INTRODUCTION
Body composition has an increasing interest in oncology for its prognostic role1,2. Body weight (BW) and fat mass (FM) gain in breast cancer survivors are a common side effect of adjuvant chemotherapy, regardless of their body mass index (BMI), that can affect their quality of life and both disease-free and overall survival3,4. Frequently, BMI is used to classify overweight and obesity in cancer2. However, BMI cannot distinguish lean and adipose tissue distribution and has been questioned as a ratio for FM estimation1,5. For these reasons, novel and reliable equations validated by dual-energy X-ray absorptiometry (DXA) could be used to assess body composition in adult cancer patients. Such equations have been recently proposed by Heymsfield et al. for skeletal muscle mass (SM)6, and by Woolcott and Bergman for relative fat mass (RFM)7, additionally validated in Northwest Mexican adults by different reference methods along with DXA5. During and after cancer treatment, body composition assessment in breast cancer survivors is essential to design strategies and implement lifestyle interventions to prevent sarcopenic obesity8.
According to the American Academy of Nutrition and Dietetics, oncology patients should receive nutrition counseling by registered dietitian nutritionists (RDNs) throughout the nutrition care process to prevent malnutrition and sarcopenic obesity9. However, diet costs can be considered a potential barrier related to healthy eating10. Identifying affordable and appealing nutrient-rich foods should be a priority when designing dietary plans for cancer patients8,11. The dynamic macronutrient meal-equivalent menu method (MEM) offers RDNs a systematic way to calculate energy and macronutrient content to design individualized nutrition plans considering standard food servings, population-specific dietary guidelines, and individual socioeconomic, educational, occupational, and cultural characteristics12. Still, information regarding grocery shopping behavior in breast cancer survivors, as well as of the costs of specialized dietary plans and their feasibility in oncology patients, is limited. Therefore, our objective was to study the effect of an individualized nutrition intervention according to socioeconomic status and grocery shopping behavior in breast cancer survivors RFM.
METHODS
Ethics, study design, and participants
The research protocol was reviewed and approved by the University of Sonora Ethics and Research Committee. This research has been conducted in full accordance with ethical principles, including the World Medical Association Declaration of Helsinki13. This is a non-randomized pre-post study, conducted in the Nutrition Area of an academic medical center in Sonora, Mexico, between November 2014 and 2017. This program emerged as an initiative within the university medical center to improve breast cancer survivors quality of life and decrease their risk of tumor recurrence, lymphedema, and mortality. The program offers free cancer-related supportive health care to patients with breast malignancies. Depending on the patients needs, interests, identified barriers, and goals, the program provides a varied range of supportive care. Women with Stages I to III breast cancer diagnosis, with previous breast surgery, chemotherapy, and radiotherapy treatment completion, were potential eligible participants. In addition, breast cancer survivors willing to pursue an adequate and specialized diet8, were invited to participate through social media, television, and radio broadcasts, word-of-mouth recommendations from one participant to another, and institutional run/fitness walk events designed to raise public breast cancer awareness. Reasons for non-participation and dropouts were location, transportation, and time barriers, related to missing nutrition appointments, change of address, city of residence, or contact information. Exclusion criteria included self-medication with nutritional supplements and herbal over-the-counter products, being in active cancer treatment, and an incomplete medical record.
At baseline, a signed consent form was obtained from all participants. Data on age, highest education level completed, occupation, and tobacco and alcohol consumption were self-reported by the volunteers. Women without a menstrual period over 12 consecutive months were considered as postmenopausal. In addition, participants answered the following questionnaires: International Physical Activity Questionnaire (IPAQ)14, Malnutrition Screening Tool (MST)15, and Subjective Global Assessment (SGA)16. Both MST and SGA are validated, easy to apply, and suitable screening tools to detect malnutrition risk in cancer patients15-16.
