Introduction
In December 2019, in Wuhan, China, a type of pneumonia associated with a new coronavirus that causes severe acute respiratory syndrome emerged; it was named SARS-CoV-2.1 By May 2020, more than 7,000 publications had been generated, including more than 150 articles on molecular aspects (according to the National Center for Biotechnology Information of the United States),2 which shows a clear interaction between clinical practice and biomedical research to find possible solutions to the health problem represented by the pandemic caused by this virus.
SARS-CoV-2 is a betacoronavirus that infects human and vertebrate epithelial cells. It has a positive single-stranded RNA of approximately 29,900 nucleotide bases covered by a lipid membrane, with more than 80 % genomic similarity to SARS-CoV-1.3 In addition, it shows similarities to the sequences of coronaviruses that infect the bat and the pangolin, as well as to those of the human immunodeficiency virus; in other words, it is a chimera virus of natural divergent evolution.4,5 These viruses are known to code for 23 proteins, among which the most widely studied are structural N-protein (nucleocapsid), S-protein (spike, crucial for its entrance to the cell, virulence and tropism), M-protein (membrane) and envelope protein.6
Usefulness and transcriptional complexity of SARS-CoV-2 mutations
Like other RNA viruses, SARS-CoV-2 mutation rate is high, which represents a real challenge for vaccine development and is associated with severe clinical presentations and geographic differences. For example, the mortality rate has been reported to be different in China (5.6 %) than outside that country (15.2 %), which could be attributed to genomic differences combined with other factors such as isolation strategies and herd immunity.7
The virus mutates to adapt to the host and thus it evolves. Thirteen genome variations contained in open reading frame (ORF) 8, ORF1ab, S, ORF3a and N regions have been identified; positions 28144 (ORF8) and 8782 (ORF1a) have mutation rates of 30.53 % and 29.47 %, respectively.8 RNA-dependent RNA polymerases, especially at position 14408, are associated with the appearance of emerging multiresistant viral phenotypes.9 Knowing the mutations will allow the development of targeted drugs, such as favipiravir, remdesivir, ribavirin for other diseases caused by RNA viruses.10
SARS-CoV-2 functional architecture is extremely complex. It is suggested that this virus’ ORFs might serve as accessory proteins to modulate viral replication. Discontinuous transcription from its transcriptome and epitranscriptome generates new genomic fusions, causing more than 40 modifications that contribute to its pathogenicity, viral cycle, successful host immune response evasion and survival.9
First SARS-CoV-2 infection target and detection tests
SARS-CoV-2 infection main targets have been reported to be nasopharyngeal tract epithelial cells.1 Therefore, the approved technique for molecular diagnosis of this virus is viral load estimation by polymerase chain reaction (PCR) in a nasopharyngeal tract sample (which can also be obtained from feces or blood) obtained with a swabbing technique, according to the Berlin protocol published on January 17, 2020.11 This type of tests, while being “fast”, require time, since viral RNA purification is essential and the interval for diagnosis is at least eight hours. To date, the United States Food and Drug Administration has approved various molecular tests.12
On the other hand, there are faster serological tests that detect high concentrations of IgG and IgM antibodies; unfortunately, most of them have low sensitivity and specificity. Since COVID-19 symptoms are similar to those of other respiratory diseases, it is essential to rule out infections with other RNA viruses.
The application of diagnostic tests and clinical data allow early, late and convalescent or recovered cases to be identified. Especially in early cases, viral load should be estimated, in addition to applying a second test to define the probability of community infection. Once the time window elapses, antibody production is intensified (approximately at second week) and serological tests can be carried out for disease control.13-15
Cellular proteins as receptors for entry of the virus into the host
Evidence indicates that SARS-CoV-2 molecular targets are cells that express angiotensin-converting enzyme 2 (ACE2, Figure 1) –involved in lowering blood pressure by catalyzing the cleavage of angiotensin II into angiotensin–,16 membrane molecule CD14717 (Figure 2) –which stimulates fibroblasts for the production of matrix metalloproteinases and is associated with spermatogenesis and embryo implantation– and serine protease TMPRSS2 (Figure 3), regulated by androgenic hormones, among other proteins.18 The most widely studied receptor is ACE2, which acts as a receptor for the glycoproteins of the virus’ spike.19 Previous data suggest SARS-CoV-2 tropism towards cells that express its target cellular proteins.
