Introduction
Colorectal cancer (CCR) is among the leading causes of cancer in the world with more than 1.2 million cases, with almost half of them dying at 1 year. In the last decades, a decrease has been observed in the number of cases, by virtue of the expansion of early detection methods, but with an increase in the population younger than 50 years, without the reason being clear1.
At the moment of diagnosis, between 20% and 30% of subjects have metastatic disease, and nearly half the patients at Stage II-III will develop recurrence, with this being the cause of death in most of them. Standard treatment for these patients is systemic chemotherapy (CT) (oxaliplatin, irinotecan, and any fluoropyrimidine) with some monoclonal antibody, either an anti-angiogenic agent such as bevacizumab, ramucirumab or aflibercept, or an epidermal growth factor receptor (EGFR) antagonist such as cetuximab and panitumumab, according to the RAS mutation status. Since some years ago, incorporation of other drugs, both cytotoxic agents and, recently, immune therapy has been investigated in selected populations2-4.
In the process of molecular research, regorafenib, a drug with anti-tumor activity in patients with advanced CCR, was identified in a Phase I trial5. Regorafenib, a multi-targeted small molecule, was shown to block the activity of several protein kinases, including angiogenesis (VEGFR1, VEGFR2, and VEGFR3), oncogenesis through KIT, RET, RAF1, and BRAF, as well as tumor microenvironment with PDGFR and FGFR. In 2013, Dr. Grothey, and Dr. Li in 2015, published two Phase III studies (the CORRECT and CONCUR trials, respectively) where they demonstrated regorafenib clinical benefit in heavily treatment-experienced patients with advanced CCR versus placebo in terms of progression-free and overall survival (OS)5,6. In both trials, patients were randomized to receive regorafenib 160 mg by the oral route at a single daily dose for 3 weeks with 1 weeks rest every 4 weeks versus placebo. At first analysis, a 23% reduction was shown in the mortality risk (HR = 0.774; 95% CI: 0.64-0.94) versus placebo, with a median of 196 and 151 days, respectively, and, finally, in the second analysis, a 21% reduction was documented in the mortality risk (HR = 0.79; 95% CI: 0.66-0.94), with a median OS of 194 and 152 days for regorafenib and placebo, respectively5,6.
The purpose of the present study is to report the efficacy and toxicity results with the use of regorafenib in a Mexican population with heavily treated metastatic CCR (mCRC).
Methods
This was a multi-center, retrospective cohort study that took place in the period from July 2016 to June 2017. Heavily treatment-experienced patients (treated with two or more lines of treatment) with metastatic and/or recurrent colon or rectum adenocarcinoma who had received at least one cycle of regorafenib were included in the study.
Demographic data such as gender, age, comorbidities, clinical symptoms at treatment initiation, performance status (ECOG scale), KRAS/NRAS status, metastatic sites, previous CT lines and use of monoclonal antibodies (bevacizumab, cetuximab, or panitumumab), date of diagnosis, date of treatment initiation, received cycles, date of progression, dose modification or interruption, adverse effects, and NCI (CTCAE v.4) toxicity grade were recorded7.
Overall response rate (complete response [CR] + partial response [PR]) and disease control rate (CR+PR + stable disease [SS]), using the RECIST 1.1 method (in most patients), were considered for efficacy parameters8.
OS was defined as the duration in time since regorafenib initiation until the date of death for any reason.
Statistical analysis
Statistical analysis was carried out with the statistical software SPSS V. 23. Frequencies were used for descriptive statistics. OS was calculated with the KaplanMeier method, and statistical comparisons were developed using the log-rank test. Statistical significance was considered when a two-sided p-value lower than 0.05 was demonstrated.