Grocery shopping consumer behavior and costs of dietary plans
Each participant completed a survey that addressed sociodemographic variables, including: if the patient received a salary or income, number of family members living in the household who received a salary or income, total weekly family income, the self-declared amount of money spent weekly on grocery shopping, and grocery shopping consumers preferences (frequency of visits by supermarket and store type, awareness of price discounts and special sales, and awareness of seasonal fruits and vegetables). Furthermore, participants saved all 1-week supermarket tickets to confirm total food weekly expense. We anticipated that perhaps not all participants could save or have their 1-week grocery tickets. Therefore, if they could not obtain the printed or online tickets, we asked them to record what they bought including the total and individual costs of their grocery purchases. Once we obtained the tickets or food purchases records from the patients, one of the researchers immediately called or went physically to the store to obtain the real individual and total costs based on the patients record to avoid discrepancies in the product cost as well. Next, this information was classified according to food groups and standard food servings17. All information regarding income and grocery expenditure from breast cancer patients were obtained in Mexican pesos (MXN) and subsequently converted into the US dollars (USD) to guarantee a universal currency18.
Weekly dietary plans costs were calculated for each participant, based on local market prices from Hermosillo, Sonora, Mexico. Costs were classified according to the following categories for individualized diet menus based on the characteristics of plant-based and animal-based diets and the preference, tolerance, and food access to legumes and dairy products, as follows: (1) plant-based diet plan including legumes and dairy products; (2) plant-based diet plan including legumes without dairy products; (3) animal protein-based diet plan including dairy products without legumes; and (4) animal protein-based diet plan excluding legumes and dairy products.
Anthropometry and body composition assessment
At baseline and every follow-up nutrition session, participants BW was measured on a digital scale (SECA® model: 285, 0-300 kg ± 0.05 Kg, Hamburg, Germany) with light and metal-free clothes, without shoes or breast prosthesis. Standing height (H) was measured using a stadiometer (SECA® model: 285, range of 30-220 cm ± 1 mm, Hamburg, Germany). Then, BMI was calculated as weight in kilograms divided by height in meters squared and categorized according to the World Health Organization (WHO) classification19. Anthropometric measurements were performed by trained RDNs and in accordance with the International Society for the Advancement of Kinanthropometry (ISAK) protocol20. Mid-upper arm (MUAC), hip, and waist circumferences (WC) were measured with a metal Lufkin tape and waisthip ratio (WHR) was calculated as the waist circumference in cm divided by the hip circumference in cm. Measurement of the triceps skinfold (TSF) thickness was done using a Harpenden Skinfold Caliper (range 0-50 mm; minimum graduation 0.2 mm, England). According to the ISAK protocol, MUAC and TSF were measured in the right arm of each volunteer20.
The RFM equation used in this study as a fat mass % estimate7, was then validated in Northwest Mexican adults by DXA, air displacement plethysmography, bioelectrical impedance analysis, and a 4-compartment model5. Both H and WC must be added in meters to the equation (Equation S1)7. Participants FM in kg were obtained from the RFM% and BW resultant. For SM assessment, we used one of the novel equations for women, recently proposed by Heymsfield et al. and validated by DXA in 12,330 participants (r2 = 0.89, p < 0.0001) (Equation S2)6. Likewise, we used anthropometric measurements to calculate the arm bone-free muscle area for women (Equation S3)21.
Nutrition counseling
Individualized dietary plans for breast cancer survivors were based on WCRF/AICR guidelines22, adapting 1.2-1.5 g/kgBW/d of dietary protein, <30% of energy/d from fat (avoiding trans and saturated fatty acid food sources), approximately 50% of energy/d from carbohydrate food sources rich in dietary fiber. The diet included 5-9 servings of fruits and vegetables per day, rich in β-carotene and Vitamins A, E, and C, as well as garlic and cruciferous vegetables for their protective effect against breast cancer recurrence, including antioxidant and antiproliferative activity in breast cancer cells8. For breast cancer patients with obesity, adjusted, ideal body weight (AdjIBW) was considered to calculate participants total protein requirement. Correspondingly, AdjIBW was calculated using ideal BW according to BMI (Equation S4)23. Resting energy expenditure was estimated using an algorithm for Mexican population24. When appropriate (BMI ≥ 25 kg/m2), a caloric restriction was considered (500-1000 kcal/d) to avoid BW and FM gain and reduce the metastatic burden in breast cancer patients8,25.