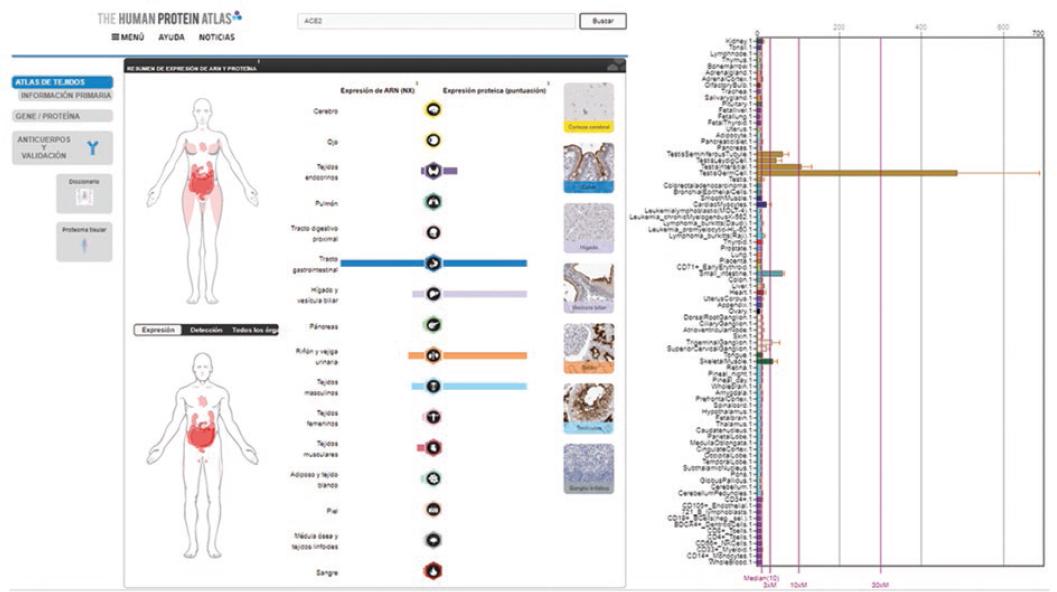
Figure 1 Anatomical sites of ACE2 protein expression that constitute potential targets for interaction with SARS-CoV-2 on each gender. The icons indicate anatomical sites of RNA expression and protein expression. ACE2 tissue expression is also exemplified by immunohistochemistry. The bar graph on the right shows relative expression in different types of cell, and overexpression in testicular cells (ochre colored bars) is highlighted. Source: The Human Protein Atlas.
