INTRODUCTION
Ovarian cancer is the seventh most common cancer in women and the eighth cause of death from cancer worldwide1. Although there are numerous risk factors associated with ovarian cancer, there are no definitive causes2. Epithelial ovarian cancer is categorized depending on its main cell type, and although these subtypes are fairly different from one another, they are given the same or very similar treatment3. Aggressive cytoreduction, when possible, and chemotherapy with carboplatin and paclitaxel are the standard treatment for ovarian adenocarcinoma4. According to the disease-free survival, ovarian cancer can be classified as platinum refractory, platinum resistant, partially platinum sensitive, and platinum sensitive5. Although most patients respond adequately to the first-line therapy, up to 85% experience a recurrence of disease, which carries a poor prognosis6. Patients with a platinum-sensitive disease can be treated again with platinum-based agents, while those with a non-sensitive disease go through several second-line therapies6.
There are two mechanisms of resistance to chemotherapy: intrinsic, which is the ability of the cancer cell to survive initial exposure to treatment and acquired, in which cells that survived initial therapy reproduce with a survival advantage7. Platins and taxanes act by interfering with deoxyribonucleic acid repair8 and microtubule polymerization9, respectively. Alterations in these cellular pathways may lead to chemoresistance in cancer cells7.
Mitotic arrest deficiency 1 (MAD1) is a protein that helps in the assembly of the mitotic spindle assembly checkpoint by recruiting MAD2 protein into the kinetochore and preventing anaphase until all chromatids are properly aligned during metaphase10. Alterations in any of the proteins of the mitotic checkpoint complex can lead to aneuploidy and tumorigenesis10. A reported single-nucleotide polymorphism in the MAD1L1 gene in nucleotide 1673 causes a G→A (rs1801368) transition that leads to the substitution of an arginine for a histidine in codon 55811. This polymorphism is prevalent in patients with advanced epithelial ovarian cancer and alters the way in which it responds to chemotherapy11. The primary objective of this work was to study the relationship between the rs1801368 polymorphism of MAD1L1 and chemoresistance in ovarian adenocarcinomas. The secondary objectives were to study if there is an association between the polymorphism and clinical stage at diagnosis, reduced disease-free period, and overall survival.
METHODS
Population
Data used in this study were retrieved from electronic hospital records from patients treated at Mexicos National Cancer Institute (INCan) from January 2005 to March 2018. The protocol was approved by INCan Institutional Review Board, with approval reference 008/044/IBI. Inclusion criteria were women older than 18 years, with an ovarian adenocarcinoma as confirmed by the institutions pathology department, with a sequenced MAD1L1 genotype, who received adjuvant or neoadjuvant treatment with carboplatin and paclitaxel and with at least 1 year of follow-up after the end of treatment. Exclusions were patients under 18, with incomplete data, with borderline tumors, whose tumor was not confirmed as a primary ovarian adenocarcinoma, with < 1 year of follow-up after the end of treatment, or who did not receive chemotherapy with platins and taxanes. A total of 367 patients with ovarian tumors were identified, from whom 142 had a primary ovarian adenocarcinoma. Of them, 118 had a sequenced MAD1L1 genotype and sufficient data for analysis and were the final patients included in the analysis.
Polymorphism determination
Deoxyribonucleic acid was isolated from peripheral blood using phenol and chloroform and precipitated in ethanol. A 241 base pair (bp) fragment from MAD1L1 exon 17 was amplified with the following primers:
Sense: 5-GTGTGAGAATTCCTGCAGGGTGACTATGACCAG-3.
Antisense: 5-GAGTCTGGATCCCTGCCACCTCCTTGGACGATGGCAGAC-3.
An allele-specific digestion was made with the restriction enzyme BsTUi (New England Biolabs), which recognizes the CGCG sequence. Digested samples were analyzed by electrophoresis. Amplified sequences of patients with the wild-type genotype (GG) had five fragments (94, 42, 50, 43, and 12 bp). The G:A substitution modifies the restriction site between 94 and 42 bp. Patients with the homozygous polymorphism (AA) had a 136 bp fragment. Heterozygous (GA) patients had both the 136 bp and the 94 bp fragments.