The nutrition intervention program was based on the MEM12. This innovative method provides RDNs with an organized way to calculate macronutrients and kcal/day into an individualized nutrition plan, providing every patient with interchangeable food choices within each mealtime, all being equivalent in energy and macronutrient content12. According to baseline sociodemographic and grocery shopping consumer behavior data and the Mexican Food Equivalent System17, standard food servings were set by the RDNs. To increase adherence and economic feasibility to the implemented nutrition plans, RDNs proposed well-known, affordable, and appealing nutrient-rich foods to each participant. All the meal options suggested by the RDNs were included in the individualized nutrition plan in common agreement with each breast cancer patient. Energy (kcal/d) and macronutrients (g/day) theoretical calculations were within the following acceptable ranges: protein ± 1 g/d, total fat ± 1 g/d, carbohydrates ± 2 g/d, and energy ± 15 kcal/d12. Patients were encouraged to consume all the food servings contained in their individual dietary plan. For each follow-up session (every 2 weeks ± 0.5 week), participants provided a self-reported single item for adherence in a 10-point Likert scale (0 = poor adherence and 10 = full adherence during the preceding period)26,27. The nutrition plan was modified and adapted according to the individuals needs in each follow-up session12.
Sample size and statistical analysis
Given the exploratory nature of this study, conducted at the initial stages of a new nutrition area in an academic medical center, an emphasis on descriptive statistics was done, as proportions for categorical variables, while means and standard deviations (SDs) were used for normally distributed continuous variables and medians and interquartile ranges (IQRs) for data not normally distributed. Furthermore, sample size was calculated based on a previous study conducted in Sonoran breast cancer patients undergoing cancer treatment and following an individualized nutrition intervention using the MEM26. The SD of the change in the outcome (BW) of the previous study was inserted in the formula to calculate sample size for a before-after study (paired t-test) with an independent continuous outcome (Equation S5)28. Twenty-three participants were required to complete the study. An additional 30% of expected volunteer loss was calculated, having as a result a total of 30 participants required to enroll in the nutrition intervention until 3 months were completed. The mean cost differences between the four dietary plan categories were analyzed by a one-way ANOVA at a 95% confidence level. Spearman correlation tests were used to measure the strength and direction of the association between two variables. Differences at baseline and 3 months after the nutrition intervention in body composition determinants were analyzed using Wilcoxon test analysis for continuous variables. A two-tailed p = 0.05 or less was considered significant. Data were processed using the statistical software NCSS® 11.0. Version.
RESULTS
From the total breast cancer population attending the academic medical center, 30% completed the 3-month nutrition intervention and met the inclusion criteria for analysis (Fig. 1). Estimated sample size was met, regardless of the dropouts due to location, transportation, and time constraints. All breast cancer survivors had undergone surgery, 73% mastectomy and 27% quadrantectomy. Sociodemographic characteristics of participants are presented and stratified by family annual income to ensure adequate sample description (Table S1). All participants were post-menopausal; their mean age was 55 years ± 10 and 30% corresponded to a middle-upper income level. Furthermore, most participants at baseline had a sedentary lifestyle and a very low risk of malnutrition (Table S1). About 36% of the volunteers had a job or ran their own business. On average, 2 ± 1 family members living in the same household periodically received a salary or income. In general, the reported amount of money spent weekly on grocery shopping ($21 ± 6 USD) was different to what the participants self-declared ($57 ± 23 USD) (r2 = 0.02, p = 0.4). Only 11.5% of the volunteers weekly family income was destined to grocery shopping.
As consumers, participants preferred to shop for groceries weekly (70%) or twice a month (30%) at supermarkets. However, the type of grocery store varied according to specific food groups (Fig. 2). Even so, not one volunteer reported making online grocery shopping or requesting a home delivery service. The main characteristic by which participants selected the type of store where they shopped for groceries was their regular prices. Product quality and store location were secondary factors considered by subjects. At the same time, only 35% of the volunteers shopped for groceries according to seasonal fruits and vegetables, special offers, or discount deals at stores.