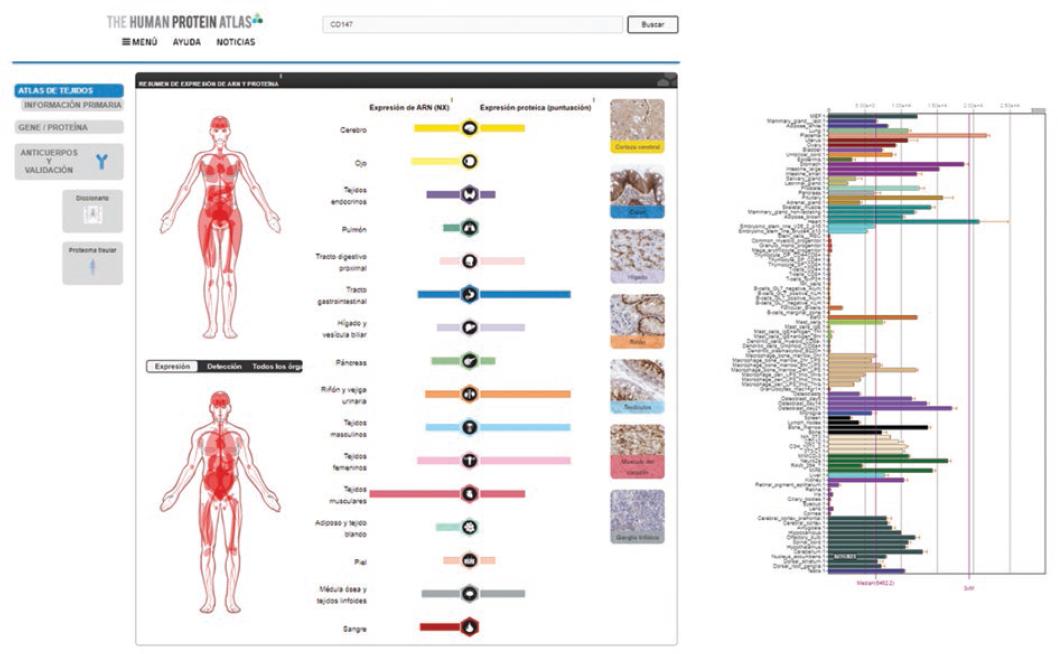
Figure 2 Anatomical sites of CD147 expression that represent potential targets for interaction with the SARS-CoV-2 virus. The bar graph on the right shows relative expression of different cell types in most part of the body. Source: The Human Protein Atlas.
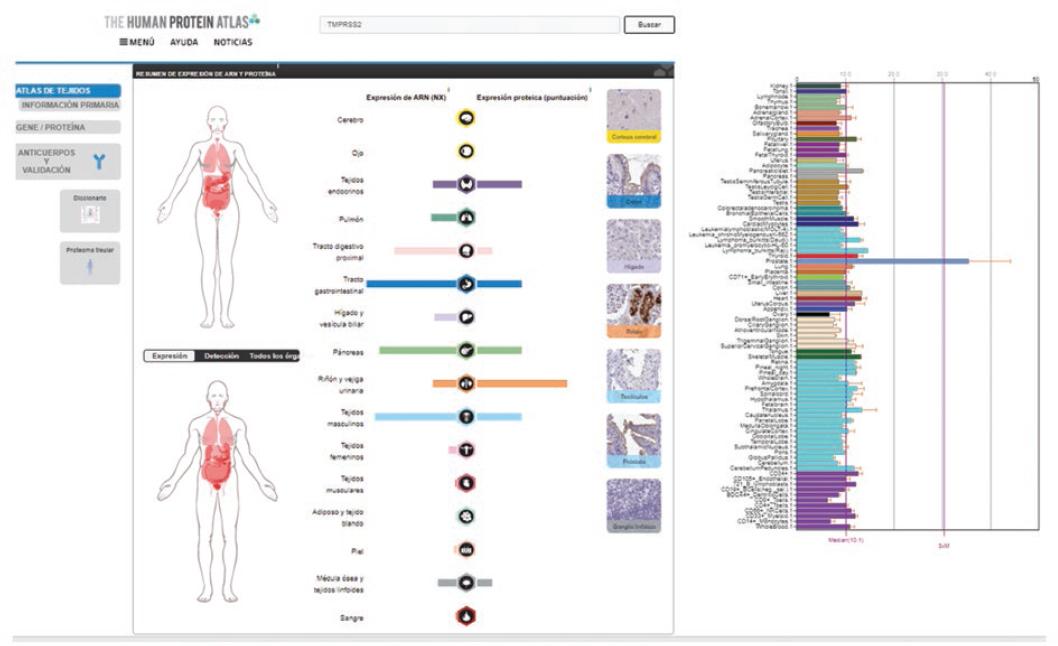
Figure 3 Anatomical sites of TMPRSS2 cellular protein expression as potential targets for interaction with the SARS-CoV-2 virus. The bar graph on the right shows different cell types relative expression in most part of the body, and higher expression in the prostate (blue bar) is highlighted. Source: The Human Protein Atlas.
