INTRODUCTION
Cardioembolic stroke is an important topic on the borderline of geriatric cardiology and vascular neurology. Cardioembolic infarction accounts for 18-25% of all ischemic strokes1-4 and its incidence is expected to rise because of the age-related incidence of cardiac embolism and an aging population and increasing life expectancy5.
Basically, there are two etiological types of non-lacunar ischemic stroke: atherothrombotic infarct and cerebral cardioembolism, which cause cardioembolic stroke, the most severe ischemic stroke subtype with high in-hospital mortality rate (6-27%)4. These two subtypes of ischemic stroke are worth distinguishing since the causes, clinical pictures, outcome, and treatment strategies are different. For instance, oral anticoagulation is indicated for secondary prevention in most patients with cardioembolism without contraindications for anticoagulant therapy whereas antiplatelets, endarterectomy, or angioplasties can be recommended in atherothrombotic stroke.
An early presumptive diagnosis of the stroke subtype can be made following a thorough history, physical examination, and imaging studies such as computed tomography (CT) or magnetic resonance imaging (MRI). However, confirmation of the etiological diagnosis requires more extensive testing.
The aim of this study was identifying the early clinical features related to cardioembolic stroke to help clinicians to improve the adequate and timely management of this neurovascular geriatric ischemic stroke subtype. As a secondary objective, we aimed to compare clinical features and early outcomes of cardioembolic ischemia versus ischemic atherothrombotic stroke to contribute to improve the early clinical differentiation of these two ischemic stroke subtypes.
METHODS
Setting and study design
Retrospective clinical study based on prospectively collected data available from an ongoing hospital-based stroke registry.
Since 1986, the Hospital Universitari Sagrat Cor (an acute-care 350-bed teaching hospital in Barcelona, Catalonia, Spain, serving a population of over 300,000 inhabitants) established a hospital-based Stroke Registry6. Data of all patients included in our stroke registry were entered following a standardized protocol with 186 items (demographic features, risk factors, clinical characteristics, laboratory and neuroimaging data, complications, and outcome). The use of the same protocol for all patients ensures completeness of the information in the database. Stroke subtypes are classified according to criteria of the Cerebrovascular Study Group of the Spanish Society of Neurology7, which is similar to the National Institute of Neurological Disorders and Stroke classification8. The study protocol was approved by the Clinical Research Ethics Committee of the hospital.
Patient selection and study procedures
The frequency of the stroke subtypes of the registry was as follows: 956 cardioembolic (20.8%), 945 atherothrombotic (20.5%), 864 lacunar (18.8%), 128 unusual cause (2.8%), 374 essential (8.1%), 761 transient ischemic attack (TIA) (16.5%), 473 cranial intracerebral hemorrhage (10.3%), 52 subarachnoid hemorrhage (1.1%), and 43 spontaneous subdural hemorrhage/spontaneous epidural hemorrhage (1%).
For the purpose of the study, consecutive patients diagnosed with cardioembolic infarction were selected from the database of the Stroke Registry. Criteria for classifying patients as having cardioembolic infarction required the presence of a medium-sized (maximal diameter of the lesion 1.5-3 cm) or large (> 3 cm) cerebral infarction, cerebral cortex involvement on the brain CT and/or MRI scans, sudden (minutes) or acute (hours) onset, stroke onset during ordinary daily activities, peak of deficit at onset, duration of focal neurological deficit > 24 h, absence of lacunar clinical syndrome, and identification of a commonly accepted cardiac source of embolus in the absence of confirmatory clinical (ipsilateral carotid bruit) or investigative results (Doppler ultrasonography, carotid angiography, or angio-MRI) of lesions (stenosis ≥ 50%) in the ipsilateral supra-aortic trunks.
Criteria for classifying patients as having atherothrombotic stroke required the presence of a medium or large cerebral infarction, absence of lacunar clinical syndromes documented by CT and/or MRI and evidence of two or more of the following manifestations: (a) presence of carotid, subclavian, vertebral bruit or absent carotid pulses or unequal radial pulses; (b) duplex sonography or angiography, showing arterial stenosis > 50% or occlusion of the symptomatic artery; and (c) evidence of clinical complications of atherothrombotic disease elsewhere, i.e., angina pectoris, previous myocardial infarction, peripheral vascular disease, femoral bruits, and absence of foot pulses.
