Introduction
Mycotoxins are metabolites produced by specific filamentous fungi that colonize several human foods and animal feeds (García and Heredia, 2013). Mycotoxins ingested by humans or animals can cause a toxic response referred as mycotoxicosis, with acute, subchronic, or chronic manifestations in animals (Streit et al., 2013). The mycotoxins most frequent in animal feedstuffs include aflatoxins, deoxynivalenol, zearalenone, fumonisins, ochratoxin A, and T-2/ HT-2 toxins (Bryden, 2012). Fungal growth and its ability to produce mycotoxins in forage is influenced by complex interactions of abiotic and biotic factors, such as the aggressiveness of the fungal species, host susceptibility, environmental factors (temperature or water availability), and agrotechnological systems (Alonso et al., 2013).
Aflatoxins produced mainly by Aspergillus flavus and A. parasiticus, are of greatest concern from a global perspective (Bhat et al., 2010). When ingested, they can cause liver necrosis, acute toxicity, cancer, or even death in humans and animals (Duarte et al., 2010). Corn (Zea mays L.), rice (Oriza sativa L.), sorghum [Sorghum bicolor (L.) Moench], barley (Hordeum vulgare L.), rye (Secale cereale L.), wheat (Triticum aestivum L.), and cottonseed (Gossypium hirsutum) are used as animal feeds and are targets of contamination (Duarte et al., 2010; Torres et al., 2014).
Fumonisins, which are produced mainly by members of Fusarium and by some Alternaria species, occur frequently in corn and other cereals (Streit et al., 2013). Fumonisins are associated with equine leukoencephalomalacia, hydrothorax, and porcine pulmonary edema syndrome (Bhatnagar and García, 2013); besides, they can affect the immune system (Streit et al., 2013).
Ochratoxin A is produced by A. alutaceus, A. carbonarius, A. niger, and Penicillium verrucosum (García and Heredia, 2013). This toxin can be found in cereal grains, beans, peanuts, and meat (Pereira et al., 2014), and it produces nephrotoxic, teratogenic, and hepatotoxic effects in certain animal species (García and Heredia, 2013).
Deoxynivalenol, also known as vomitoxin, is produced by F. graminearurn and F. culmorum and is found on cereal crops worldwide (Wood, 1992). In animal husbandry, this toxin alters the immune system (Streit et al., 2013) and is associated with feed refusal as well as vomiting (Binder et al., 2007).
Zearalenone is a nonsteroidal, estrogenic mycotoxin produced by F. graminearum, F. culmorum, and F. heterosporum, which are common contaminants of cereal crops worldwide (Wood, 1992; García and Heredia, 2013). This toxin is associated with reproductive problems in animals and probably in humans.
T-2/HT-2 toxins are produced mainly by F. sporotrichoides, F. poae, and other Fusarium species (Wood, 1992). They are associated with lethal toxicosis in dairy cattle that have consumed moldy corn (Wood, 1992; García and Heredia, 2013).
Contamination of feeds with mycotoxin occurs when the conditions are favorable for fungal growth. Animal production systems in temperate and arid or semi-arid regions, in México, rely on cattle systems representing more than 95 % of the value of ruminant products (Améndola et al., 2006). The state of Nuevo León in northern México is located within one of the oldest and most established cattle feeding regions (Olsen et al., 2006). Between 1993 and 2003, six northern Mexican states, which have an arid or semiarid climate in approximately 74 % of their territory, exported 0.91 million cattle per year to the USA, sharing 94 % of the national export of this type of product (Améndola et al., 2006).
Mycotoxins in feeds are of both economic and health concerns because of the reduction of animal production and their accessibility to humans through animal products. To the best of our knowledge, no data in the scientific literature describe the levels of mycotoxins in feeds in the state of Nuevo León. Thus, the objective of this study was to determine the presence and levels of selected mycotoxins in alfalfa, sorghum, and grass retailed in this state.
