Introduction
In the context of sustainable agriculture, the incorporation of nanotechnology in the design and manufacture of innovative fertilizers is a strategy with the potential to significantly increase crop production and encourage the development of profitable agriculture, thus feeding the world's population, which has a high growth rate (Guo, White, Wang, & Xing, 2018).
Recent advances in nanotechnology have led to the development of nanomaterials of different sizes and shapes. These advances are the basis to synthesize particles with unique properties and directed to specific applications; however, its use in agriculture, especially in the protection and production of plants, is an area little explored by the scientific community (Khot, Sankaran, Maja, Ehsani, & Schuster, 2012). Nevertheless, as the study of nanotechnology advances, the potential use of nanostructured materials to generate controlled-release fertilizers and other strategies to improve the efficiency in the use of fertilizers has generated a great interest in the scientific community, governments, industry and the public sector (Milani, França, Balieiro, & Faez, 2017).
Lee, Heng, Ng, Chan, and Tan (2011) mention that the definition of nanoparticles depends on each discipline; some define nano objects as those materials that present one, two or three dimensions in the range of 0.1 to 100 nm. On the other hand, in fertilizers the definition seems to be in use, because there are patents and publications that have denominated "nano" to those materials that present different properties to those of the bulk material and that have one, two or three dimensions inferior to 1 000 nm (Rai, Ribeiro, Mattoso, & Duran, 2015).
According to Liu and Lal (2015), nanofertilizers are expected to significantly improve crop growth and yield, as well as plant use efficiency, and reduce nutrient losses, adverse environmental impacts, application frequency, and labor costs. The above due to the fact that the nanofertilizers could prolong the longevity of nutrients in the environment, which would maintain a continuous and effective supply for the crops during a longer growth period (Guo et al., 2018).
Polymers are a class of soft materials with numerous and versatile mechanical and chemical properties that can be applied in agriculture. Smart agricultural supplies (controlled or slow-release agrochemicals and super absorbents) and bio absorbents form an expanding niche using polymer technology (Milani et al., 2017). Recent studies refer to the use of biodegradable polymers and their compounds as materials for the controlled release of agrochemicals. Milani et al. (2017) mention some polymers that, due to their specific application capacity, uses, useful life and economic viability, have been studied in recent years as matrices for controlled release in agriculture.
A key aspect to consider when formulating slow-release nanomaterials is that their components must be biodegradable and must not form toxic substances upon degradation (Kumar, Ashfaq, & Verma, 2018); furthermore, nanofertilizers must release nutrients on demand to prevent them from prematurely turning into chemical/gaseous forms that plants cannot absorb. To achieve this, nutrients must be prevented from interacting with soil, water, and microorganisms, and it must be ensured that nutrients are released only when the plant can internalize them directly (de Rosa, Monreal, Schnitzer, Walsh, & Sultan, 2010).
Considering the above, the aim of the present work was to elaborate and characterize calcium-loaded gelatin nanoparticles, and to evaluate their behavior as a controlled release system.
Materials and methods
Chemical substances
In the production of the nanoparticles, gelatin type A (Sigma-Aldrich, USA), Ca(NO3)2·4H2O (Meyer, Mexico) and citric acid (Merck, Germany) were used as crosslinking agents. Deionized water was used in all experiments and analyses (18 MΩ·cm-1).
Synthesis of calcium-loaded gelatin nanoparticles
To synthesize the nanoparticles, 3.8 g of Ca(NO3)2·4H2O were weighed and dissolved in 300 mL of deionized water; simultaneously, 4.5 g of gelatin were dissolved in 700 mL of deionized water at 80 °C. Before being mixed together, both solutions were filtered through 0.45 µm PVC membranes (Millipore, Ireland). The resulting solution was kept in agitation for 2.5 h at 80 °C and sonicated for 15 min in an ultrasonic bath (TI-H-5, Elma, Germany). Subsequently, 1.2 g of citric acid was added to the solution and kept in agitation for 12 h at room temperature. Finally, the solution was sonicated for 15 min and filtered with a PVC membrane with a pore diameter of 0.22 µm (Millipore, Ireland).