All patients were admitted to the hospital within 48 hours of the onset of symptoms and did not receive thrombolytic therapy. On admission, demographic characteristics, salient features of clinical, and neurological examination and results of laboratory tests (blood cell count, biochemical profile, serum electrolytes, and urine analysis), chest radiography, 12-lead electrocardiography, and brain CT and/or MRI were recorded, as well as other clinical investigations performed at the discretion of the neurologist in charge. The registry included medical complications respiratory, cardiac, urinary, renal, and vascular- and mortality during the acute phase of the disease. The degree of clinical disability at discharge from the hospital was evaluated according to the modified Rankin scale9.
Statistical analysis
Continuous data were summarized as mean and standard deviation (SD), and categorical data were summarized as frequency and percentages. The distributions of variables in patients of both groups were compared with the Chi-square (χ2) test or the Fishers exact test for categorical variables and the Students t-test for quantitative variables. For all analyses, p < 0.05 was taken to indicate significance.
Covariates with p < 0.20 in the univariate testing were entered into three multivariable logistic regression models with a stepwise selection method, in which cardioembolic stroke (vs. atherothrombotic infarction) was the dependent variable. Model 1 was based on demographics and cardiovascular risk factors, to which clinical features and vascular topography (model 2) and complications (model 3) were added. The odds ratio (OR) and 95% confidence interval (CI) were calculated for the final statistically significant variables independently associated with cardioembolic stroke as well as in atherothrombotic stroke. The accuracy of model 3 to identify cardioembolic infarction was assessed using the receiver operating characteristics (ROC) curve. The sensitivity, specificity, and positive and negative predictive values were calculated. Statistical analyses were computed using the package SPSS (Version 20 for Mac; SPSS Inc., Chicago, IL, USA).
RESULTS
The crude cohort consisted of 4,597 consecutive patients diagnosed with acute stroke. Following etiological investigation, 956 patients were classified as cardioembolic infarction and 945 as atherothrombotic infarction. The results of differences between cardioembolic and atherothrombotic groups by univariate analysis are presented in table 1. Overall, female gender, 85 years or older, atrial fibrillation (AF), ischemic heart disease, and congestive heart failure were significantly more frequent risk factors in the cardioembolic group, whereas hypertension, diabetes, peripheral vascular disease, heavy smoking, hyperlipidemia, and previous TIA were significantly more frequent in the atherothrombotic group.
Table 1 Results of univariate analysis: differences between patients with cardioembolic infarction versus atherothrombotic stroke
| Variables | Cardioembolic (n = 956) | Atherothrombotic (n = 945) | p value |
|---|---|---|---|
| Demographics | |||
| Female patients | 602 (63%) | 471 (49.8%) | |
| Age, years, mean (SD) | 80 (9.14) | 77.01 (9.75) | 0.000 |
| 85 years old or more | 317 (33.2%) | 219 (23.2%) | 0.001 |
| Risk factors (%) | |||
| Hypertension | 513 (53.6) | 643 (68.0) | 0,001 |
| Diabetes mellitus | 177 (18.5) | 307 (32.5) | 0,001 |
| Ischemic heart disease | 224 (23.4) | 66 (7.0) | 0,001 |
| Atrial fibrillation | 711 (74.3) | 132 (14.0) | 0.000 |
| Congestive heart failure | 100 (10.4) | 48 (5.1) | 0.000 |
| History of transient ischemic attack (TIA) | 96 (10.0) | 133 (14.1) | 0.007 |
| History of cerebrovascular disease | 174 (18.2) | 190 (20.1) | 0.286 |
