A preliminary contribution (Delgadillo et al. 2015) noted that several states in central Mexico were poorly explored and their moss floras were not well known. Guanajuato, Querétaro, and Zacatecas received some recent attention, but Aguascalientes remained unexplored until 2013. Collections reported by Delgadillo et al. (2015) listed the names of 45 moss species and varieties, including seven previously known for the flora of Aguascalientes; collecting effort and niche modelling analysis suggested a potential richness of 91 species for the state when a broader exploration were conducted. The earlier report also proposed that this moss flora was an extension of the dry land flora of Jalisco and Zacatecas, but no further comment was offered because of the limited floristic information available. With no other publications on the subject, additional field work was conducted to gain insight on the size and diversity of the state moss flora. This contribution updates the previous list, re-examines the distribution of the species, and provides a key for the identification of known species in Aguascalientes.
Materials and methods
In August 2014 and September 2015, the authors collected about 165 moss specimens, mainly in north- and southwestern localities in Aguascalientes (Table 1, Figure 1). Abandoned fields (San Antonio Montoya), riparian vegetation or stream beads (Ojo de Agua, Charco Azul), and scrubland vegetation elsewhere were the source of our collections. In addition, 140 miscellaneous specimens from the herbarium of Universidad Autónoma de Aguascalientes (HUAA), served to update the floristic list for that state. All specimens were identified and deposited in the Bryophyte Collection of the National Herbarium (MEXU), with duplicates for HUAA and other herbaria.
Table 1 Moss collecting sites in Aguascalientes in 2014, 2015. Specimen numbers preceded by PP were collected by Paola Peña. Four-digit numbers belong to collections by C. Delgadillo.
| Specimen Number | Localities | Coordinates (N – W) | Elev. (m) |
|---|---|---|---|
| PP 297-305; 7704-7720 | Cerro El Salteador | 21° 44’ 37’’ - 102° 31’ 34’’ | 1950-2000 |
| 7675-7677 | San Antonio Montoya | 21° 56’ 32’’ - 102° 04’ 00’’ | 2040 |
| 7678-7683 | Cerca de Ojo de Agua | 22° 01’ 29’’ - 101° 56’ 35’’ | 2000 |
| 7684 | Cerca de Charco Azul | 22° 02’ 56”- 102° 01’36” | 1950 |
| PP 321-323; 7744-7747 | 17 km W La Congoja | 22° 09’ 8” - 102° 39’32” | 2520 |
| PP 306-320; 7734-7743 | 10 km S La Congoja | 22° 11’ 31” - 102° 38’15” | 2580 |
| PP 324-327; 7748-7754 | 10 km NW San José de Gracia | 22° 09’ 36” - 102° 22’16” | 2290 |
| 7694-7695 | San Gil | 22° 11’ 51’’ - 102° 01’ 16’’ | 2030 |
| PP 328-336; 7755-7769 | Boca del Túnel de Potrerillo | 22° 13’ 48” - 102° 26’49” | 2020 |
| PP 288; 7693 | Tepezala | 22° 13’ 22’’ - 102° 11’ 08’’ | 1980 |
| PP 289-291; 7696 | Cerca de La Boquilla | 22° 15’ 32’’ - 102° 22’ 48’’ | 1960 |
| PP 292-293; 7697 | Cerca de La Boquilla. | 22° 14’ 59’’ - 102° 23’ 49’’ | 2010 |
| PP 294-295; 7698-7703 | Cerca de La Boquilla | 22° 15’ 01’ - 102° 24’ 16’’ | 2080 |
| PP 284-287; 7685-7692 | Las Pilas | 22° 15’ 15’’ - 102° 10’ 40’’ | 2000 |
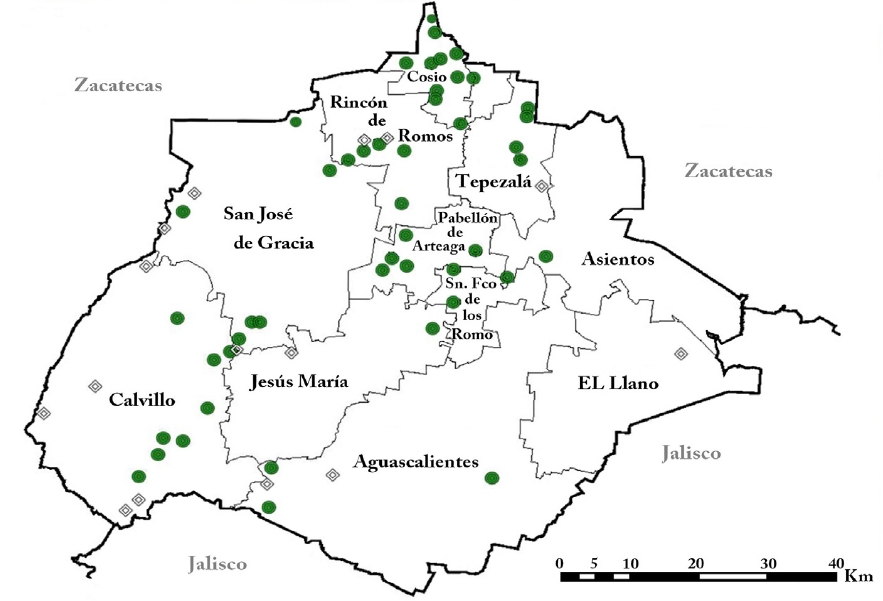
Figure 1 Collecting localities in Aguascalientes. Localities reported by Delgadillo et al. (2015) are indicated by diamonds. Solid circles represent recent collections by the authors and by HUAA personnel.