A Nano Spray Dryer B-90 (Büchi, B-90, Switzerland) with a 4 µm grid was used to generate the nanoparticles. The drying temperature was 100 °C at a pressure of 3 500 Pa and 100 % nozzle opening. To obtain the nanoparticles without calcium loading (control), the methodology mentioned above was used, but without the addition of calcium nitrate in the process.
Scanning electron microscopy
The morphology and size of the particles were studied with a scanning electron microscope (JSM-6390LV, JEOL, Japan). Prior to analysis, the samples were coated with gold-palladium by sputtering (Desk IV, Denton Vacuum). The micrographs were obtained at a magnification of 10 000 X with an accelerating voltage of 20 kV. The average size was calculated from the measurement of 400 particles with the Scandium software (Olympus, ResAlta Research Technologies, Golden, CO, USA, 2010).
Fourier transform infrared spectroscopy
To evaluate possible interactions between calcium load and gelatin within the nanoparticle, FTIR determinations were performed with the Cary 630 equipment (Agilent Technologies, Santa Clara, USA). A total attenuated reflectance (TRA) cell, a range of 4 000 to 600 cm-1 and a resolution of 4 cm-1 at 32 scans were used for the analyses.
Calcium quantification and release testing
The quantification of total calcium contained in the nanomaterials was performed with a inductively coupled plasma atomic emission spectroscopy equipment (ICP-OES 725, Agilent Technologies, USA). Prior to the determinations, the samples were subjected to the wet digestion procedure proposed by Alcántar-González and Sandoval-Villa (1999).
Calcium release through time was determined following the methodology proposed by Miranda-Villagómez et al. (2019), using an atomic absorption spectrometer (PinAAcle 900, PerkinElmer, USA). For the determination, several solutions were prepared with 0.30 g of the encapsulated material and 50 mL of deionized water, which were kept in constant agitation for 30, 60, 90 and 120 min, 24, 48 and 72 h. At the same time, the release of Ca2+ by Ca(NO3)2·4H2O and the uncharged nanoparticles (control) was evaluated.
Statistical analysis
The data obtained, from the quantification and release of calcium, were subjected to an analysis of variance and, subsequently, to a Fisher mean comparison test (P ≤ 0.05), for which the statistical software Design Expert ver 8.0 was used. (Stat-Ease, Inc. MN, USA). The analyses were performed in triplicate and the results presented as mean ± standard deviation.
Results and discussion
Scanning electron microscopy
The size and shape of the nanoparticles obtained in this research are the result of the synthesis conditions and the technique used, since these two factors provide the means to adapt the properties of the materials to a specific application (Li, Anton, Arpagaus, Belleteix, & Vandamme, 2010).
Figure 1 shows the morphology and size distribution of gelatin particles with and without calcium loading. Figures 1A and 1C show that, independently of calcium incorporation, both groups of particles had defined perimeters, with mostly spherical shapes and smooth surfaces. In terms of size and distribution (Figures 1B and 1D), no changes were observed when Ca2+ ions were incorporated, since the average particle sizes, with and without charge, were 680 and 698 nm, respectively.