| Previous cerebral hematoma | 11 (1.1) | 9 (1.0) | 0.674 |
| Chronic obstructive pulmonary disease | 84 (8.8) | 97 (10.3) | 0.269 |
| Chronic renal disease | 57 (6.0) | 32 (3.4) | 0.008 |
| Peripheral vascular disease | 91 (9.5) | 81 (8.6) | 0.476 |
| Chronic liver disease | 20 (2.1) | 22 (2.3) | 0.724 |
| Hyperlipidemia | 130 (13.6) | 234 (24.8) | 0.000 |
| Heavy smoking (> 20 cigarettes/day) | 42 (4.4) | 123 (13.0) | 0.000 |
| Clinical features (%) | |||
| Sudden onset | 625 (65.3) | 401 (42.4) | 0.000 |
| Headache | 73 (7.6) | 116 (12.3) | 0.001 |
| Dizziness | 27 (2.8) | 51 (5.4) | 0.005 |
| Early seizures | 17 (1.8) | 17 (1.8) | 0.970 |
| Nausea/vomiting | 62 (6.5) | 84 (8.9) | 0.048 |
| Altered consciousness | 264 (27.6) | 186 (19.7) | 0.000 |
| Motor symptoms | 762 (79.6) | 718 (76.0) | 0.056 |
| Sensory symptoms | 346 (36.2) | 360 (38.1) | 0.381 |
| Visual disturbances | 202 (21.1) | 225 (23.8) | 0.158 |
| Speech disturbances | 589 (61.5) | 518 (54.8) | 0.003 |
| Ataxia | 40 (4.2) | 78 (8.3) | 0.000 |
| Cranial nerve palsy | 43 (4.5) | 64 (6.8) | 0.031 |
| Vascular topography (%) | |||
| Middle cerebral artery involvement | 620 (64.8) | 518 (54.8) | 0.000 |
| Anterior cerebral artery | 45 (4.7) | 35 (3.7) | 0.278 |
| Posterior cerebral artery involvement | 77 (8.0) | 87 (9.2) | 0.367 |
| Anterior choroidal artery | 4 (0.4) | 13 (1.4) | 0.026 |
| Vertebral artery | 19 (2.0) | 57 (6.0) | 0.000 |
| Basilar artery | 23 (2.4) | 91 (9.6) | 0.000 |
| Internal carotid involvement | 24 (2.5) | 67 (7.1) | 0.000 |
| Posteroinferior cerebellar artery | 9 (0.9) | 15 (1.6) | 0.206 |
| Complications (%) | |||
| Neurological | 126 (13.2) | 115 (12.2) | 0.513 |
| Respiratory | 138 (14.4) | 107 (11.3) | 0.044 |
| Renal | 17 (1.8) | 14 (1.5) | 0.612 |
| Urinary | 86 (9.0) | 107 (11.3) | 0.092 |
| Cardiac events | 86 (9.0) | 33 (3.5) | 0.000 |
| Vascular | 27 (2.8) | 11 (1.2) | 0.010 |
| Hemorrhagic events | 27 (2.8) | 10 (1.1) | 0.005 |
Sudden-onset and altered consciousness were significantly more frequent clinical features in the cardioembolic group, and headache, and ataxia were significantly more frequent among atherothrombotic patients. The distribution of lesions according to the vascular topography and medical complications were similar within groups. Early outcomes reported a significant higher percentage of patients with mild neurological deficits at discharge in atherothrombotic infarction compared to cardioembolic patients. The in-hospital mortality rate was also significantly higher in the cardioembolic group than in atherothrombotic patients (22.8% vs. 12.7%) (p = 0.000).
The results of multivariate analysis are shown in table 2. The first logistic regression model based on demographics and cardiovascular risk factors reported that AF (OR 15.87), ischemic heart disease (OR 3.11), and female gender (OR 1.58) were independently associated with cardioembolic stroke, whereas hypertension, diabetes, hyperlipidemia, and previous TIA were predictors of atherothrombotic infarcts. In the second regression model, the aforementioned factors (OR 15.75; OR 3.14; OR 1.53, respectively), and sudden-onset (OR 1.98) were predictors of cardioembolic stroke. The third model, in which medical complications were added, reported that AF (OR 15.75), ischemic heart disease (OR 3.12), sudden onset (OR 1.97), cardiac events (OR 1.69), and female gender (OR 1.56) were independent variables related to cardioembolic stroke. Other variables including hypertension, diabetes, hyperlipidemia, visual disturbances, ataxia, infarction in the territory of the anterior choroidal artery, internal carotid and arterial basilar involvement, urinary complications, and mild neurological deficit at hospital discharge, were independent variables associated with atherothrombotic patients.
