Introduction
The family Tetraodontidae comprises 193 species in 28 genera (Fricke et al. 2021). The fishes in this family are characterized by having intestines with a distensible ventral diverticulum, a modified mandible with 4 dental plates (2 upper and 2 lower), and dorsal and anal fins supported by rays (McEachran and Fechhelm 2005). They inhabit tropical and temperate seas around the world; some species are oceanic, others are coastal, and others can foray into estuaries, whereas some are strictly freshwater species (Shipp 2002). In various parts of the world, tetraodontids are commercially important and support coastal fisheries. Culturally, they are highly valued in Asian cuisine. However, some species have a toxin called tetrodotoxin, which is a potent neurotoxin found mainly in the liver, gonads, stomach, and skin (Smith-Vaniz et al. 1999).
Within the Tetraodontidae, the genusLagocephalusSwainson, 1839 is made up of 9 valid species, but there is evidence of a greater number of species yet to be described (Matssura 2015), which indicates that a taxonomic review of the genus is needed.Lagocephalusspecies are characterized by the presence of a ventrolateral skinfold along the body; a broad silvery-white band on the sides, at eye level, running from snout to caudal peduncle; a divided lateral line, with ventral and lateral extensions; and 2 openings in the nasal organ (Matssura 2010).
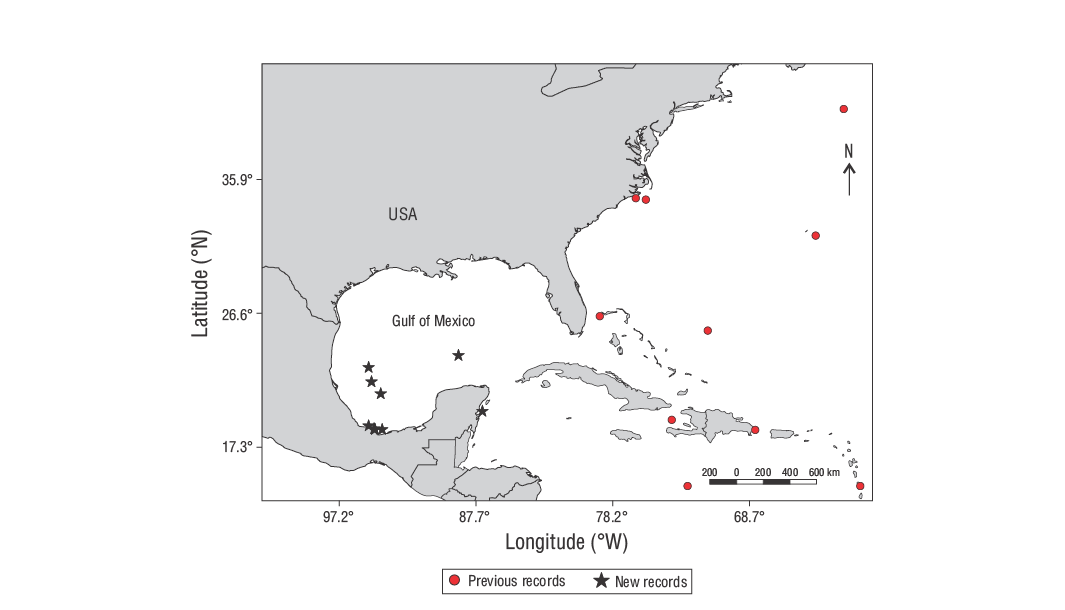
In the western Atlantic Ocean, the genusLagocephalusis represented by 3 species:Lagocephalus laevigatus, with an amphiatlantic distribution;Lagocephalus guentheri, with sporadic records in northeastern Brazil; andLagocephalus lagocephalus, with a broader distribution, in both Atlantic coasts and along the Indo-Pacific coast (Aguilera et al. 2018). Records ofL. lagocephalusin the western Atlantic Ocean are scarce and sporadic (Shipp 1974, Smith-Vaniz et al. 1999); to date, there is no evidence of its presence within the Gulf of Mexico (McEachran and Fechhelm 2005, McEachran 2009). This study documents the first records ofL. lagocephalusin the Gulf of Mexico and contributes to a better understanding of the ichthyofaunistic diversity in Mexico.