Statistical analysis
Patients were divided into platin sensitive and non-platin sensitive and then statistically analyzed. Patients were also analyzed according to wild-type versus polymorphic MAD1L1. Platin sensitive was defined as a patint who had a recurrence of disease after 1 year of the end of treatment or who did not experience recurrence after treatment. Non-platin sensitive was defined as a patient who experienced a recurrence of disease before 1 year of the end of treatment, or who had progression of disease. Statistical analysis was carried out with RStudio (Version 1.1.456. Boston, MA). p < 0.05 was set to establish statistical significance. Continuous variables were compared using Students t-test. Categorical variables were compared using Chi-squared test. Survival curves were analyzed with the KaplanMeier estimator and the log-rank test. For the logistic regression analysis, MAD1L1 was dichotomized as wild type versus polymorphic.
RESULTS
A total of 118 patients were included in this study, with a mean age of 52.12 years (range 18-79 years) and a mean body mass index (BMI) of 27.18 kg/m2 (range 16.01-50.22 kg/m2). The median initial CA125 level was 935.50 U/mL with an interquartile range (IQR) of 2610.70 U/mL. The median initial HE4 level was 50.40 U/mL, with an IQR of 46.35 U/mL. The median tumor size was 11.50 cm, with an IQR of 12.13 cm.
A total of 89 patients (75.42%) presented with initial 0 Eastern Cooperative Oncology Group (ECOG) performance status, while 21 (17.80%) presented with ECOG 1, 7 (5.93%) with ECOG 3, and 1 (0.85%) with ECOG 4. The most common histological subtype was high-grade papillary serous (n = 58, 49.15%), followed by endometrioid (n = 21, 17.80%), clear cell carcinomas (n = 11, 9.32%), and low-grade papillary serous (n = 7, 5.93%). Other tumors (mixed histology, mucinous, etc.) accounted for 21 cases (17.80%). As to the clinical stage, 25 patients (21.19%) were diagnosed at Stage I, 5 (4.24%) at Stage II, 57 (48.31%) at Stage III, and 31 (26.27%) at Stage IV. A total of 113 patients (95.76%) were taken to cytoreduction (initial or interval), from which 44 (60.2%) were optimally cytoreduced at some point. Neoadjuvant treatment was given to 74 (62.71%) patients and adjuvant treatment to 90 (76.27%). Patients could receive neoadjuvancy and/or adjuvancy. There was a recurrence or progression of disease in 78 patients (66.10%), from which 16 (20.51%) were peritoneal, 23 (29.48%) nodal, 27 (34.61%) distant, and 12 (15.38%) elsewhere, or undetermined. As to chemotherapy sensitivity, 60 patients (50.85%) were classified as platin sensitive and 58 (49.15%) as non-platin sensitive. The MAD1L1 genotype was the wild type in 26 (22.03%) patients, heterozygous in 49 (41.53%) patients, and homozygous polymorphic in 43 (36.44%) patients (Table S1), table 1 we compared MAD1L1 genotype versus the International Federation of Gynecology and Obstetrics clinical stage at diagnosis, tumor histology, and cytoreduction. Patients with the wild-type genotype had a higher frequency of early-stage disease, with 12 patients in Stage I or II (46.16%). Conversely, for the non-wild-type genotype, there were 74 patients (81.32%) in Stages III or IV; this had a statistically significant value (p = 0.019). As to histology, wild-type genotype tumors were more likely endometrioid (n = 10, 38.46%), while non-wild type genotype tumors were most likely high-grade serous papillary (n = 52, 57.14%); this had a statistically significant value (p = 0.006). There was a similar distribution of cytoreductions between groups, with 25 (96.15%) in patients with the wild-type genotype and 88 (95.65%) in the polymorphic group (p = 1.000). Optimal cytoreduction was achieved in 23 (92%) patients with the wild-type genotype and 62 (72.45%) patients with the polymorphic genotype (p = 0.062).