Table 2 Results of multivariate analysis: variables independently associated with cardioembolic infarction
| Regression models | Coefficient (β) | Standard error | Odds ratio (95% confidence interval) | p value |
|---|---|---|---|---|
| First model: demographics and risk factors | ||||
| Atrial fibrillation | 2.76 | 0.125 | 15.87 (12.43-20.27) | 0.000 |
| Ischemic heart disease | 1.136 | 0.182 | 3.115 (2.179-4.452) | 0.000 |
| Female gender | 0.461 | 0.122 | 1.586 (1.249-2.013) | 0.000 |
| Hyperlipidemia | −0.382 | 0.156 | 0.682 (0.502-0.926) | 0.014 |
| History of TIA | −0.446 | 0.188 | 0.641 (0.443-0.926) | 0.018 |
| Diabetes mellitus | −0.637 | 0.139 | 0.529 (0.403-0.694) | 0.000 |
| Hypertension | −0.678 | 0.125 | 0.508 (0.397-0.649) | 0.000 |
| Second model: demographics, risk factors, clinical features, and vascular topography | ||||
| Atrial fibrillation | 2.757 | 0.131 | 15.749 (12.193-20.342) | 0.000 |
| Ischemic heart disease | 1.144 | 0.185 | 3.139 (2.184-4.512) | 0.000 |
| Sudden onset | 0.682 | 0.124 | 1.978 (1.552-2.521) | 0.000 |
| Female gender | 0.425 | 0.125 | 1.529 (1.196-1.956) | 0.001 |
| Hyperlipidemia | −0.325 | 0.159 | 0.722 (0.529-0.986) | 0.040 |
| Diabetes mellitus | −0.58 | 0.142 | 0.560 (0.424-0.740) | 0.000 |
| Visual disturbances | −0.588 | 0.153 | 0.556 (0.412-0.750) | 0.000 |
| Hypertension | −0.656 | 0.129 | 0.519 (0.403-0.668) | 0.000 |
| Ataxia | −0.661 | 0.286 | 0.516 (0.295-0.905) | 0.021 |
| Basilar artery involvement | −1.098 | 0.299 | 0.334 (0.185-0.600) | 0.000 |
| Internal carotid involvement | −1.198 | 0.317 | 0.302 (0.162-0.562) | 0.000 |
| Anterior choroidal artery | −1.886 | 0.74 | 0.152 (0.036-0.646) | 0.011 |
| Third model: demographics, risk factors, clinical features, vascular topography, and complications | ||||
| Atrial fibrillation | 2.757 | 0.133 | 15.747 (12.145-20.417) | 0.000 |
| Ischemic heart disease | 1.137 | 0.188 | 3.118 (2.157-4.509) | 0.000 |
| Sudden onset | 0.676 | 0.125 | 1.966 (1.538-2.512) | 0.000 |
| Cardiac events | 0.526 | 0.269 | 1.693 (0.999-2.869) | 0.050 |
| Female gender | 0.445 | 0.127 | 1.56 (1.217-2.000) | 0.000 |
| Hyperlipidemia | −0.333 | 0.161 | 0.717 (0.523-0.983) | 0.039 |
| Visual disturbances | −0.57 | 0.156 | 0.566 (0.417-0.768) | 0.000 |
| Mild neurological deficit at discharge | −0.514 | 0.147 | 0.598 (0.448-0.798) | 0.000 |
| Visual disturbances | −0.57 | 0.156 | 0.566 (0.417-0.768) | 0.000 |
| Diabetes | −0.582 | 0.144 | 0.559 (0.422-0.74) | 0.000 |
| Ataxia | −0.639 | 0.287 | 0.528 (0.301-0.926) | 0.026 |
| Hypertension | −0.666 | 0.13 | 0.514 (0.398-0.663) | 0.000 |
| Basilar artery involvement | −1.193 | 0.309 | 0.303 (0.166-0.555) | 0.000 |
| Internal carotid involvement | −1.24 | 0.318 | 0.289 (0.155-0.54) | 0.000 |
| Anterior choroidal artery | −1.745 | 0.754 | 0.175 (0.04-0.766) | 0.021 |
Model 1: Hosmer-Lemeshow goodness-of-fit test 0.169, cardioembolic versus atherothrombotic subjects were correctly classified in 80.5% of cases.
