotrophic factors has been associated with several pathologies, including major depressive disorder (MDD)29. In addition, ketamine inhibits GluN2B-containing NMDARs in astrocytes (reviewed in25). Recent evidence supports that NMDAR antagonism and non-NMDA ketamine actions are involved in ketamine's antidepressant effects21.
RISKS ASSOCIATED WITH HALLUCINOGEN DRUG USE
The adverse effects shared by different hallucinogens include the "hallucinogen persisting perceptual disorder" (HPPD), drug interactions, and potential adverse cardiac effects. The most recent version of the diagnostic and statistical manual for psychiatric disorders (DSM-5) includes the description of HPPD, which refers to the re-experience of the perceptual changes ("flashbacks") a long time after cessation of the drug consumption, which is sufficiently intense to cause a serious disturbance or impairment in function30. The flashbacks can be unpleasant and emotionally traumatic and may co-occur with physical changes, including nausea, vomiting, mydriasis, headache, and drowsiness.
Several drug interactions between classic hallucinogens and other drugs have been described. For example, the combination of hallucinogens and lithium or tricyclic antidepressants (TCAs) exacerbates the neurochemical changes produced by hallucinogens. As a result, this drug combination intensifies the perceptual and emotional experience, which may increase the probability of panic attacks and transitory psychotic states31. In addition, the acute administration of SSRI or MAO inhibitors potentiates the response to LSD and can produce a serotonin syndrome32. This potentially life-threatening condition includes neuromuscular, autonomic, and cognitive symptoms. In the initial stage, individuals show agitation, insomnia, nausea, diarrhea, tremor, and dilated pupils; symptoms can progress to hyperreflexia, excessive sweating, and clonus. Patients with severe symptoms, such as body temperature over 38.5°C, confusion, delirium, sustained clonus, and rhabdomyolysis, must be referred to the hospital33.
Another negative long-term effect of hallucinogens use is the chronic activation of 5-HT2B receptors in the heart, which can produce valvular heart disease (VHD). Due to tolerance development, users are unlikely to consume hallucinogens daily; however, VHD could be a risk when hallucinogens are consumed in a "microdosing" regimen, in which users consume a low (but not neglectable) dose every day, sometimes multiple times a day34.
As to ayahuasca's risk effects, people with gastrointestinal disorders can have them exacerbated due to vomit and diarrhea. This can produce a severe electrolyte imbalance requiring immediate medical attention. In addition, people with a personal or familial history of psychiatric disorders are more vulnerable to having a psychotic experience35.
Although not very common, one of the risks related to psilocybin-mushrooms consumption is a toxic response that includes gastrointestinal symptoms, hypertension, hyperreflexia, tachycardia, and liver or renal failure. Mushroom poisoning may require medical intervention or emergency hospitalization. As it occurs with ayahuasca, hallucinogenic mushrooms may also precipitate some forms of psychosis in people with a family history of psychiatric disorders7.
Users, after mescaline intake, may also show signs of a toxic syndrome including hyperreflexia, tachycardia, ataxia, seizures, mydriasis, sialorrhea, hyperthermia, and paresthesia. Due to its long-lasting effects, mescaline can produce a prolonged psychotic state with schizophrenia-like symptoms. Although extremely rare, peyote ingestion might cause damage to the lower esophagus tissue due to severe vomiting17.
Ketamine, at high doses, produces hypertension, tachycardia, chest pain, anesthesia, muscular rigidity, anxiety, paranoia, and psychosis, without loss of consciousness. This intoxication is usually known as the K-hole. Besides being very unpleasant, it makes people vulnerable to injuries and attacks due to losing body sensations and the inability to move. Ketamine produces tolerance and, unlike classic hallucinogens, it has high dependence liability. In addition, long-term use of ketamine can produce bladder and liver toxicity36.