Results
The list of taxa in Table 2 contains 50 new records for the moss flora of Aguascalientes. These are unmarked under “A” to distinguish them from previously reported records (Delgadillo et al. 2015), and bring the total number to 95 taxa, i.e., four species above the previously estimated number. Most of the species are also known from Jalisco or Zacatecas so that the Aguascalientes moss flora may indeed represent an extension of the flora from those states (Delgadillo et al. 2015).
Table 2 Mosses from the state of Aguascalientes. A, species previously recorded by Delgadillo et al. (2015); those without a mark are new state records. The records for Jal (Jalisco) and Zac (Zacatecas) were cited by Sharp et al. (1994) or come from specimens at MEXU (H). Sb = substrate: R, rock; S, soil; Sr, soil-covered rocks; T, trunk; r, root
| A | TAXA | Jal | Zac | H | Sb |
|---|---|---|---|---|---|
| X | Aloina hamulus (Müll. Hal.) Broth. | X | X | * | S S, Sr |
| Anacolia laevisphaera (Taylor) Flowers | X | X | * | S | |
| Anoectangium aestivum (Hedw.) Mitt. | X | X | * | S | |
| Anomobryum conicum (Hornsch.) Broth. | R | ||||
| X | Anomobryum julaceum (Gaertn., Meyer & Schreb.) Schimp. | X | X | * | S |
| Anomobryum plicatum Cardot | X | * | S | ||
| Aongstroemia orientalis Mitt. | X | S | |||
| Archidium donnellii Austin | X | * | S | ||
| X | Barbula orizabensis Müll. Hal. | X | S | ||
| X | Brachymenium mexicanum Mont. | X | X | * | R, S, Sr |
| X | Brachymenium systylium (Müll. Hal.) A. Jaeger | X | T | ||
| Brachythecium frigidum (Müll. Hal.) Besch. | Sr | ||||
| Brachythecium ruderale (Bird.) W. R. Buck | X | Sr | |||
| X | Braunia andrieuxii Lorentz | X | X | * | R, S, Sr |
| Braunia plicata (Mitt.) A. Jaeger | T | ||||
| X | Braunia secunda (Hook.) Bruch & Schimp. | X | X | * | R |
| Bryoerythrophyllum inaequalifolium (Taylor) R. H. Zander | X | X | * | S | |
| Bryoerythrophyllum recurvirostrum (Hedw.) Chen | X | * | S | ||
| var. recurvirostrum | |||||
| Bryoerythrophyllum recurvirostrum var. aeneum (Müll. Hal.) | X | S | |||
| R. H. Zander | |||||
| X | Bryum argenteum Hedw. | X | X | * | S |
| X | Bryum billarderi Schwägr. | X | X | * | S |
| X | Bryum chryseum Mitt. | X | X | * | S, Sr |
| Bryum coronatum Schwägr. | S | ||||
| X | Campyliadelphus chrysophyllus (Brid.) Kanda | S | |||
| Campylopus albidovirens Herz. | X | S | |||
| Campylopus flexuosus (Hedw.) Brid. | X | S | |||
| X | Campylopus pilifer Brid. | X | X | * | R, Sr |
| X | Ceratodon purpureus (Hedw.) Brid. subsp. stenocarpus | X | S | ||
| (Bruch & Schimp.) Dixon | |||||
| X | Crossidium crassinervia (De Not.) Jur. | X | * | Sr | |
| X | Didymodon australasiae (Hook. & Grev.) R.H. Zander | X | X | * | R, S |
| X | Didymodon revolutus (Cardot) R.S. Williams | X | X | S | |
| X | Didymodon rigidulus var. gracilis (Schleich. Ex Hook. & | X | X | * | S |
| Grev.) R.H. Zander | |||||
| X | Didymodon rigidulus var. icmadophilus (Schimp. ex Müll. | X | X | * | S |
| Hal.) R.H. Zander | |||||
| X | Didymodon rigidulus var. rigidulus | X | X | * | S, Sr |
| Entodon beyrichii (Schwägr.) Müll. Hal. | X | X | * | R | |
| X | Entosthodon apiculatopilosus (Cardot) Fife | X | * | S | |
| Entosthodon jamesonii (Taylor) Mitt. | S | ||||
| Entosthodon obtusatus (Schimp.) Fife | S | ||||
| Entosthodon obtusifolius Hook. f. | X | * | S | ||
| Epipterygium immarginatum Mitt. | X | * | S | ||
| X | Erythrodontium longisetum (Hook.) Paris | X | T | ||
| X | Erythrodontium squarrosum (Hampe) Paris | X | * | R | |