Model 2: Hosmer-Lemeshow goodness-of-fit test 0.239, cardioembolic versus atherothrombotic subjects were correctly classified in 81.5% of cases.
Model 3: Hosmer-Lemeshow goodness-of-fit test 0.08, cardioembolic versus atherothrombotic subjects were correctly classified in 81.8% of cases.
According to these models, cases of cardioembolic infarction versus atherothrombotic stroke were correctly classified in 80.5% of the cases for model 1, 81.5% of the cases for model 2, and 81.8% of the cases for model 3.
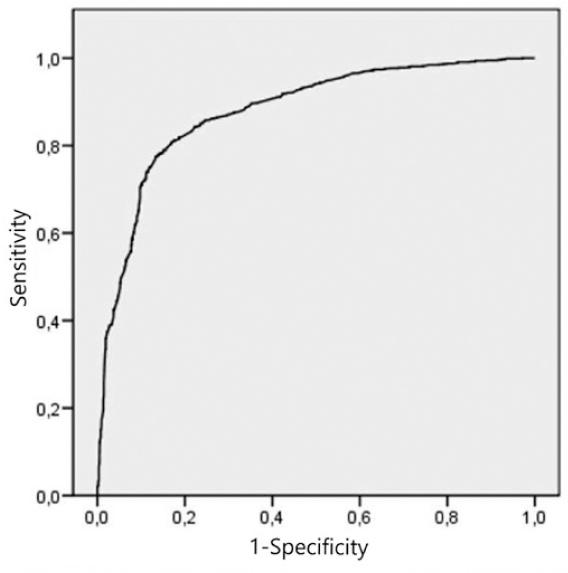
Figure 1 shows the ROC curve of the accuracy of the regression model based on demographics, cardiovascular risk factors, clinical features, vascular topography, and complications. The area under the curve was 0.879. The sensitivity was 78%, specificity was 85%, positive predictive value was 84%, and negative predictive value was 79%.
DISCUSSION
The results of the present study confirm that cardioembolic stroke is a severe neurovascular geriatric ischemic stroke subtype that presents a lower frequency of mild functional limitation and higher rates of neurological impairment at hospital discharge, and higher in-hospital mortality, coinciding with other series in the literature10-15.
In our study, AF is the most significant cerebrovascular risk factor associated with cardioembolic stroke. This is due to the fact that non-valvular AF is the leading cause of underlying heart disease as stated in different series of cardioembolic infarction14-16. Both, permanent and paroxysmal AF increase the risk of cardioembolic stroke17. AF leads to inadequate contraction of the atria, resulting in stasis of blood and, consequently, clot formation within the left atrial appendage, with subsequent risk of cerebral embolization. The prevalence of AF is globally increasing caused by improved survival rates of patients with heart disease, and the increase in age of the population. Thus, the number of AF -related strokes may triple in the next decades in high-income countries3.
The presence of coronary heart disease and other types of peripheral vascular disease are considered markers of increased risk for subsequent cardiovascular and cerebrovascular events in ischemic stroke3,4. In our study, ischemic heart disease (found in 23.4% of patients) was independently associated with cardioembolic stroke in all three logistic regression models (OR = 3.11 in the first model; OR = 3.13 in the second model and OR = 3.12 in the third model). In these cases, isolated ischemic heart disease (with a left ventricular aneurysm, left ventricular ejection fraction <40%, or with akinesia/dyskinesia of 2 or more segments), or associated with AF was a well-defined structural cardioembolic source. In addition, ischemic heart disease, mainly angor pectoris without associated structural heart disease, may also be a manifestation of generalized atherosclerotic disease and was found in 7% of patients with atherothrombotic infarctions. However, in these cases, the presence of ischemic heart disease was merely coincidental; it was an epiphenomenon that did not imply a cardioembolic risk.