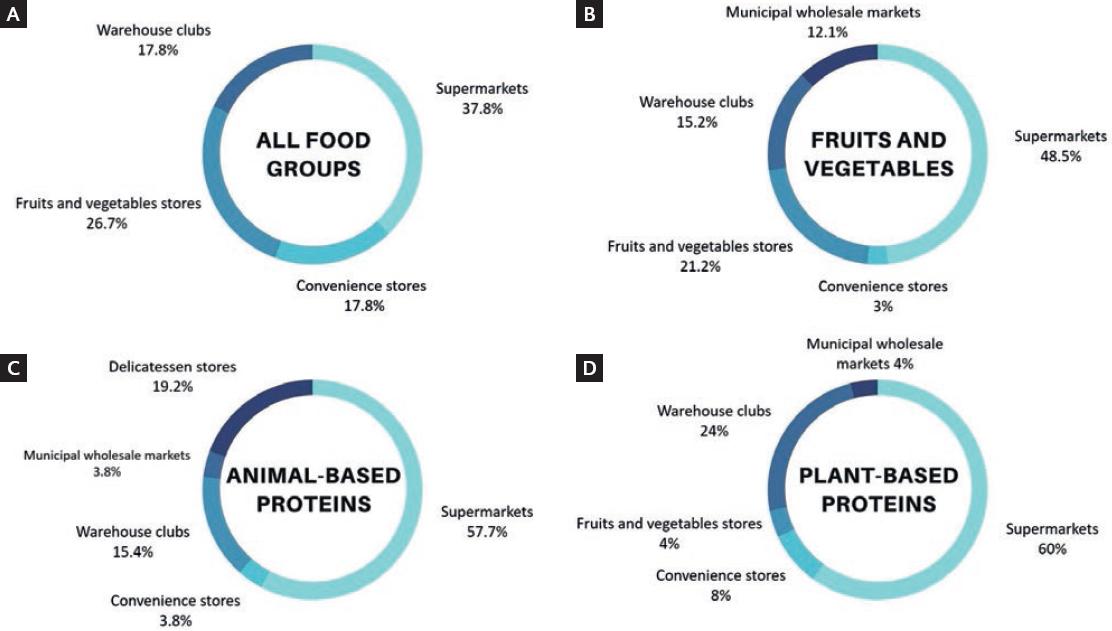
Figure 2 Types of grocery stores preferred by breast cancer survivors according to all and specific food groups (n=33). (A) all food groups; (B) fruits and vegetables; (C) animal-based proteins; (D) plant-based proteins.
The individualized dietary plans contained 6 ± 0.5 standardized servings of fruits and vegetables per day (3 servings of fruits and 3 servings of vegetables per day) (Table S2). Dietary adherence self-reported by subjects was 8.3/10 ± 0.6. Food groups servings distribution and average prices, and weekly dietary plans cost adjusted to 1500 kcal/d, were determined and grouped into 1 of 4 categories according to the patients preferences, feasibility, and theoretical adherence (Table S2). Diets higher in plant-based sources of protein cost less than animal protein-based diet plans (Table S3). The costs of all dietary meal plan categories were suitable for the amount of money the patients spent on groceries, according to all 1-week supermarket tickets (r2 = 0.35, p = 0.04).
On average, participants estimated resting energy expenditure was 1462 ± 197 kcal/d, and the estimated total energy expenditure was 2047 ± 276 kcal/d. When appropriate, a caloric restriction of 538 ± 337 kcal/d was considered, providing a total of 1509 kcal/d ± 170 per dietary plan. Based on the participants BW, 1.3 ± 0.3 g/kg/d of dietary protein was included in their individualized nutrition plans. When adjusting by AdjIBW in subjects with obesity, they were expected to consume 1.5 ± 0.2 g/kg/d of protein instead. Mean macronutrient distribution, grams, and energy in the individualized dietary plans were as follows: protein 26% ± 1, 97 ± 11 g/d, and 390 ± 46 kcal/d; fat 29% ± 2, 50 ± 7 g/d, and 447 ± 62 kcal/d; and carbohydrates 45% ± 2, 169 ± 21 g/d, and 676 ± 85 kcal/d.