| Fabronia ciliaris var. ciliaris | X | X | * | T | |
| X | Fabronia ciliaris var. polycarpa (Hook.) W.R. Buck | X | T | ||
| Fabronia ciliaris var. wrightii (Sull.) W.R. Buck | X | X | * | T | |
| X | Fabronia macroblepharis Schwägr. | X | * | T | |
| X | Fissidens bryoides Hedw. | X | X | * | Sr |
| X | Fissidens crispus Mont. | X | X | S, Sr | |
| Fissidens elegans Brid. | Sr | ||||
| X | Fissidens guianensis Mont. | X | S | ||
| X | Fissidens sublimbatus Grout | S | |||
| Funaria hygrometrica var. calvescens (Schwägr.) Mont. | X | X | * | S | |
| Funaria hygrometrica var. hygrometrica | X | X | * | S | |
| Globulinella globifera (Hampe) Steere | X | S | |||
| X | Grimmia elongata Kaulf. | X | R | ||
| Grimmia involucrata Cardot | X | * | R | ||
| X | Grimmia laevigata (Brid.) Brid. | X | R | ||
| X | Grimmia longirostris Hook. | X | X | * | R, Sr |
| X | Grimmia ovalis (Hedw.) Lindb. | X | * | R | |
| X | Grimmia pulla Cardot | X | R, Sr | ||
| Gymnostomum aeruginosum Sm. | X | * | Sr | ||
| Haplocladium microphyllum (Hedw.) Broth. | X | * | R | ||
| X | Hennediella heteroloma (Cardot) R.H. Zander | X | X | * | r |
| Hennediella stanfordensis (Steere) Blockeel | R | ||||
| Hyophila involuta (Hook.) A. Jaeger | X | X | * | R | |
| X | Jaffueliobryum arsenei (Thér.) Thér. | X | * | R | |
| X | Leptodontium flexifolium (Dicks..) Hampe | X | X | * | S |
| X | Leskea angustata Taylor | X | X | * | T |
| Lindbergia mexicana (Besch.) Cardot | X | * | T | ||
| Orthotrichum bartramii R.S. Williams | T | ||||
| Orthotrichum diaphanum Schrad. ex Brid. | X | * | T | ||
| Orthotrichum pycnophyllum Schimp. | X | X | * | T | |
| Plaubelia sprengelii var. stomatodonta (Cardot) R.H. Zander | X | X | * | S | |
| X | Pleuridium mexicanum Cardot | X | * | S | |
| X | Pogonatum campylocarpon (Müll. Hal.) Mitt. | X | X | * | S, Sr |
| X | Pogonatum oligodus (Kunze ex Müll. Hal) Mitt. | X | * | S, Sr | |
| Pohlia nutans (Hedw.) Lindb. | S | ||||
| Pohlia oerstediana (Müll. Hal.) A.J. Shaw | X | * | S | ||
| Pseudocrossidium crinitum (Schultz) R.H. Zander | X | * | S | ||
| X | Pseudocrossidium replicatum (Taylor) R.H. Zander | X | X | * | S, Sr |
| Ptychomitrium chimborazense (Spruce ex Mitt.) A. Jaeger | X | * | R | ||
| Syntrichia bartramii (Steere) R.H. Zander | S | ||||
| X | Syntrichia chisosa (Magill, Delgad. & L. R. Stark) R.H. Zander | X | * | R, S | |
| X | Syntrichia fragilis (Taylor) Ochyra | X | X | * | R, S, Sr, T |
| Syntrichia obtusissima (Müll. Hal) R.H. Zander | X | * | Sr | ||
| Syntrichia pagorum (Milde) J.J. Amann | X | * | S, T | ||
| Timmiella anomala (Bruch & Schimp.) Limpr. | X | X | * | S | |
| X | Tortula acaulon (With.) R.H. Zander | X | * | S | |
| X | Tortula atrovirens (Sm.) Lindb. | X | * | S | |
| Tortula brevipes (Lesq.) Broth. | R | ||||
| Trichostomum brachydontium Bruch | X | X | * | S | |
| Trichostomum crispulum Bruch | X | * | S | ||
| Trichostomum subangustifolium (Thér.) R.H. Zander | S | ||||
| Weissia controversa Hedw. | X | X | * | S | |
| Weissia ligulaefolia (E.B. Bartram) Grout | S |
The moss flora of Aguascalientes contains numerous taxa from dry land areas. Among them, the Pottiaceae are rather frequent, with 34 species and varieties for a 36 % of the total state moss flora. The Bryaceae, with 11 species, only make a 12 %. The Grimmiaceae, with seven species (ca. 7 % of the total), are also noteworthy because they are rock inhabitants and more diverse than fifteen other families that are represented by five or less in the state moss flora. At the generic level mosses are represented by 43 genera, or nearly 13 % of the Mexican moss flora.