Approximately 2.5% of patients with acute myocardial infarction experience a stroke within 2-4 weeks of the infarction, and 8% of men and 11% of women will have an ischemic stroke within the next 6 years3,4,17,18.
Other risk factors for cardiovascular disease such as hypertension, diabetes mellitus, and dyslipidemia are more frequent among atherothrombotic ischemic stroke patients, as reported in the previous studies4,19. When all of these risk factors and stroke mechanisms are considered together, they account for 60-80% of the population-attributable risk of ischemic stroke20.
Of interest, female gender is an independent predictor of cardioembolic infarction in all three regression models, as reported in literature, possibly due to the longer life expectancy of women, and this higher biological age may lead to an increased likelihood of developing AF, ischemic heart disease, and other related heart diseases21-23.
Sudden onset to maximal deficit and non-lacunar clinical syndrome are also significant predictors of cardioembolic infarction4,24,25. This is in line with the study of Timsit et al., who reported that sudden onset of neurological deficit was more frequent in cardioembolic stroke (79%) than in lacunar (38%) or atherothrombotic infarction (46%)26. The embolus blocks suddenly so that the onset of symptoms is abrupt and neurological deficits are maximal at this moment. Conversely, visual disturbances and ataxia are more frequent among atherothrombotic stroke patients.
It is noteworthy that vascular involvement of the basilar trunk, the internal carotid artery and the anterior choroidal artery are less frequent in cardioembolic infarction compared to thrombotic infarction. This might be due to the fact that cerebral embolism usually occludes more distal cerebral arteries, such as the middle cerebral artery or the posterior cerebral artery4. In contrast, hemodynamically significant stenosis at the supra-aortic trunk level or at the vertebrobasilar territory level is common in atherothrombotic infarction.
It should be noted that our results confirm that cardioembolic stroke is the most severe subtype of ischemic stroke, with a high mortality rate, and a higher rate of complications, particularly cardiac events27,28 compared to lacunar infarctions and the other subtypes of cerebral ischemia24. Appropriate treatment measures are essential for secondary stroke prevention, as many cardiac conditions pose a high stroke recurrence risk. In cardioembolic stroke, oral anticoagulation should be started according to the severity of the infarction, and its cerebral extension1-3.
The comparative study of the evolution of demographic characteristics and clinical and outcome data in patients with cardioembolic stroke overtime was not the aim of this study. However, this evolution in our stroke registry was already analyzed in a previous study in which, over a 19-year study period, we observed significant changes in patients with a first-ever cardioembolic stroke that included an increase in the age of patients (the percentage of very old patients increased from 16% to 38.2%), in frequency of hypertension (from 40.5% to 60.8%), and use of echocardiography (from 39.7% to 73.9%), while the frequency of heavy smoking > 20 cigarettes/day (from 9.2% to 2%) and length of hospital stay decreased29. It should be noted that the lack of improvement in the early prognosis of patients with cardioembolic stroke during this study period (both mortality and neurological impairment) can be explained by the increased prevalence of major cardiovascular risk factors due to progressive ageing of the population29.
Limitations of the present study include the retrospective analysis of data based on a single-center stroke registry, and hospital referral selection bias cannot be excluded. Laboratory, neuroimaging, and neuropsychological variables were not included in multivariate analysis, which would have provided a more robust regression model30. Strengths of the study are the large number of patients analyzed the systematic evaluation of predictors of cardioembolic infarction based on risk factors, clinical features, vascular topography, and outcome from a previously validated stroke registry.
We conclude that cardioembolic stroke is a geriatric neurovascular entity, and its early identification is important in triaging medical resources, choosing among treatment modalities, and predicting clinical outcomes.











 text new page (beta)
text new page (beta)