Materials and methods
The Gulf of Mexico is a partially isolated body of water, with a surface area of 1,138,980 km2. It is connected to the Atlantic Ocean and the Caribbean Sea and delimited by an imaginary line from Key West (USA) to Cape Catoche (Mexico) (McEachran and Fechhelm 1998).
Specimens of the oceanic puffer,L. lagocephalus, were caught in 2018, 2020, and 2021. In 2018, catches came from 4 mid-water fishing sets, made with a 25/25 NMWT-type net on 28 May, 28 July, and 1-2 August in oceanic waters of the exclusive economic zone of the Gulf of Mexico (Fig. 1), at depths between 30 and 126 m (119°35ʹ45.68ʺ N, 87°15ʹ19.00ʺ W; 22°50ʹ29.23ʺ N, 95°09ʹ37.14ʺ W; 21°50ʹ30.64ʺ N, 94°57ʹ43.69ʺ W; 21°00ʹ41.29ʺ N, 94°19ʹ09.50ʺ W), during the research cruises JCFINP1805 and JCFINP1807 aboard the R/VDr. Jorge CarranzaFraser of the National Institute of Fisheries and Aquaculture. Another adult specimen was caught on 13 August 2018 at the edge of the continental shelf and the slope of the Yucatán Peninsula (23°40ʹ28.56ʺ N, 88°55ʹ9.48ʺ W), at approximately 312 m depth, during the cruise JCFINP1807, with a surface longline and a circle hook baited with the vermilion snapper,Rhomboplites aurorubens. The specimens are in the process of being deposited in the Colección Nacional de Peces del Instituto de Biología of the National Autonomous University of Mexico (UNAM, for its acronym in Spanish). In 2020 and 2021, 8 specimens were caught with a bottom longline with No. 6-10 hooks (Del Moral-Flores et al. 2020) on 12 and 27 December 2020, 10 and 21 April 2021, 24 June 2021, and 17 December 2021, in the southwestern Gulf of Mexico, Tuxtlas region, Veracruz. The catch area corresponds to depths of 250, 280, and 180 m (Fig. 1). Specimens were deposited in the Colección Ictiológica de la Facultad de Estudios Superiores Iztacala, UNAM (CIFI). Taxonomic determination was made using taxonomic keys (Shipp 2002, McEachran and Fechhelman 2005). Weight (Wt), biometric measurements, and meristic features were based on Jribi and Bradai (2012). We examined 3 specimens to extract and weigh the gonads (gonad weight, Wg) and obtained the gonadosomatic index (GSI), which was calculated as a percentage of the Wg/(Wt - Wg) ratio (Devlaming et al. 1982).
Results
The collected specimens were determined as follows:
Class: Actinopterygii
Order: Tetraodontiformes Regan 1929
Family: Tetraodontidae Bonaparte, 1832
Species:Lagocephalus lagocephalus(Linnaeus, 1758) (Figs. 2-3, Table 1).

Figure 2. Lagocephaluslagocephalusspecimens caught in the southwestern Gulf of Mexico (CIFI record No. 1780, 360-398 mm standard length), Veracruz, Mexico.

Figure 3. Sexual dimorphism ofLagocephalus lagocephaluscaught in the southwestern Gulf of Mexico. (a) Specimens in reproductive phase, female (top) and male (bottom), with their respective gonads; (b) ventral region showing the urogenital area for each sex.