The list contains such taxa as Anomobryum conicum that seem rare or may be infrequently collected while others such as Aloina hamulus and Didymodon spp., are rather frequent in the region. The flora also includes a significant number of high elevation taxa that are known to occur in Jalisco or in the alpine regions of central Mexico; Aongstroemia orientalis and species of Grimmia are in this group. Most species live on soil or rocks. The number of epiphytes is comparatively small (14 species, Table 2) because, in addition to low rainfall, tree cover is sparse and may offer scarce protection against desiccation. Species of Fabronia, Leskea, Lindbergia, and Orthotrichum usually occupy these habitats.
Hennediella stanfordensis, only known from the state of Guerrero, is represented by two poorly preserved specimens from San Francisco de los Romo area, but the identification is facilitated by a differentiated papillose leaf margin with thick-walled elongated cells, and a mucro standing against an entire leaf apex. Jaffueliobryum arsenei and Ptychomitrium chimborazense have narrow ranges; the former is known from Zacatecas to Querétaro, but in Aguascalientes it seems more frequent although it is officially recognized as rare (Delgadillo 1996). The distribution of the latter in Mexico extends from Zacatecas to Puebla, but collections have been occasional, only. Archidium donnellii, Pleuridium mexicanum, and Tortula acaulon are the only cleistocarpic mosses known for this state. Anomobryum plicatum and Entosthodon obtusatus are tentatively included in the list because specimen identification was uncertain due to immature or sterile material available. There are no species restricted to Aguascalientes, but its flora includes Grimmia involucrata, G. pulla, Hennediella heteroloma, Jaffueliobryum arsenei, and Trichostomum subangustifolium, endemic to Mexico.
Although most species are cited in Sharp et al. (1994), the keys introduced in this contribution reflect recent taxonomic and nomenclatural arrangements. They should facilitate the identification of mosses from Aguascalientes. For this purpose, observations in the following paragraph are to be considered.
Moss keys usually distinguish the erect from the prostrate habit. Such growth forms may be labeled as “acrocarpous” and “pleurocarpous”, as defined in standard floras and glossaries, but they have been omitted in the keys to avoid confusion. Broadly speaking, such terms describe the position of the perichaetium in a branching system. In the acrocarpous mosses, a single perichaetium is formed at the end of a primary module of a branch system; in the pleurocarpous mosses one or more perichaetia are produced per primary or secondary module, at the end of lateral innovations that lack branch primordia or developed branches. A third moss growth type that is seldom mentioned is termed “cladocarpous” where the primary modules end in vegetative growth and the perichaetia are produced at the end of lateral branches of secondary or tertiary branches (La Farge-England 1996). Withey (1996) suggests that the cladocarpous condition is a special form of pleurocarpy. In Aguascalientes, the species of Braunia may be considered as acrocarpous while Anoectangium aestivum is cladocarpous. However, because of their prostrate or erect condition, they have been termed pleurocarpous and acrocarpous, respectively, in standard taxonomic or floristic manuals.