Accessible database information
A search in different databases was carried out to determine other potential infection targets. The Human Protein Atlas20 was chosen, where all human proteins in cells, tissues and organs are mapped by integrating experimental results obtained from various genomic and histological tools; as well as BioGPS, which brings together information related to gene expression in different sites of the human body.21 In an in silico analysis, the expression of ACE2 and CD147 was observed on various anatomical sites (Figures 1 to 3); ACE2 was observed in respiratory tract epithelial cells.18-22
Receptor expression in specific cells: the testes
A recent study showed that ACE2 expression is higher in a small population of type II alveolar cells, which are involved with pneumonia clinical presentation; mononuclear cell alteration, small thrombosed vessels, megakaryocyte activation and platelet aggregates were also observed.23 Other investigations show that deterioration is general and that it also involves the urinary system,24 the central nervous system neurotropic mechanism25 and enterocytes,26 which shows that these cells possess viral genomes and express the aforementioned receptors. In addition to the above, CD147 expression shows that numerous organs and tissues might be susceptible to SARS-CoV-2 infection. The high expression of these cellular proteins in the testes stands out. No viral sequences were detected in the only report on the search for SARS-CoV-2 in testicles analyzed on autopsies; however, it should be noted that the search was performed using in situ hybridization, a technique that is not comparable with PCR.27
Similarities of SARS-CoV-2 and its latency with other RNA viruses
Parotiditis (mumps) RNA virus infection is very similar to that of COVID-19: it affects epithelial cells (where viral load increases and causes viremia), it is transmitted by droplets of saliva from the carrier, either by direct or indirect contact with fomites and biofluids, and shows tropism towards cells of the salivary glands, of the central nervous system, testicles and ovaries; a parotiditis sequel is orchitis, which is associated with infertility.28
Therefore, we should wonder whether the prodromal symptoms of influenza, other respiratory diseases (such as mumps) and those of SARS-CoV-2 infection are just a coincidence. In this context, ACE2 high expression in the testes could be correlated with SARS-CoV-2 infection latency; however, there is no clear evidence on this association. Therefore, we consider the need to investigate ACE2 expression in testicular tissue on autopsies of individuals who die from COVID, as well as SARS-CoV-2 sequences detection by PCR, in order to determine if chronic or latent infection existed. A sequel of viral infections (with DNA and RNA viruses) is latency, as in hepatitis29 and herpes.30 In COVID-19 patients who did not show symptoms, viral RNA was not identified with commercial tests practiced during follow-up (more than four weeks);31 however, with Sherlock’s technology, whose detection limit is 100 copies/mL, in nasal and anal samples, 75 % of positive results were obtained for S gene and 41.6 % for ORF genes.32
The referred data indicate SARS-CoV-2 sequences persistence for at least two months in the respiratory and digestive tracts (latent infection) and that anal swab can be another follow-up test, hence the importance of innovating and combining more sensitive and specific clinical and molecular resources, in order to rule out false positive and false negative results to the extent possible.33 So far, information on latent SARS-CoV-234 infection and its consequences is insufficient.
It would be desirable that COVID-19 recovered patients, as well as intensive care and cardiopulmonary resuscitation personnel (without a parotiditis history) who directly interact with individuals with SARS-CoV-2 infection could undergo molecular studies (including spermatobioscopy), in order to rule out sperm damage and absence of latent infection. Therefore, it is urgent for more evidence on the subject to be available. During the writing of this document, a report in this regard was published.35
Conclusions
Given the comorbidities of the Mexican population, SARS-CoV-2 infection represents a huge challenge and a “Pandora box” and, therefore, the possibility of new pathophysiogenic mechanisms should not be ruled out. SARS-CoV-2 involvement could be suggested if there was coincidence of infection in testicular cells that express this virus’ receptors.











 text new page (beta)
text new page (beta)