Table 1. Morphometric and meristic data ofLagocephalus lagocephalusspecimens caught in the Gulf of Mexico. The corresponding registration numbers (CIFI) are shown.
| Morphometric data | CIFI 1780 | CIFI 1780 | CIFI 1781 | CIFI 1850 | CIFI 1900 |
| Total length (mm) | 478 | 432 | 486 | 587 | 570 |
| Standard length (mm) | 398 | 360 | 396 | 484 | 462 |
| Fork length (mm) | 440 | 405 | 441 | 537 | 519 |
| Head length (mm) | 105 | 104 | 114 | 126 | 134 |
| Maximum body height (mm) | 140 | 101 | 98 | 133 | 109 |
| Eye diameter (mm) | 21 | 23 | 22 | 27 | 24 |
| Pre-dorsal length (mm) | 279 | 262 | 283 | 310 | 312 |
| Preanal length (mm) | 285 | 261 | 294 | 352 | 318 |
| Snout length (mm) | 42 | 40 | 52 | 56 | 54 |
| Postorbital length (mm) | 39 | 39 | 44 | 46 | 56 |
| Interorbital length (mm) | 43 | 40 | 48 | 50 | 49 |
| Caudal fin length (mm) | 101 | 86 | 104 | 128 | 127 |
| Pectoral fin length (mm) | 78 | 67 | 78 | 98 | 94 |
| Peduncle caudal height (mm) | 27 | 24 | 34 | 28 | 27 |
| Dorsal fin height (mm) | 60 | 55 | 55 | 78 | 81 |
| Anal fin height (mm) | 58 | 48 | 60 | 70 | 75 |
| Weight (g) | 845 | 1,080 | 955 | 1,900 | 1,655 |
| Meristic data | |||||
| Dorsal fin | 14 | 14 | 14 | 14 | 14 |
| Anal fin | 14 | 13 | 13 | 13 | 13 |
| Pectoral fin | 15 | 16 | 15 | 16 | 16 |
| Caudal fin | 10 | 11 | 10 | 11 | 11 |
Meristic and morphometric data of the examined non-inflated specimens are shown in Table 1. The body is elongated, fusiform, and narrow; its height, between 2.8 and 4.2 times the standard length (SL). The interorbital distance is equal to or less than the length of the snout. The mouth is terminal, with 2 strong dental plates in each jaw. The snout is blunt and its length is between 2.2 and 2.6 times the cephalic length. The eye is round and its diameter is 15.0 to 19.0 times in SL; it comprises between 18% and 22% of the cephalic length. The nostrils are located in the middle part of the snout. The dorsal fin and anal fin are falcate; the insertion of the first precedes the second. The caudal fin is black and emarginate; the ventral lobe is longer than the dorsal lobe. Pelvic fins are absent.
Dorsolateral coloration is dark and delimited ventrally at the upper margin of the eye. The sides have a wide, whitish-to-greyish, iridescent stripe that runs from the snout to the base of the caudal fin; ventrally, it is white, mainly in the abdominal area where the stomach distends when inflated. The dorsal, anal, and caudal fins are black.
During the catch and handling of the 3 specimens (CIFI registration No. 1925) caught on 17 December 2021, 2 males and 1 female, we observed the release of gametes. The GSI was high in each case: 3.63, 4.39, and 5.68. Testicles are elongated, consistent, and cream-colored. Ovaries are larger and more robust, vascularized, yellowish in color, and creamy, and oocytes are visible to the naked eye and occupy the entire gonad (Fig. 3a). Externally, males show a conical genital papilla, whereas females have a distended oviductal pore (Fig. 3b).