Discussion
The Aguascalientes floristic list might contain a larger number of species for various reasons. There are many field sites that require exploration, but are privately owned and are not readily accessible. On the other hand, farmland, cattle ranches, and industrial facilities are common and represent heavily disturbed areas whose value in moss diversity studies is virtually nil. Frequent disturbance favors the arrival of tolerant species such as Bryum argenteum, B. chryseum or Funaria hygrometrica while many other species are displaced from their original substrates. Despite the need for additional exploration, because the predicted number of species is essentially the same as the number of taxa recovered in our sampling, we conclude that species richness for the state has been conveniently evaluated.
With a land surface area of about 5,471 km2 (García de Miranda & Falcón de Gyves 1984), Aguascalientes is one of the smallest states in Mexico. In spite its small size, its moss flora holds species or groups of species with peculiar features. There is a group of species that occupies disturbed areas whose frequency varies in response to human activities, as mentioned above. In this regard, the eastern and central portions of the state harbor cities, industry, highways, farm and cattle ranges that reduce or modify species dynamics depending on soil management practices. Group composition may vary with the geography, climate, and vegetation of the state; the northern areas are dominated by the dry land species while the western elevations incorporate highland taxa. Detailed mapping and phytosociological evaluations are required in this and neighboring states to detect associations. One such association may be represented by mosses growing on limestone or soils derived from calcareous rocks. Aloina hamulus, Didymodon revolutus, Globulinella globifera, and Pseudocrossidium replicatum are frequently found together in the same areas so that by finding one species the presence of others may be predicted. The frequency of sites where these species occur perhaps reflect the extent of human interference in natural moss communities.
The cleistocarpic species, represented by Archidium donnellii, Pleuridium mexicanum y Tortula acaulon, illustrate the structural and functional diversity of mosses in Aguascalientes. These species may be more frequent in disturbed sites because, in the absence of a dehiscent system, capsules may open by mechanical disturbance by cattle or agricultural equipment. The presence of such species as Jaffueliobryum arsenei marks the existence of other peculiar features in the area. For instance, the monoicous condition termed “cryptoicous” observed in J. arsenei is characterized by the position of both sex organs on the same stem. The perichaetial leaves enclose a small male branch growing from the vaginula, at the base of the sporophyte. The same condition has been described in Ptychomitrium (Deguchi 1977, Deguchi & Takeda 1986) and Jaffueliobryum (Churchill 1987); both genera are part of the Aguascalientes moss flora.
From the geographical point of view, diversity studies may benefit from the use of environmental indicators. The introductory contribution (Delgadillo et al. 2015) indicated that the potential number of species in the flora would be about 91moss taxa. Because of the information in this contribution, this may be regarded as a well-known flora. Nevertheless, further exploration elsewhere may confirm or expand knowledge of the distribution of individual taxa and assist in the evaluation of the conservation status of rare or endangered species.
Key to the known moss species in Aguascalientes
Mosses with prostrate or lateral sporophytes
| 1. Costa short, double or absent | 2 |
| 2. Leaves ecostate | 3 |
| 3. Leaf apex with a hyaline hair | Braunia plicata |
| 3'. Leaf apex not hyaline | 4 |
| 4. Leaf margin revolute in the basal 1/3 | Braunia andrieuxii |
| 4'. Leaf margin revolute in the basal ? | Braunia secunda |