Materials and methods
Forage samples
One hundred and twenty forage samples (40 alfalfa, 40 sorghum, and 40 grass) were collected from farms and commercial locations of the metropolitan area of Monterrey (25° 40’17” N, 100° 18’31” W), Nuevo León, from June to November 2013 and January 2014. Samples were transported to the laboratory and processed within a week.
Sample extraction
Forage samples (1 kg) were ground with a mechanical grinder and homogenized prior to extraction. To quantify total aflatoxins, fumonisin, T-2/HT-2 toxins, and zearalenone, 10 g of ground sample were mixed with 50 mL of methanol/water (7:3, v/v), and the mixture was shaken vigorously for 3 min. For ochratoxin extraction, samples (10 g) were mixed with 40 mL of methanol/water (1:1, v/v); and for deoxynivalenol extraction, samples (10 g) were mixed with 100 mL of distilled water. Samples were filtered through a Whatman No.1 paper, and the solution was used for mycotoxin determinations.
Mycotoxin assays
Total aflatoxins, deoxinivalenol, fumonisins, ochratoxin, T-2/HT-2 toxins, and zearalenone were quantified using Neogene mycotoxin kits (Veratox®). These kits are based on a direct, competitive enzyme-linked immunoassay, and the procedure used followed the manufacturer’s instructions. Briefly, 100 μL of the extracted sample and 100 μL of horseradish peroxidase-conjugated solution (toxin-HRP) were added to wells of a microwell plate provided in the kit. Aliquots (100 μL) were then transferred to wells coated with specific mycotoxin antibody, and the plate was incubated at room temperature for 2 min for aflatoxin quantification, 10 min for ochratoxin, and 5 min for the other toxins. The wells were washed five times with distilled water, and 100 μL of K-Blue substrate solution was added. After incubation for 3-5 min at room temperature, the reaction was stopped by the addition of 100 μL of Red Stop solution. The absorbance (650 nm) was measured using a NEOGEN®Stat Fax® 4700 microstrip reader. The mycotoxin concentrations were calculated using external standards for each mycotoxin provided by the kit (Roze et al., 2013). The detection limit of the kit is 1.4 ppb, 0.1 ppm, 0.2 ppm, 1 ppb, 10 ppb for total aflatoxins, deoxinivalenol, fumonisins, ochratoxin and zearalenone, respectively (determined by the mean average of 10 mycotoxin free sample plus two standard deviations; http://foodsafety.neogen.com/en/veratox#mycotoxins).
Statistical analysis
Simple random sampling was used and each sample was analyzed in duplicate. The KruskalWallis test was used to determine if all medians were equal (H0) or at least two medians were different (p≤0.05), followed by Kruskal-Wallis Multiple-Comparison Z-Value test (Dunn’s Test) to know if the medians were significantly different. The Number Cruncher Statistical System version 6.0 software (NCSS, LLC) was used.
Results and discussion
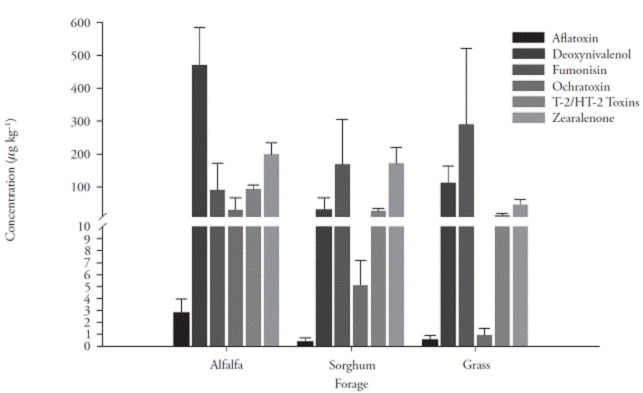
The mycotoxin levels in feeds ranged from not detectable (ND) to 6.4 μg kg-1 for aflatoxin, ND to 1.1 μg kg-1 for deoxynivalenol, ND to 1.82 μg kg-1 for fumonisin, ND to 273 μg kg-1 for ochratoxin, ND to 139 μg kg-1 for T-2/H2 toxins, and ND to 463.4 μg kg-1 for zearalenone. Alfalfa contained higher levels of mycotoxins, as compared to sorghum and grass. The average contents of aflatoxins, ochratoxin, deoxynivalenol, fumonisins, T-2/HT-2 toxins, and zearalenone present in alfalfa, sorghum, and grass are shown in Figure 1.