Results
Forty-five heavily treatment-experienced patients with metastatic or recurrent colon or rectum adenocarcinoma were included according to the clinical criteria of the oncologist. Table 1 describes patients clinical characteristics; 27 were males and 18 females, with a mean age of 51 ± 10.3 years. ECOG performance status was 1 in all 45 patients. History of diabetes mellitus was found in 9% (4 cases), and of systemic arterial hypertension in 18% (8 cases). Primary tumor localization was the colon in 23 patients (51%) and the rectum in 22 (49%). As for disease extension, 24 patients were classified at Stage IV at initial diagnosis, with 21 having unresectable distant recurrence. Of the latter, two patients with Stage IIA colon adenocarcinoma had been treated with radical surgery and observation, six cases with Stage III colon cancer had been treated with radical surgery and adjuvant CT, four patients with Stage III rectum cancer had been treated with radical surgery and adjuvant CT-radiotherapy (RT), and nine patients with locally-advanced rectum cancer had been treated with neoadjuvant CT-RT followed by radical surgery and adjuvant CT. The main sites of metastasis were the liver in 24 cases (53%), the lung in 21 (46%), retroperitoneal adenopathies in 10 (22%), peritoneal carcinomatosis in seven patients (15%), and ovary in five cases (11%). In 13 patients, three or more metastatic sites were identified, with liver, lung, and retroperitoneal lymph nodes being the most common combination. KRAS oncogenes determination was obtained in 35 patients, which was wild-type in 16 (46%). In these 16 patients, the determination was broadened to NRAS in 13, with no mutation being identified in any of them. All patients received CT in the setting of first-line treatment for metastatic disease with oxaliplatin-based combinations in 30 cases (67%), irinotecan in 12 cases (27%), oxaliplatin and irinotecan (FOLFOXIRI) in two patients (4%) and fluoropyrimidine alone in one case (2%). Second-line CT was administered in 38 patients (84%), third-line in 13 (29%), and fourth-line in 2 (4%). Monoclonal antibodies were administered as first line in 30 cases (bevacizumab in 20, cetuximab in 8 and panitumumab in 2), 20 patients received it at second line (bevacizumab in 19 and cetuximab in 1 case), and ten cases received it as third line (bevacizumab in 7 and cetuximab in 3) (Fig. 1).
Table 1 Clinical characteristics
| Variable | n (45) | % |
|---|---|---|
| Age | 51 years (29-73) | |
| < 65 years | 37 | 82 |
| > 65 years | 8 | 18 |
| Gender | ||
| Male | 27 | 60 |
| Female | 18 | 40 |
| Comorbidity | ||
| Hypertension | 8 | 18 |
| Diabetes M. | 4 | 9 |
| Performance status | ||
| ECOG 1 | 45 | 100 |
| Localization | ||
| Colon | 23 | 51 |
| Rectum | 22 | 49 |
| KRAS | ||
| Mutated | 19 | 43 |
| Non-mutated | 16 | 46 |
| Not determined | 10 | 21 |
| Metastatic sites | ||
| Liver | 24 | 53 |
| Lung | 21 | 46 |
| Retroperitoneal lymph nodes | 10 | 22 |
| Peritoneal carcinomatosis | 7 | 15 |
| Ovary | 5 | 11 |
| Other | 15 | 33 |
| Stage at initial diagnosis | ||
| IV | 24 | 53 |
| II-III | 21 | 47 |

Figure 1 Lines of treatment with chemotherapy and monoclonal antibodies in 45 Mexican patients with metastatic colon and rectum cancer.
All 45 patients received at least one regorafenib cycle. In 20, it was started at a dose of 160 mg/day, in 18, at 120 mg/day and in 7, at a dose of 80 mg/day. In 3 of the latter, the dose was escalated to 120 mg/day from cycle 2 and thereafter. Mean cycle number was 4 (range: 1-13). In 17 patients (38%) there was a time interval ≤ 18 months since metastatic disease diagnosis until regorafenib was started, and in 28 patients (62%), this time interval was longer than 18 months.
Treatment efficacy was locally assessed by each investigator with tomography according to the policies of each hospital, with most evaluations being carried out every 2 regorafenib cycles. In no patient was a CR observed, in 4 cases (9%) PR was obtained, in 21 cases (47%), there was SS, in 15 cases (33%), there was progressive disease and, in 5 cases, imaging assessment was not possible (all 5 patients only received a single regorafenib cycle, two withdrew their consent to continue with the treatment, other patient had regorafenib discontinued owing to clinical deterioration, and the remaining two patients died at home, with the cause being unknown). Disease control (SS plus PR) was achieved in 56% of patients (Table 2). In two patients with lung metastases, cavitations in these lesions were observed since the first response to regorafenib evaluation; one of these patients had partial reduction and 10 cycles, and the second had SS with seven treatment cycles (Fig. 2).