Table 1 FIGO clinical stage, histology, and cytoreduction according to MAD1L1 genotype in patients with ovarian adenocarcinoma, treated at the National Cancer Institute Mexico, from 2005 to 2018 (n = 118)
| Variable | GG | GG or GA | p-value** | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| FIGO clinical stage | |||||
| I | 11 | 42.31 | 14 | 15.38 | 0.019 |
| II | 1 | 3.85 | 4 | 4.40 | |
| III | 11 | 42.31 | 46 | 50.55 | |
| IV | 3 | 11.54 | 28 | 30.77 | |
| Histological subtype | |||||
| High-grade papillary serous | 6 | 23.08 | 52 | 57.14 | 0.006 |
| Low-grade papillary serous | 4 | 15.38 | 3 | 3.30 | |
| Endometrioid | 10 | 38.46 | 11 | 12.09 | |
| Clear cell | 4 | 15.38 | 7 | 7.69 | |
| Mixed histology/other | 2 | 7.69 | 19 | 20.88 | |
| Cytoreduced | |||||
| Yes | 25 | 96.15 | 88 | 95.65 | 1.000 |
| Optimal cytoreduction* | |||||
| Yes | 23 | 92.00 | 62 | 70.45 | 0.062 |
*Including only patients taken to cytoreduction.
**p-value calculated using Chi-squared test. FIGO: International Federation of Gynecology and Obstetrics; MAD1: mitotic arrest deficiency 1.
In the comparative analysis shown in Table S1, the median tumor size in the non-platin-sensitive group was 6 cm (IQR 2.85 cm) and 16 cm (IQR 9.5 cm) in the platin-sensitive group (p < 0.001). The most prevalent histological subtype in the non-sensitive group was high-grade serous papillary (n = 44, 75.86%), with no low-grade tumors. Contrary, the sensitive group had 19 (31.67%) high-grade serous papillary tumors, 16 endometrioid (26.67%), 9 clear cell tumors (15%), 2 low-grade serous papillary tumors (3.33%), and 14 (23.33%) classified as other or mixed (p < 0.001). No patients in the non-sensitive group presented at Stage I disease, 1 patient (1.72%) presented at Stage II, and the rest at Stage III (n = 35, 60.34%) or IV (n = 22, 37.93). In the sensitive group, there were 26 patients (43.33%) in Stage I, 3 patients (5%) in Stage II, 22 in Stage III (36.67%), and 9 patients (15%) in Stage IV (p < 0.001). In the non-sensitive group, 53 patients (91.38%) were initially cytoreduced, from whom 29 cytoreductions (54.72%) were optimal. On the other hand, in the sensitive group, 60 patients (100%) were cytoreduced, from whom 56 (93.33%) were optimal. There was p = 1.000 for being taken to cytoreduction and p < 0.001 for the cytoreduction being optimal. Neoadjuvant treatment was given to 53 patients (89.93%) in the non-sensitive group and 21 (35%) in the sensitive group (p < 0.001). By definition, every patient in the non-sensitive group had a recurrence or progression of disease (n = 58, 100%), while 20 patients (33.33%) did so in the sensitive group (p < 0.001). As for the MAD1L1 genotype, in the non-sensitive group, 7 patients (12.07%) had the wild-type genotype, 23 (43.10%) the heterozygous genotype, and 26 (44.83%) the homozygous polymorphic. In the sensitive group, 19 patients (31.67) had the wild-type genotype, 24 (40%) the heterozygous genotype, and 17 (28.33%) the homozygous polymorphic (p = 0.024).
In the multivariate regression model adjusted by age, BMI, initial CA125, and histological subtype, we found that having a MAD1L1 polymorphic allele increased the risk of being non-sensitive to chemotherapy (risk ratio 4.623, 95% confidence interval [CI] 3.285-5.960, p = 0.025).
The median disease-free survival for the whole cohort was 12.05 months (Q1-Q3 4.01-35.13). The median disease-free survival for patients with the wild-type MAD1L1 was 46.93 months, while it was 10.4 months for patients with at least one polymorphic allele; this was statistically significant (p = 0.049) (Fig. 1).