All samples showed multiple contaminations with mycotoxins, with at least two mycotoxins in the same sample. Alfalfa showed the highest incidence of multiple contaminations. The six mycotoxins analyzed were found in 15 of 40 alfalfa samples (37.5 %), whereas there was a lower frequency of contamination in samples of sorghum, 1 of 40 (2.5 %), and grass: 3 of 40 (7.5 %). Five mycotoxins were detected in 35 %, 40 %, and 25 % of alfalfa, sorghum, and grass samples, respectively. Four and three different mycotoxins where detected in 25 %, 32%, and 20% and 2.5%, 25%, and 20% of alfalfa, sorghum, and grass samples, respectively. Only 10 % of the grass samples had two mycotoxins.
Aflatoxins
These toxins were detected in 62 % of the alfalfa samples, with a mean level of 2.77 μg kg-1. Fewer positive samples were found in the grass (45 %; 0.5 μg kg-1) and sorghum samples (40 %; 0.36 μg kg-1) (Table 1).
Table 1 Incidence and concentration of mycotoxins in alfalfa, sorghum, and grass, in Nuevo León, México.
| Mycotoxin | Forage | #Samples | Positive samples (%) | Mean (μg kg-1) |
| Aflatoxin | ||||
| Ochratoxin | ||||
| Zearalenone | ||||
| T-2/HT-2 | ||||
| Deoxynivalenol | ||||
| Fumonisin |
Different upper case letters indicate significant differences (p≤0.05) between forages.
Fumonisins
Fumonisins were found in 54 % of the forage samples, with a range between 0.09 and 0.29 mg kg-1 (Table1). Grass showed the highest mean level (0.29 mg kg-1), followed by sorghum (0.16 mg kg1) and alfalfa (0.09 mg kg-1). The Krustal-Wallis test indicates that there were no significant differences (p>0.05).
Ochratoxin
This toxin was found in 97 of the 120 (80 %) forage samples, with a range between 0.92 and 32.74 μg kg-1. Similar to aflatoxins, ochratoxin was detected mainly in alfalfa (39/40 samples) and in 37/40 and 21/40 samples of sorghum and grass, respectively (Table 1). Besides, alfalfa showed the highest mean concentration (32.74 μg kg-1), followed by sorghum (5.09 μg kg-1) and grass (0.92 μg kg-1).
T-2/HT-2 toxins
All 120 forage samples contained T-2/HT-2 toxins and there were significant differences (p≤0.05) between forages (Table 1). Thus, higher mean concentrations were detected in alfalfa (93.7 μg kg-1) as compared to sorghum (30.2 μg kg-1) and grass (17.6 μg kg-1).
Zearalenone
All 120 forage samples contained zearalenone. Higher mean levels were found in alfalfa and sorghum (199 μg kg-1 and 171 μg kg-1, respectively), as compared to grass (41 μg kg-1) (Table 1).
Deoxynivalenol
Alfalfa contained the highest mean level of deoxynivalenol (0.47 mg kg-1), followed by grass and sorghum forage (0.11 mg kg-1 and 0.03 mg kg-1, respectively) (Table 1).
Aflatoxins are among the mycotoxins with significant impacts in a wide range of agricultural commodities (Robledo et al., 2001). Since aflatoxin contamination is difficult to avoid, limits of aflatoxin content in food and feeds were established in several countries to protect human and animal health (Torres et al., 2010), but the maximum limits vary among countries (Afsah‐Hejri et al., 2013). For example, the maximum limits of aflatoxins in cereals for human consumption established by the Mexican Secretary of Health (NOM-188-SSA1-2002; García and Heredia, 2013) and by the United States Environmental Protection Agency are 20 μg kg-1, whereas the European Food Safety Authority has set a limit of 10 μg kg-1 (Afsah‐Hejri et al., 2013). If this concentration is exceeded, the contaminated cereal can be used only for animal feed and there are maximum limits (μg kg-1): poultry 100, pork 100200, cattle 100-300 (García and Heredia, 2013).