| 2'. Costa double, short, usually not extending beyond mid-leaf | 5 |
| 5. Leaves not decurrent. Alar cells short, quadrate | Entodon beyrichii |
| 5'. Leaves with broad decurrencies. Alar cells mainly oblate | 6 |
| 6. Leaves oblong-ovate. Seta yellow to orange, exostome smooth | Erythrodontium longisetum |
| 6'. Leaves suborbicular to shortly oblong-ovate. Seta red, exostome striate | Erythrodontium squarrosum |
| 1'. Costa simple, slender or strong, reaching mid-leaf or beyond | 7 |
| 7. Alar cells quadrate, in a small group. Costa ending in an inconspicuous abaxial spine | 8 |
| 8. Leaves with broad decurrencies | 9 |
| 9. Leaves strongly plicate, alar cells inflated; seta papillose | Brachythecium frigidum |
| 9'. Leaves weakly plicate or only so at base, alar cells quadrate to rectangular; seta smooth | Brachythecium ruderale |
| 8'. Leaves base rounded, without decurrencies | Campyliadelphus chrysophyllus |
| 7'. Alar cells undifferentiated to oblate in a broad proximal region. Costa smooth or rugose | 10 |
| 10. Stem and branch leaves differentiated in size and form Paraphyllia smooth | Haplocladium microphyllum |
| 10'. Stem and branch leaves similar, except in size. Paraphyllia papillose | 11 |
| 11. Leaf cells inconspicuously papillose | 12 |
| 12. Papillae borne on distal ends of leaf cells. Endostome segments as long as the teeth | Leskea angustata |
| 12'. Papillae borne on cell luminae. Endostome as a fine papillose membrane | Lindbergia mexicana |
| 11'. Leaf cells smooth | 13 |
| 13. Leaves ovate | 14 |
| 14. Leaves abruptly acuminate, margins entire to long-dentate | Fabronia ciliaris var. ciliaris |
| 14'. Leaves gradually acuminate, margins entire to irregularly dentate | Fabronia ciliaris var. polycarpa |
| 13'. Leaves lanceolate to ovate or triangular | 15 |
| 15. Leaves lanceolate to oblong-ovate | Fabronia ciliaris var. wrightii |
| 15'. Leaves oblong-lanceolate to narrowly triangular | Fabronia macroblepharis |
Mosses with erect stems and apical sporophytes
| 1. Stems julaceous, leaves imbricate, dry or moist | 2 |
| 2. Leaves ovate-lanceolate, with an obtuse apex and excurrent costa | Anomobryum conicum |
| 2'. Leaves oblong or oblong-ovate with an obtuse to rounded apex obtuse; costa ending below the apex to subpercurrent | Anomobryum julaceum |
| 1'. Stems not julaceous, leaves erect, sparse or crowded | 3 |
| 3. Costa excurrent, ending as an awn or hyaline hair | 4 |
| 4. Costa ending as an awn | 5 |
| 5. Costa broad | 6 |
| 6. Leaf section with hyalocysts and chlorocysts alternating in the abaxial region | Campylopus albidovirens |
| 6'. Leaf section with groups of abaxial stereids | Campylopus flexuosus |
| 5'. Costa narrow | 7 |
| 7. Sporophytes cleistocarpous | 8 |
| 8. Spores large, more than 100 µm in diam | Archidium donnellii |
| 8'. Spores small, less than 50 µm in diam | Tortula acaulon |
| 7'. Sporophytes stegocarpous | 9 |
| 9. Leaf margin strongly revolute | Pseudocrossidium crinitum |
| 9'. Leaf margin plane | 10 |
| 10. Leaves rosulate, at the stem apex, with a strong red awn | Brachymenium mexicanum |
| 10'. Leaves not in a rosette, with a fine yellowish awn | Bryum chryseum |
| 4'. Costa ending in a hyaline hair | 11 |
| 11. Costa broad, occupying 1/2 to 1/3 of the leaf base | Campylopus pilifer |
| 11'. Costa narrow | 12 |
| 12. Distal leaf cells hyaline | Bryum argenteum |
| 12'. Distal leaf cells not hyaline | 13 |
| 13. Photosynthetic filaments of costa 2-12 cells long | Crossidium crassinervium |
| 13'. Costa without photosynthetic filaments | 14 |
| 14. Leaf margin with one to several rows of elongated cells | Brachymenium systylium |
| 14'. Marginal leaf cells and inner cells similar | 15 |
| 15. Leaf cells in distal half rhomboidal, marginal teeth obtuse-rounded | Entosthodon apiculatopilosus |
| 15'. Leaf cells in distal half with other shapes, marginal teeth not rounded | 16 |
| 16. Leaf section with an U- or V-shaped adaxial channel | 17 |