In total, 18L. lagocephalusspecimens were caught. Nine of these specimens were juveniles (21-30 mm SL and 1-2 g) caught in the oceanic part of the Gulf of Mexico and Caribbean waters off Mexico: one of them (30 mm SL and 2 g) at 30 m depth (JCFINP1805) and the remaining 8 (24-30 mm SL and 2-3 g) between 67 and 126 m depth (JCFINP1807). One specimen was an adult (500 mm SL and 1,400 g) caught with longline in the oceanic part at 312 m depth (JCFINP1807). Eight specimens were adults caught in the coastal region of Veracruz, southwestern Gulf of Mexico, during bottom fishing: 2 specimens (360-398 mm SL, CIFI 1780) on 12 December 2020, near the town of Zapotitlán, at a depth close to 280 m (18°46ʹ56.70ʺ N, 95°09ʹ40.20ʺ W); 1 specimen (396 mm SL, CIFI 1781) on 27 December 2020, near the town of Zapotitlán, at a depth close to 280 m (18°32ʹ19.99ʺ N, 94°43ʹ42.38ʺ W); 1 specimen (484 mm SL, CIFI 1850) on 21 April 2021, near the town of Zapotitlán, at a depth close to 180 m (18°32ʹ19.99ʺ N, 94°43ʹ42.38ʺ W); 1 specimen (462 mm SL, CIFI 1900) on 24 June 2021, at a depth between 160 and 300 m (18°34′32.10″ N, 94°46′58.32″ W); and 3 specimens (CIFI 1925) on 17 December 2021, at a depth close to 180 m (18°34′32.10″ N, 94°46′58.32″ W). Specimens from the Veracruz coast were caught between 160 and 300 m depth, where the dominant substratum is rocky with sand patches. Oceanic specimens were part of the catch of fishing sets made in mid-water, which was dominated by species of the Myctophidae family; juvenile stages of species of Bothidae, Acanthuridae, and Congridae; and invertebrates such asPyrosomasp.
Discussion
Records ofL. lagocephalusin the western Atlantic Ocean are sparse. There are records of the species in Canada (Scott and Scott 1988), Florida (USA) and Curaçao (Shipp 1974), North Carolina (USA) (Rohde et al. 1995), Bermuda (Templeman 1962), and Brazil (Santos-Sampaio et al. 2001). There is a record in the northeastern part of Yucatán that must be verified (GIBF 2021), as it could have resulted from a misperception ofL. laevigatus, whose frequency is predominant in the coastal region. Schmitter-Soto et al. (2000) had not recorded the presence ofL. lagocephalusin Caribbean waters off Mexico until the year 2000.
There is good knowledge of ichthyofaunistic diversity in the Gulf of Mexico (Hoese and Moore 1998; McEachran and Fechhelm 1998, 2005). In the last review, 1,541 fish species were recorded (McEachran 2009). Within this diversity, the Tetraodontidae family is represented by 9 species (McEachran 2009), but the present record would increase the number of known species in said sea. Shipp (2002) noted thatL. lagocephalusis present in the Gulf of Mexico, without detailing any records, probably based on the area of distribution of the species. The presence ofL. lagocephaluscould have been obviated due to its habits, as it is frequently present in oceanic waters, which makes its catch and study difficult. Oceanographic anomalies could influence the distribution of the species, since adult specimens were caught after norte events, which could have displaced the specimens towards the coastal zone. Juvenile catches in the oceanic part possibly resulted from the gyres and meanders of the Yucatan Current (Carrillo et al. 2017), which introduce water from the Caribbean Sea into the Gulf of Mexico (Muhling et al. 2013). An example of this is the presence of a juvenile in the Caribbean Sea off the coast of Quintana Roo, Mexico. In addition, the reproduction ofL. lagocephalusin the Gulf of Mexico was evidenced based on the high values of the GSI and the state of maturity of gonads (in reproductive state) (Sánchez-Cárdenas et al. 2007).
The depth at which the specimens were caught coincides with the great depth at which a specimen was caught in the northeastern part of Brazil (Santos-Sampaio et al. 2001). Records indicate that in addition to being strictly pelagic,L. lagocephaluscan reach depths close to 1,000 m.
In Mexican waters, the first record ofL. lagocephaluscorresponds to the description of the subspeciesLagocephalus lagocephalus nigridorsumby Fowler (1944) based on several small specimens that were caught north of the Islas Marías, in the eastern Pacific (Castro-Aguirre and Lachica-Bonilla 1973). Further studies are needed to understand the validity of the extant subspecies in Mexican coasts.
Recently, as a result of their medical relevance when poisonous species are consumed erroneously, the impact of invasive species, and the management and conservation of puffer fish, genetic studies have been done to try to recognize and clarify intra- and interspecific relationships within the family Tetraodontidae (Turan et al. 2017, Giusti et al. 2019).











 texto en
texto en