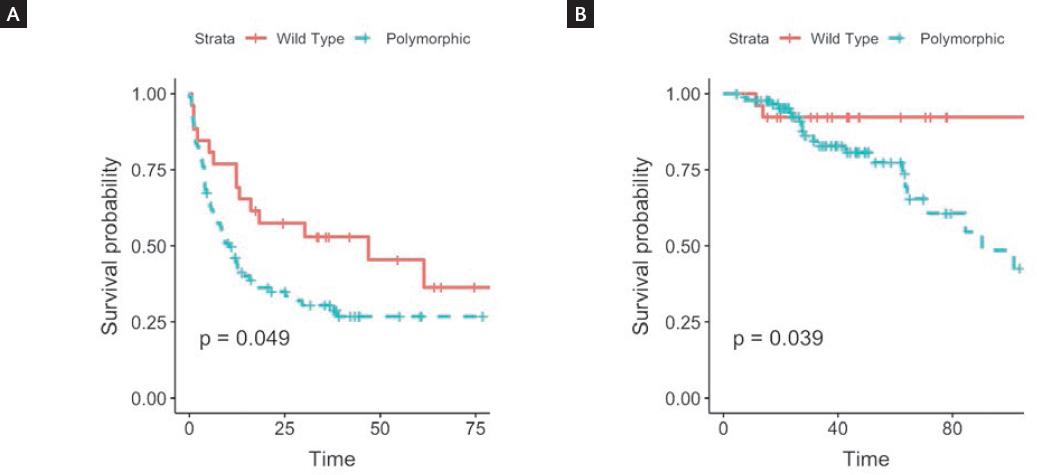
Figure 1 Disease-free survival (A) and overall survival (B), according to MAD1L1 genotype (wild type vs. polymorphic).
The median overall survival for the whole cohort was 38.68 months (Q1-Q3 23.02-62.06). Due to the low number of deaths in patients with the wild-type genotype, we could not calculate a median overall survival in this group, while patients with at least one polymorphic allele had a median overall survival of 90.43 months; this was statistically significant (p = 0.039) (Fig. 1). When these survival curves were estimated without dichotomizing MAD1L1 genotype, the statistical significance was maintained. Disease-free survival was 46.9 months for the wild type, 12.2 for heterozygous polymorphic, and 9.2 for homozygous polymorphic (p = 0.017). The Median overall survival was not available for the wild type; it was 101.5 months for the heterozygous polymorphic, and 84.6 months for the homozygous polymorphic (p = 0.032).
In a multivariable Cox regression model adjusted by age, BMI, initial CA125, and histological subtype, we found that having a MAD1L1 polymorphic allele conferred a higher hazard ratio (HR) of recurrence or progression of disease (HR 1.473, 95% CI 1.077-2.013; p = 0.015).
DISCUSSION
Our main findings are the association between polymorphic MAD1L1 genotype and chemoresistance, higher clinical stage at diagnosis, decreased disease-free survival, and decreased overall survival. Although the homozygous polymorphic genotype was associated to the worst overall outcomes, the presence of a single polymorphic allele was sufficient to worsen the prognosis.
We found that having a polymorphic MAD1L1 allele decreased the probability of being sensitive to chemotherapy. It is difficult to determine the resistance to which of the two chemotherapeutic agents the MAD1L1 polymorphism correlates, but it seems logical to assume that since paclitaxel targets microtubules and MAD1 relates to the functionality of the mitotic checkpoint complex, a polymorphism could confer patients with resistance to taxanes. One study found that cells that overexpress MAD1L1 are resistant to microtubule agents12. However, the rs1801368 polymorphism does not alter the expression of MAD1L1 but rather affects the way in which MAD1 recruits MAD2 to cause a metaphase arrest.
Studies on the MAD1L1 rs1801368 polymorphism are scarce, although most of them agree that it leads to chromosomal instability, aneuploidy, and tumorigenesis13. For example, a study by Guo et al. reported that the MAD1L1 rs1801368 polymorphism decreased the capacity of MAD1 to bind to MAD2 and promote mitotic arrest, which elevates the risk for lung cancer14. A different study, by Zhong et al., found that the polymorphism confers risk for colorectal cancer development15. Furthermore, reduced levels of MAD2 have been associated with poor outcmes and chemoresistance in ovarian cancer16,17.
The fact that the polymorphism causes chromosomal abnormalities leads to important clinical implications. Aneuploidy has been previously associated with chemoresistance18 and poor prognosis in ovarian adenocarcinoma19. Furthermore, studies as far back as that of Friedlander et al. have shown a significant correlation between ploidy and clinical stage, where they found that all of their examined diploid ovarian adenocarcinomas were in Stage I or II, and all late-stage tumors were aneuploid20 (Fig. 2).

Figure 2 A and B: Proposed mechanism of the effect of the MAD1L1 polymorphism on clinical responses. The polymorphism substitutes an arginine for a histidine in the second leucine zipper in the mitotic arrest deficiency (MAD1) protein, which leads to decreased MAD2 recruitment. This affects the function of the mitotic checkpoint complex, which leads to tumorigenesis, aneuploidy, and chromosomal instability. The final consequences are a higher clinical stage at diagnosis and chemoresistance, leading to a more aggressive tumor with poor prognosis.