Table 2 Response to regorafenib treatment in 45 Mexican patients with metastatic colon and rectum cancer
| Response | Number | Percentage |
|---|---|---|
| Complete response | 0 | 0 |
| Partial response | 4 | 9 |
| Stable disease | 21 | 47 |
| Progressive disease | 15 | 33 |
| Non-evaluable | 5 | 11 |
| Disease control | 25 | 56 |
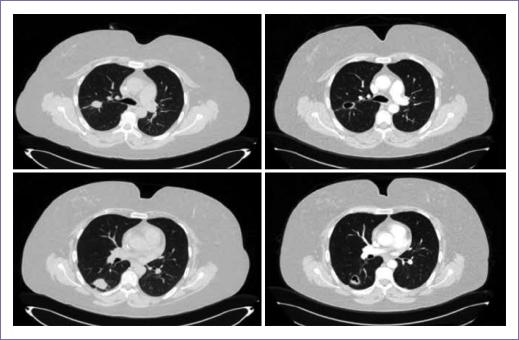
Figure 2 46-year old female patient with rectum adenocarcinoma with multiple pulmonary metastases treated regorafenib 160 mg/day. A-B: August 17, 2016 computerized axial tomography (TAC). C-D: October 11, 2016 TAC (after 2 treatment cycles) with both cavitated lung metastases being observed.
Overall survival was assessed in months, with 30% of patients remaining alive 12 months after regorafenib was started. Median overall survival was 6.0 months (Fig. 3). Five patients in whom response assessment was not possible died within the first 2 months after regorafenib treatment was started.
A sub-analysis was performed based on the time elapsed since metastatic disease diagnosis until regorafenib treatment was started; patients were divided in two groups: (1) those who started regorafenib < 18 months after diagnosis, and (2) those who started regorafenib more than 18 months after diagnosis. Among Group 1 patients, 40% were still alive at 12 months, with a median OS of 8.9 months, whereas Group 2 patients had a median OS of 3.0 months (p = 0.001; HR = 4.5; 95% CI: 1.82-11.22 (Fig. 4).

Figure 4 Median survival according to the months elapsed since diagnosis until regorafenib treatment initiation.
When survival was analyzed according to the number of metastatic sites, patients with ≤ 3 metastatic sites (n = 35) had a median OS of 6.8 months versus 3.9 months in those patients with > 3 metastatic sites (n = 10) (p = 0.116).
Toxicity was recorded in 36 patients, with Grade 3 being observed for hand-foot syndrome (HFS) in 4 cases (11%), fatigue in 2 (5.5%), and stomatitis, constipation, nausea, and transaminase elevation in one patient each (2.8%). Two patients with systemic arterial hypertension had uncontrolled hypertension that required adding a second antihypertensive drug. Four patients had the regorafenib dose reduced from 160 to 120 mg/day, and 2 of them had a second dose reduction from 120 to 80 mg, all owing to treatment toxicity.
Discussion
Clinical characteristics of the patients included in the present study are in similar ranges to those described by the CORRECT5 and CONCUR6 clinical trials, including gender distribution, with 60% of males and 40% of females, which is close to the reported distribution of 62%-38%5 and 63%-37%6, respectively. In our study, patient age range was 29-73 years, in comparison with 54-675 and 50-66 years6, respectively, with younger Mexican population with mCRC being observed, and with nearly 50% being 50-year old or younger. Primary tumor localization in the colon was observed in 51% of subjects in our study versus 64% and 58% in the referred trials. It should be noted that, since hospitals in our study are nation-wide referral centers, higher numbers of patients with rectum tumors are received, due to the need for greater hospital infrastructure. It is important pointing out that the population that participated in the CONCUR trial was 100% of Asian origin, very different to the CORRECT trial population, which mostly was from North America (United States and Canada) and Western Europe, with only 15% of Asian origin.
ECOG performance status was recorded in 100% of our patients, in comparison with only 48% in the CORRECT trial and 26% in the CONCUR trial5,6. This simple, but highly important clinical assessment provides additional prognostic information. Patients with an ECOG 0 performance status (practically asymptomatic) have higher treatment tolerance as regards dose and duration, which can positively impact on outcomes. In our group, no patient who received regorafenib had an ECOG 0 performance status, which suggests that lower tolerance to regorafenib treatment might be expected.