| 17. Basal marginal cells uniformly thin-walled | Grimmia elongata |
| 17'. Transverse walls of marginal basal cells thicker than the longitudinal walls | 18 |
| 18. Leaves unistratose except at margins; costa with two guide cells; seta curved | Grimmia pulla |
| 18'. Leaves distally bistratose, costa with 2-6 guide cells; seta straight | Grimmia longirostris |
| 16'. Leaf section concave, without an obvious adaxial channel | 19 |
| 19. Basal leaf cells oblate toward margin | Grimmia laevigata |
| 19'. Basal leaf cells quadrate to short-rectangular | 20 |
| 20. Capsule immersed to emergent | 21 |
| 21. Calyptra pilose | Orthotrichum diaphanum |
| 21'. Calyptra smooth | Grimmia involucrata |
| 20'. Capsule exserted | 22 |
| 22. Capsule cylindrical, with a spongiose neck | Bryum coronatum |
| 22'. Capsule ovoid-cylindrical, with a smooth neck | 23 |
| 23. Leaves unistratose | 24 |
| 24. Calyptra mitrate, deeply lobed | Jaffueliobryum arsenei |
| 24'. Calyptra cucullate | 25 |
| 25. Leaf apex emarginate, hair point dentate | Syntrichia obtusissima |
| 25'. Leaf apex obtuse or rounded, hair point not dentate | 26 |
| 26. With foliose axillary propagulae | Syntrichia pagorum |
| 26'. Without specialized propagulae | Tortula brevipes |
| 23'. Leaves partly or wholly bistratose | 27 |
| 27. Leaf blade with bistratose patches | Syntrichia bartramii |
| 27'. Leaf blade completely bistratose | Grimmia ovalis |
| 3’. Costa ending below leaf apex or as a mucro | 28 |
| 28. Mid-leaf cells thick-walled, vermicular | Anomobryum plicatum |
| 28'. Distal mid-leaf cells thick- or thin-walled, not vermicular | 29 |
| 29. Leaves distichous, equitant, with two vaginant laminae | 30 |
| 30. Limbidium present, uni- to tristratose | 31 |
| 31. Leaf cells strongly bulging, in distinct rows | Fissidens crispus |
| 31'. Leaf cells slightly or not bulging, not in rows | 32 |
| 32. Leaf cells pluripapillose | Fissidens elegans |
| 32'. Leaf cells smooth | 33 |
| 33. Limbidium present in all laminae, nearly extending to leaf apex | Fissidens bryoides |
| 33'. Limbidium intramarginal, usually restricted to the vaginant laminae | Fissidens sublimbatus |
| 30'. Limbidium absent | Fissidens guianensis |
| 29'. Leaves in more than two rows, with a single lamina | 34 |
| 34. Leaves rosulate | Bryum billarderi |
| 34'. Leaves distributed along the stem | 35 |
| 35. Leaves secund, margins erose-denticulate | Aongstroemia orientalis |
| 35'. Leaves not secund, margins entire or dentate | 36 |
| 36. Leaf cells smooth | 37 |
| 37. Leaves dimorphous, the lateral larger than the dorsal | Epipterygium immarginatum |
| 37'. Leaves not dimorphous | 38 |
| 38. Leaves with adaxial lamelae | 39 |
| 39. Lamelae in section ending in a simple square apical cell | Pogonatum oligodus |
| 39'. Lamelae ending in two flask-shaped cells (geminate) | Pogonatum campylocarpum |
| 38'. Leaves without lamelae, sometimes with filaments or cell masses | 40 |
| 40. Leaf cells plane, not bulging | 41 |
| 41. Leaves unistratose | 42 |
| 42. Sporophytes cleistocarpous | Pleuridium mexicanum |
| 42'. Sporophytes stegocarpous | 43 |
| 43. Plants paroicous | 44 |
| 44. Leaves serrate in distal half, cells thick-walled | Pohlia nutans |
| 44'. Leaves serrate to serrulate near apex, cells thin-walled | Pohlia oerstediana |
| 43'. Plants gonioautoicous or cladautoicous | 45 |
| 45. Stomata immersed | Orthotrichum bartramii |
| 45'. Stomata superficial | Orthotrichum pycnophyllum |
| 41'. Leaves bistratose | 46 |
| 46. Calyptra mitrate | Ptychomitrium chimborazense |
| 46'. Calyptra cucullate | Timmiella anomala |
| 40'. Leaf cells mammillose or bulging | 47 |
| 47. Leaves spatulate, with axillary gemmae | Hyophila involuta |
| 47'. Leaves with other shapes | 48 |
| 48. Leaf apex cucullate | 49 |
| 49. Leaves oblong-ovate, costa slightly spurred | Globulinella globifera |
| 49'. Leaves lingulate, costa and lamina with photosynthetic filaments | Aloina hamulus |
| 48'. Leaf apex open | 50 |