We found an association between MAD1L1 genotype and clinical stage at diagnosis, in which having at least one of the MAD1L1 polymorphic alleles correlated with more advanced clinical stage. Since clinical stage is considered to be part of the predictors for chemoresistance in ovarian adenocarcinoma21, it may be a mediator in the causal pathway from the polymorphism to chemoresistance. In our study, evidence to this is that after performing a Baron and Kenneys mediation analysis, we found an association between MAD1L1 genotype and chemoresistance (p = 0.024, Table S1), MAD1L1 and clinical stage (p = 0.019, table 1), and clinical stage and chemoresistance (p < 0.001, not shown), and the overall association between MAD1L1 and chemoresistance was lost when adjusting by clinical stage (p = 0.156, not shown).
Histological subtype is also classically described as being closely associated to chemoresistance. For example, although high-grade serous papillary adenocarcinomas tend to respond well initially to chemotherapy21, they have a higher rate of recurrence22 with progressively increased resistance to chemotherapy23. In our study, high-grade serous papillary tumors were more likely resistant to chemotherapy and had lower disease-free survival and lower overall survival. Furthermore, most high-grade serous papillary tumors had polymorphic MAD1L1 genotype. However, the association between MAD1L1 and chemoresistance was maintained even when adjusting by histology in the regression model.
Optimal cytoreduction is one of the most important prognostic factors in ovarian adenocarcinomas; that is, patients who are taken to optimal cytoreduction have a higher overall survival24. In our study, both groups of patients were taken to cytoreduction with similar rates. Although it did not achieve statistical significance, there was a clear tendency for the polymorphic group to less likely reach optimal cytoreduction.
A previous study by our group showed that the rs1801368 MAD1L1 polymorphism was associated with resistance to chemotherapy in ovarian adenocarcinomas but found no association between the polymorphism and disease-free survival or overall survival11. Our current study found statistically significant association for both. Since the polymorphism is closely associated to serous papillary histology and to more advanced disease at diagnosis, lower disease-free survival and overall survival are expected.
Weaknesses of our study are its retrospective nature, the fact that our study included all histological subtypes of adenocarcinomas and that there was insufficient information to determine whether chemoresistance is directed toward platins, taxanes, or both. Furthermore, since the number of deaths among patients with the wild-type genotype was low, we could not calculate a median survival for this group. Our group previously published a study on the same MAD1L1 rs1801368 polymorphism that focused more on mechanistic aspects and failed to find associations on most clinical variables, probably due to a reduced sample size11. Our current study performed in a different group of patients, analyzed more in-depth the clinical implications of the MAD1L1 rs1801368 polymorphism, as well as its association to chemotherapy, validating previous findings, and is the first to correlate the polymorphism with disease-free and overall survival.
The MAD1L1 rs1801368 polymorphism significantly worsens prognosis in patients with ovarian adenocarcinoma, most likely through indirect effects such as chromosomal instability that leads to chemoresistance and more advanced disease at diagnosis, although independent of histology. Traditional therapy for ovarian cancer with platins and taxanes may not be the optimal therapeutic target in patients carrying the MAD1L1 rs1801368 polymorphism. Although our study only included women who were treated with platins and taxanes, future research should focus on the effect of novel therapies on women with this polymorphism. For example, bevacizumab, an anti-vascular endothelial growth factor monoclonal antibody, has shown improved prognosis as a first-line therapy and as a treatment for recurrence in ovarian cancer25,26. Likewise, olaparib, a PARP inhibitor, has shown up to 70% decrease in recurrence when used as maintenance therapy27. With these new treatment options and appropriate research, it is likely that women with the MAD1L1 rs1801368 polymorphism will be able to receive more adequate, individualized therapy.
SUPPLEMENTARY DATA
Supplementary data are available at Revista de Investigación Clínica online (www.clinicalandtranslationalinvestigation.com). These data are provided by the corresponding author and published online for the benefit of the reader. The contents of supplementary data are the sole responsibility of the authors.
SUPPLEMENTARY MATERIAL










 nueva página del texto (beta)
nueva página del texto (beta)