THERAPEUTIC POTENTIAL OF HALLUCINOGENS
The initial research analyzing the potential therapeutic value of classic hallucinogens conducted in the 1950s and 19,602 had low scientifical standards and questionable methodology, mainly due to poor standardized diagnostic techniques. During the recent "second wave" of psychedelics research, the studies aimed for more rigorous experimental designs and higher criticism of results. In 2006, a double-blind, randomized, and controlled study conducted on healthy subjects described that a high dose of psilocybin (30 mg) significantly improved psychological well-being over 2 months37. The subsequent studies mainly focused on psilocybin's potential benefits in patients with anxiety, depression, and emotional suffering and distress associated with a terminal illness (cancer). For example, Moreno et al. 2006, described the reduction of clinical symptoms in patients diagnosed with obsessive-compulsive disorder after receiving up to four psilocybin sessions38. In another study with a randomized crossover design, psilocybin significantly reduced the psychiatric distress of patients with advanced-stage cancer39,40. In addition, administering two doses of psilocybin (10 and 25 mg) to patients with treatment-resistant depression significantly reduced depressive symptoms throughout 6-months41. Other studies have shown that psilocybin increases abstinence in patients with alcohol and nicotine dependence42,43. Although these studies reported positive results, the therapeutic potential of hallucinogens remains controversial due to the lack of proper control conditions, discrepancies in the dose administered based on participants' weight variability, the difficulty of conducting double-bind studies, and other methodologic variables. Recent studies have shown that low doses of several hallucinogen compounds have therapeutical potential as antidepressant drugs. According to the DSM 5, the criteria for diagnosing MDD include the occurrence of five or more of the following symptoms in a week, not attributable to other medical conditions: depressed mood, loss of interest and pleasure in daily activities, changes in body weight (loss or gain), sleep disturbances, fatigue, restlessness or psychomotor retardation, feeling worthless or excessive guilt, decreased concentration, suicidal thoughts, or attempts. In addition, it is necessary to rule out a history of maniac or hypomanic episodes and consider that depression in children and adolescents may include irritable mood30.
Because complex alterations in neurotransmitter levels occur in MDD, different classes of antidepressants exist with various mechanisms of action: Monoamine oxidase inhibitors, TCAs, atypical depressants, serotonin modulators, serotonin-norepinephrine reuptake inhibitors, and SSRIs. Each class has a different pharmacological profile and side effects. Despite their efficacy and wide clinical use, these medications usually take weeks or months to produce their therapeutic effect, and some people remain unresponsive to them.
Preclinical studies conducted in the 1900s showed that NMDAR inhibition produced antidepressant-like effects in rodents, suggesting that NMDAR could be a target for antidepressant medications in humans. Later, Berman and coworkers reported that a 40-min infusion of ketamine (0.5 mg/kg) at a subanesthetic dose produced clear antidepressant effects 4 h after infusion in patients with MDD44. Because ketamine's rapid onset of action might provide a significant advantage for patients having suicidal ideations, other clinical trials followed soon, confirming the efficacy of ketamine in producing antidepressant actions in patients unresponsive to other pharmacotherapies. Ketamine has also proved effective in patients with bipolar depression. Ketamine and the available esketamine nasal spray are usually combined with other antidepressant drugs. Due to the high dependence liability of ketamine, its use requires medical supervision.
Although there is evidence that administering ayahuasca decoction to patients with resistant MDD improves their clinical symptoms up to 21 days after the infusion intake45, scientific evidence supporting the therapeutic effect of DMT is scarce. The main limitation is the variability of ingredients in the ayahuasca drink used in clinical settings. Therefore, future studies using pharmacologically pure compounds are needed to determine if controlled and supervised ayahuasca use has therapeutic effects.
CONCLUSION
The interest in studying the potential therapeutic effects of hallucinogens emerged in the 1950s and 1960s but yielded non-conclusive results and stopped for more than two decades. The current interest in this topic warrants preclinical and clinical unbiased research using a rigorous and validated methodology in controlled settings to determine hallucinogens' efficacy and safety profile. This is particularly important because non-medical hallucinogen use appears to be outpacing evidence-based research. In addition, the microdosing practice to allegedly enhance creativity, productivity, and mood has become increasingly popular. Clinical trials should address concerns about long-term effects and potential risks associated with psychedelics consumption, such as psychiatric sequelae in vulnerable populations and the possibility of experiencing a serotonin syndrome due to some drug-drug interactions.
There is evidence that hallucinogens, especially ketamine, have antidepressant effects in people with MDD resistant to other treatments and in people with suicidal ideations, requiring a fast-acting antidepressant agent. However, current data supporting the effectiveness of psychedelic compounds in treating some psychiatric conditions are limited. Beyond doubt, psychedelic research will continue growing with unbiased studies and higher rigorous methodologic standards.











 text new page (beta)
text new page (beta)