As for the KRAS mutation test results, information was obtained only for 35 patients, with 46% with wild-type reported. If the entire group is considered, KRAS results distribution was 36%, 42%, and 22% for wild-type, mutated status and unknown status, respectively, with similar distribution to that in the described trials, where percentages were 41%, 54% and 5%5 and 37%, 34%, and 29%6, respectively, which indicates that information about this biomarker in the Mexican population is similar to that reported in both these studies on regorafenib.
In our group, 29% of patients had more than 3 metastatic sites, with the liver, the lung and the retroperitoneum being the most common sites of metastasis. In our group, the percentage was lower in comparison with 49% in the CORRECT trial5 and 38% in the CONCUR trial6.
Therapy with monoclonal antibodies, anti-angiogenic agents or anti-EGFR drugs is accepted as standard of care at 1st, 2nd, and even 3rd line, usually in combination with CT. Its use is increasingly widespread, but that does not mean it should be used in 100% of patients. In our group, 34% of patients did not receive any biological therapy anytime during their evolution, with the rate of 41% of patients in the CONCUR trial being similar. It is important highlighting this, since an overall survival advantage was observed in that trial, with a 69% reduction (HR: 0.31-95% CI) in favor of patients treated with regorafenib without a history of having received any monoclonal antibody. In the CORRECT trial, the use of bevacizumab was reported in 80% of cases and panitumumab or cetuximab in 43%. Overall, 100% of patients received at least one monoclonal antibody. For this reason, a survival advantage could not be demonstrated in this group with regard to non-exposure to any targeted therapy.
When efficacy parameters are assessed, disease control in the present study, with 54% of the 45 included patients, is superior to those reported in the CORRECT trial, with 41%5, and in the CONCUR study, with 51%6. Both in our study and the two referred trials, no CR were observed, but PRs did, with 9% in our study versus 1%5 and 4%6. In other published studies, such as the REBECCA and CONSIGN trials, data on disease control or response rates were not reported, and comparing the described results with those in these studies was therefore not possible9,10. In an oral presentation at the 2016 ASCO-GI congress, the experience on the use of regorafenib in heavily treatment-experienced patients with mCRC was described, with attention being drawn by the fact that some patients with lung metastases with cavitations managed to live longer in comparison with patients with no such cavitations. In our study, two patients had cavitations in pulmonary metastases detected since the first assessment, with longer duration on regorafenib (7 and 10 cycles). Survival for these patients was 12 and 9 months.
In terms of overall survival, a median OS of 6.0 months was achieved, ranging from 1 to 16 months. Thirty percent of patients were still alive 12 months after regorafenib treatment was started. Our OS is comparable to that in the CORRECT trial, which had a median OS of 6.4 months (3.6-11.8 months) and 38% of patients alive at 9 months of treatment. In the Asian trial, a median OS of 8.8 months was observed (7.3-9.8 months). Although in none of both referred trials can the study populations be compared with the Mexican population owing to their ethnic origins, our results resemble those of the CORRECT trial. In the OS analysis as related to some parameters, two different types on results were identified, namely, higher tumor burden (due to a larger number of metastatic sites: 1-2 vs. > 3), with a median OS of 6.8 versus 3.9 months being observed in favor of the lower number of metastatic sites (although with p = 0.118); and on the other hand, the time interval since the detection of metastasis until regorafenib was started. In 28 patients, the interval was equal to or longer than 18 months, with a median OS of 8.9 months versus only 3.0 months for the 17 patients with an interval shorter than 18 months, with p < 0.05.
In our study, there were limitations to obtain all the information related to regorafenib-associated toxicity, given that data were retrospectively collected. Information could be obtained for 36 patients, with Grade 3 or most reported events being HFS, fatigue, stomatitis, transaminase elevations, constipation, nausea and arterial hypertension. As regards Grade 3 or most commonly reported events in the CONCUR trial, these were HFS in 16%, arterial hypertension in 11% and rash in 4%, whereas Grade 3 or higher HFS, fatigue, diarrhea, high blood pressure, rash and desquamation were reported in more than 5% of the CORRECT trial population.
Conclusion
Regorafenib is an active and effective drug in Mexican patients with mCRC at third line or beyond after progression with treatments based on CT ± monoclonal antibodies. Best candidates to receive regorafenib are patients with an ECOG 0-1 performance status, an interval longer than 18 months since the diagnosis of metastatic disease until treatment initiation and no previous exposure to any of the monoclonal antibodies indicated for this disease.











 text new page (beta)
text new page (beta)