| 50. Leaf cells short, quadrate | 51 |
| 51. Leaves long-lanceolate; capsule erect, smooth | Didymodon rigidulus var. icmadophilus |
| 51'. Leaves lanceolate; capsule inclined, sulcate | Ceratodon purpureus var. stenocarpus |
| 50'. Leaf cells irregularly shaped | 52 |
| 52. Capsule inclined, asymmetric | 53 |
| 53. Capsule curved, inclined to pendent; neck not flattened | Funaria hygrometrica var. hygrometrica |
| 53'. Capsule nearly erect to inclined, gradually narrow, with a flat neck | Funaria hygrometrica var. calvescens |
| 52'. Capsule erect and symmetric | 54 |
| 54. Leaves lingulate to spatulate, apex rounded to obtuse, crenulate | Entosthodon obtusatus |
| 54'. Leaves oblong to obovate, apex entire | 55 |
| 55. Leaf apex acute to acuminate | Entosthodon jamesonii |
| 55'. Leaf apex rounded-acute | Entosthodon obtusifolius |
| 36’. Leaf cells papillose | 56 |
| 56. Papillae simple at both cell ends | Anacolia laevisphaera |
| 56'. Papillae variable in number and arrangement in leaf cells | 57 |
| 57. Costa with two stereid bands; the adaxial smaller or absent | 58 |
| 58. Leaf margin strongly involute | 59 |
| 59. Leaf cells bulging, with low papillae | Weissia controversa |
| 59. Leaf cells strongly bulging, with columnar papillae | Weissia ligulaefolia |
| 58. Leaf margin plane, erect or revolute | 60 |
| 60. Adaxial costal epidermis absent | Leptodontium flexifolium |
| 60. Adaxial costal epidermis present | 61 |
| 61. Adaxial costal epidermis smooth | 62 |
| 62. Leaf cells papillose on both surfaces | Gymnostomum aeruginosum* |
| 62. Leaf cells smooth on the adaxial surface | Plaubelia sprengelii var. stomatodonta* |
| 61. Adaxial costal epidermis papillose | 63 |
| 63. Leaves ligulate to oblong, leaf apex rounded to obtuse | 64 |
| 64. Leaf margin revolute | 65 |
| 65. Leaf margin revolute nearly to apex; axillary gemmae multicelluar | Barbula orizabensis |
| 65'. Leaf margin entirely revolute; axillary gemmae unicellular | Bryoerythrophyllum inaequalifolium |
| 64'. Leaf margin plane or erect | 66 |
| 66. Capsule without peristome | Trichostomum subangustifolium |
| 66'. Cepsule with peristome | 67 |
| 67. Leaf base differentiated, apex cucullate | Trichostomum crispulum |
| 67'. Leaf base not differentiated, apex rounded to acute | Trichostomum brachydontium |
| 63'. Leaves oblong-lanceolate to lanceolate, leaf apex acute or rounded | 68 |
| 68. Leaf apex acute, ending in a sharp hyaline cell | Bryoerythrophyllum recurvirostrum var. recurvirostrum |
| 68'. Leaf apex rounded, with several teeth | Bryoerythrophyllum recurvirostrum var. aeneum |
| 57'. Costa with abaxial stereid band only | 69 |
| 69. Perichaetium lateral; leaves keeled | Anoectangium aestivum |
| 69'. Perichaetium apical; leaves nearly plane | 70 |
| 70. Basal leaf cells differentiated, hyaline | Didymodon australasiae |
| 70'. Basal leaf cells similar to mid-leaf cells | 71 |
| 71. Costa without hydroids | 72 |
| 72. Leaves short-ovate, costa spurred | Didymodon revolutus |
| 72'. Leaves long-lanceolate, costa no spurred | 73 |
| 73. Gemmae frequent; distal leaf margin bistratose | Didymodon rigidulus var. rigidulus |
| 73'. Gemmae rare; distal leaf margin occasionally bistratose | Didymodon rigidulus var. gracilis |
| 71'. Costa with hydroids | 74 |
| 74. Leaf margin spirally revolute | Pseudocrossidium replicatum |
| 74'. Leaf margin plane or revolute | 75 |
| 75. Costa with a distal cellular pad | Tortula atrovirens |
| 75'. Costa with 1-2 layers of adaxial over the guide cells | 76 |
| 76. Leaf border differentiated | 77 |
| 77. Intramarginal leaf border unistratose | Hennediella heteroloma |
| 77'. Leaf with a bistratose margin of elongated cells | Hennediella stanfordensis |
| 76'. Leaf border not differentiated | 78 |
| 78. Leaves firm, bistratose, with foliose gemmae | Syntrichia chisosa |
| 78'. Leaves fragile, unistratose, without gemmae | Syntrichia fragilis |











 text new page (beta)
text new page (beta)