In Vietnam, a survey was carried out, between 2004-2011, on corn and it showed that 27 % of 10 172 samples were contaminated with aflatoxins (58 μg kg-1; Skrinjar et al., 1992). A similar survey of stored sorghum in Brazil showed aflatoxin B1 (7-33 μg kg-1) in 12.8% of 104 samples (Charoenpornsook and Kavisarasai, 2006). In Thailand, aflatoxin B1 (7.5 μg kg-1) was detected in 23 of 25 (92 %) feedstuff samples (Buckle, 1983); whereas Amigot et al. (2006) detected aflatoxin in alfalfa, maize, and sorghum: 3.75±0.5 μg kg-1, 2.56±0.51 μg kg-1, and 2.82±1.51 μg kg-1, respectively. These results are similar to the aflatoxin concentration (1.21 μg kg-1) found in 49 % of the samples in our study, and its levels were higher in alfalfa, followed by sorghum and grass. Aflatoxin was found in many samples, but the levels were within the range allowed by Mexican and other international regulations for animal feeds.
In the US, the maximum limit for total fumonisins (FB1+FB2+FB3) in products used for human consumption is 2-4 mg kg-1 (Streit et al., 2012), for cattle feed is 60 mg kg-1 and for poultry feed is 100 mg kg-1 (Driehuis et al., 2008a Bhat et al., 2010; Afsah‐Hejri et al., 2013). Studies carried out on animal feed in Malaysia (Petzinger and Weidenbach, 2002), on cereals and feed samples in Croatia (Hussein and Brasel, 2001), and on feeds and feed ingredients in Asia (Bhatnagar and García, 2013) detected low levels (0.261-2.4 mg kg-1, 2.3 mg kg-1, and 0.39-1.01 mg kg-1, respectively) of these toxins. Similarly, in our study, low levels (0.09-0.290 mg kg-1) of fumonisins were detected in the forage samples analyzed.
Cereals and other agricultural products are the most prominent ochratoxin source in the human diet, and carryover of this toxin from feeds to animal food products can occur. Thus, aflatoxins in poultry can have a carryover ratio of 0.02 % in eggs, and in ruminants 1 % to 3 % in milk (Driehuis et al., 2008a; Peel et al., 2011, Volkel et al., 2011). Regulations in several countries show that the maximum permissible levels of ochratoxin in raw cereals and cereal products for human consumption are 5 μg kg-1 and 3 μg kg-1, respectively (Naicker et al., 2007). Variations in the frequencies and concentrations of this toxin are reported in some countries. A study in South Africa showed a mean of 5.2 μg kg-1 of ochratoxin A in 3 of 7 grass samples (Lincy et al., 2008). A higher incidence (30 % of samples) and levels (10.48-12.35 μg kg-1) of this toxin were found in animal feedstuff from Thailand (Charoenpornsook and Kavisarasai, 2006). In Yugoslavia, concentrations of this toxin varied from traces to 400 μg kg-1 in feed samples of hay, dried and fresh alfalfa, pelleted pulp, and silage (Skládanka et al., 2010). In our study, levels of ochratoxin A ranged from 0.9 to 32.7 μg kg-1, and the mean was 12.9 μg kg-1, which exceeded the maximum limit (5 μg kg-1) set for ochratoxin in cereal products according to EU legislation.
Corn, maize, wheat, barley, rice, oats, millet, and sorghum can be contaminated by zeralenone (Zain, 2011), whereas T-2/HT-2 toxin contamination is rare on wheat and maize. However, in our study, zearalenone and T-2/HT-2 toxins were detected in all samples analyzed of alfalfa, sorghum and grass (Table 1).