At enrollment, obesity and overweight were present in 91% of the study population. Participants lost 3% of BW (p < 0.001) and 1.3% of RFM (p < 0.001) after the 3-month nutrition intervention (Table S4). At baseline, breast cancer volunteers WHR represented an increased risk for poor health outcomes, but not when the 3-month nutrition intervention ended (Table S4)29. The proportion of participants with a healthy weight increased from 9% to 21%. Breast cancer survivors with obesity had a greater BW loss (3.6 kg ± 0.3, p < 0.05) compared to participants with healthy BW or overweight19. A decrease in MUAC and TSF, but not in arm muscle area, was observed at the end of the study, meaning that in the arm area, there could have been greater mobilization of fat mass but not of skeletal muscle mass. On the contrary, according to Heymsfield et al. recent equation6, skeletal muscle mass decreased during the intervention (Table S4), which may partially compromise the quality of life and prognosis of breast cancer survivors1.
DISCUSSION
Weight loss in breast cancer survivors, particularly fat mass reduction in the abdominal area30, as reported in the present study, can improve breast cancer patients quality of life and decrease mortality, morbidity, and tumor recurrence risk, as described in many studies1,3,30. This was not an intervention focused only on caloric restriction to promote weight loss. Breast cancer survivors with healthy weight were encouraged to maintain it with special attention to avoid an increase in fat mass. Therefore, it is still important that they adhere to dietary guidelines to avoid sarcopenic obesity and nutrient deficiencies8. These are two key reasons why breast cancer survivors with a healthy weight were not excluded from the study. This is the 1st time that RFM is applied in oncology nutrition, with prior validation by four body composition methods in Northwest Mexican adults, as is our study population5. RFM has proven to be a better predictor than BMI for body fat percentage5, dyslipidemia, and metabolic syndrome31, probably due to the incorporation of WC in the equation and its relationship with abdominal and visceral fat29. A decrease in RFM may aid for dyslipidemia and metabolic syndrome prevention and should be considered a beneficial health outcome for breast cancer survivors at risk for sarcopenic obesity. Based on our results and previous studies5,31, this equation could be used in daily clinical nutrition practice for oncology patients, as a non-invasive, practical, and low cost tool for body composition assessment and follow-up.
This study evaluates dietary intervention exposure, reflected in a decrease in RFM and other body composition changes, with beneficial outcomes for breast cancer survivors, contrary to another observational study carried out in Sonora4. Furthermore, breast cancer survivors had a poor prognosis at baseline based on WHR cutoffs29 since central obesity increases the risk of mortality, dyslipidemia, and metabolic syndrome in these patients30,31. At the end of the study, this risk was no longer prevalent in the participating breast cancer population, also related to our RFM results and better health outcomes. The MEM is a methodological strength of this study because it guarantees strict calculation of energy and macronutrients to match theoretical and actual g/day and kcal/day translated into seven interchangeable meal options within each mealtime to provide patients with an individualized, yet flexible, nutrition plan, and also has been recently used in a similar population12,26.
People with obesity or overweight are frequently malnourished in the setting of disease, injury, and/or consumption of high-energy poor-quality diets such that overnutrition and malnutrition may coexist. The risk screening procedure is the first mandatory step in any diagnostic process to identify malnutrition and when possible, should always be carried out in breast cancer survivors32. Based on our findings, SGA considered well-nourished most of the volunteers in our study population, while they were also classified with obesity or overweight according to BMI. Our results are consistent with findings from the previous studies in breast cancer survivors using SGA to screen malnutrition risk, where obesity and overweight were highly prevalent (62-86%) and 71-76% of participants were classified as well-nourished according to SGA33-35. Then, SGA scores should be taken in the context of other data on patient nutritional status, as in the case of body composition, to provide a more reliable prognosis for patients with obesity and overweight.
Based on our findings, we could notice that dietary protein was not enough to prevent skeletal muscle mass loss. Although skeletal muscle mass was not the main outcome of this study, we did not expect that by following breast cancer patients dietary guidelines, specifically 1.5 g/kg/d of protein intake8, the lean tissue compartment would have decreased. This is one of the limitations of the study that can be improved for future interventions in breast cancer survivors. Physical activity in breast cancer survivors, especially resistance training, is crucial to prevent lean tissue loss3. Therefore, future interventions should also consider strategies to maintain or increase muscle mass such as exercise programs and protein supplementation1. Thus, breast cancer survivors attending this program should be encouraged to accomplish a physical activity level according to current guidelines (>150 min/wk)36, and their physical and health condition to decrease lymphedema and injury risk3. Furthermore, when possible, skeletal muscle mass should be measured rather than estimated, to avoid over- or underestimation.
The sample size of this study was limited due to its exploratory nature and because it was conducted at the initial stages of the newly created nutrition area in an academic medical center. It was calculated for methodological and ethical reasons, as well as to plan for human and financial resources. Since the estimated sample size was met, the results of this study are statistically valid. It is advisable that future studies focused on body composition assessment and follow-up, apply the RFM equation in large study populations within a stronger study design5. In our study population, location, transportation, and time constraints appeared to be a problem. Therefore, to reach more breast cancer survivors in the future and prevent dropouts, considering telemedicine and telenutrition, especially home-based programs37, could improve cancer patients participation and accessibility3.
In general, healthier diets have been related with higher diet costs per calorie10,11. However, our study shows that using methods to individualize dietary interventions, such as the MEM, additionally considering individuals grocery shopping consumer behavior and socioeconomic status, can overcome common limitations to encourage a healthy and feasible lifestyle among breast cancer survivors. Furthermore, our findings suggest that it is achievable for this population to carry out diet plans high in fruits and vegetables, whole grains, and good sources of protein, when RDNs identify affordable and appealing nutrient-rich foods for inclusion. In this sense, it is imperative for RDNs to integrate all the baseline information provided by breast cancer patients regarding their socioeconomic, educational, occupational, dietary, and cultural background into the dietary plan, through an empathic and systematic way for patients to feel guided into making better decisions within their personal means. By preventing unaffordable nutrition plans and social isolation, dietary plans following the MEM can empower breast cancer patients to make healthy and informed food choices, improving adherence, dietary habits, nutritional status, and overall health12,26. This is one of the first studies to identify grocery shopping consumer behavior in breast cancer survivors, to design dietary plans according to the patients preferences, family income, and energy nutrient requirements38.
Breast cancer survivors shopped for groceries mainly in supermarkets rather than in local food markets, making this a risky situation that may compromise their health as well as regional and nationwide food security and economic development. Growth of local food markets is expected to generate public benefits, and identifying situations to adopt broader presence of local food markets is a cost-effective tool for accomplishing policy goals. Therefore, it is essential to propose remedial actions to promote better regulatory practices that can encourage consumers to benefit directly from buying in local food markets and enhance food sustainability39. On the other hand, since online grocery shopping has recently increased during the COVID-19 pandemic40, future studies should consider diet quality in this setting and online grocery shopping consumer behavior for a more in-depth investigation on individualized nutrition interventions tailored for cancer survivors regarding food costs and affordability.
It was more accurate to obtain information regarding frequency, type of store, and actual grocery expense from the total weekly tickets each breast cancer survivor facilitated to her RDN, instead of relying on what they self-declared. The dietary plans proposed in this intervention, according to the four categories in which they were subclassified, were based on the characteristics of plant-based and animal-based diets as well as the patients preferences, tolerance, and food access to legumes and dairy products. Still, according to the environment setting of our volunteers and their preferences, plant-based diet plans cost less than diet plans higher in animal protein sources. Plant-based diets including proteins from legumes, nuts, and seeds have recently received much attention as a cost-effective way to improve diet quality within all socioeconomic levels10. Nevertheless, other studies have found that adopting a plant-based diet minimally increased food costs in breast cancer survivors38 and general population10. Breast cancer survivors can benefit in the short and long term by encouraging them to prioritize nutrient rich foods and grocery shopping at local markets, as well as having a more analytical and in-depth nutritional education.
In summary, nutrition counseling according to the MEM, socioeconomic status, and grocery shopping consumer behavior decreased RFM and body weight in breast cancer survivors attending an academic medical center.











 text new page (beta)
text new page (beta)