Current regulations regarding the maximum limits of zearalenone in foods and feeds in countries vary between no regulations to more than 1000 μg kg-1 (Afsah‐Hejri et al., 2013; Bhatnagar and García, 2013). Values less than 300 μg kg-1 in feeds are recommended for dairy cattle (Reyes-Velázquez et al., 2008), although ruminants are more resistant to the negative effects of mycotoxins (Wu et al., 2014). In vivo studies showed that zearalenone is rapidly metabolized in animals and humans and eliminated mainly as water-soluble glucuronides (García and Heredia, 2006). Therefore, consumption of low-dose zearalenone-contaminated feedstuffs by dairy cows does not pose a human health hazard. However, animal health and their food products can be altered when ruminants consume feed contaminated with mycotoxin for long periods (García and Heredia, 2006; Afsah‐Hejri et al., 2013). Feed contamination by this toxin is reported in several countries. Samples of sorghum imported into Japan from 2001 to 2006 had a zearalenone contamination rate of 52.5 % (84/160), with values of 60-7,260 μg kg-1 (Aoyama et al., 2009). Cow feedstuffs in Argentina were contaminated with this toxin (1,200-3,006 μg kg-1) according to Zain (2011), whereas in South Africa, all samples of pasture grasses contained mycotoxin (mean, 2,500 μg kg-1), which is higher than the allowable concentration for dairy and beef cattle (Lincy et al., 2008). In our study, this toxin was detected in all samples, but the concentrations were lower (49.1-199.5 μg kg-1).
Data regarding the prevalence of T-2/HT-2 toxins in commodities and feed in Mexico are limited (García and Heredia, 2013). In a study performed in Nayarit, only one sample (5 %) of forage maize was contaminated (7 μg kg-1) according to Reyes-Velázquez et al., 2008). However, in our study there was 100 % incidence of T-2/HT-2 toxins in the samples and higher concentrations (17.6-93.7 μg kg-1). Our results are similar to those reported in: Croatia, 6.09-67.68 μg kg-1 in 76 % of the feed samples; Thailand, 6.91 μg kg-1 in all the feed samples (Buckle, 1983), in India, 12-130 μg kg-1 in all the sorghum samples (Klarić et al., 2009).
Deoxynivalenol is the mycotoxin most commonly found (20-100 %) in forage feeds (particularly in corn) and in ingredients used in concentrate feeds (Driehuis et al., 2008b; Skrinjar et al., 1992). In our study, deoxynivalenol was found in alfalfa (92.5 %), sorghum (25 %), and grass (67.5 %). Although a high incidence of deoxynivalenol was found, the levels were relatively low (0.035-0.47 mg kg-1) and at allowable levels (0.5-1.0 mg kg-1), according to Aoyama et al. (2009). Our results are similar to those reported in Europe for feed samples of alfalfa (0.187±0.059 mg kg-1), maize (0.167 ±0.051 mg kg-1), and sorghum (0.029±0.015 mg kg-1) (Amigot et al., 2006) as well as compound feed (0.348-2.40 mg kg-1) (da Silva et al., 2000).
In our study, all samples showed two or more mycotoxins, which is similar to results from other countries, where two or more mycotoxins were found in 75-100 % of samples (Streit et al., 2012). The co-occurrence of multiple mycotoxins observed in our study could be due to: 1) most fungi are able to simultaneously produce various mycotoxins, 2) commodities can be contaminated by several fungi, and 3) feed can be composed by various contaminated commodities (Streit et al., 2012). The fact that a specific feed carries more than one mycotoxin could affect animal health even at low doses, and risk assessment analysis must be carried out in order to reduce o eliminate human harm by its exposure.
Conclusion
To the best of our knowledge, this is the first study to provide data regarding the status of mycotoxin contamination of forage used for animal feedstuffs in the state of Nuevo León. Most mycotoxins were detected at levels allowed according to international regulations. However, most samples were contaminated with more than two mycotoxins, which could be a risk for animal and human health. These data show the need to establish appropriate control measures to reduce the risk of contamination of feeds, particularly when mixtures with forage susceptible to fungal contamination are used.











 text in
text in