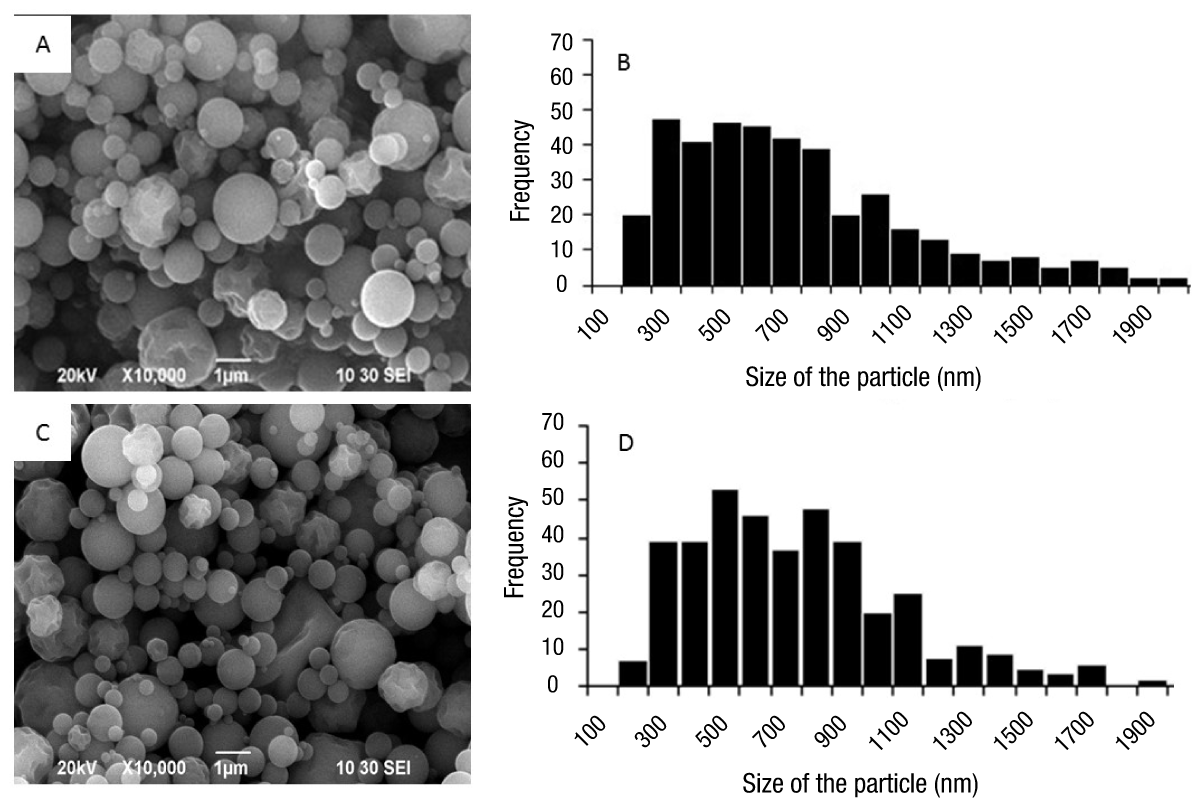
Figure 1 Morphology and size distribution of gelatin nanoparticles with and without calcium loading. Micrography of calcium loaded (A) and non-loaded (C) particles. Histogram of particle size distribution with calcium load (B) and without load (D).
The size and morphology of the particles can be attributed to factors such as the nature of the polymer used and its concentration, the diameter of the grid hole used in the Nano Spray Dryer B-90 equipment (Beck-Broichsitter et al, 2012; Schafroth, Arpagaus, Jadhav, Makne, & Douroumis, 2012), the drying temperature, the gas flow or feed rate (Richard & Benoit, 2000) and the solutes content within each drop formed before drying (Kumar, Bhanjana, Sharma, Sidhu, & Dilbaghi, 2014). In the latter, it has been found that there is a close correlation between the size of the drop to be sprayed and the size of the solid particle obtained after drying, as reported by Schmid, Arpagaus, and Friess (2011) in work done with bovine serum albumin. On the other hand, Li et al. (2010) mention that the particle size distribution of sodium chloride, obtained with the same type of equipment, was related to the concentration of the sample.
Fourier transform infrared spectroscopy
Figure 2 shows the FTIR spectra of calcium nitrate and nanoparticles, with and without load. The spectrum of the unloaded (control) particles revealed characteristic absorption bands of the gelatin at 1 633 cm-1 (amide I, C=O stretching) and 1 529 cm-1 (amide II, N−H bending and C−N stretching) (Lai, Chenga, Yang, & Yen, 2018). On the other hand, the absorption bands recorded in the range of 1 300 to 1 500 cm-1 in the Ca(NO3)2·4H2O spectrum are allocated to asymmetrical stretching of the N−O bonds in the nitrate ions (Ofer-Rozovsky et al., 2019).
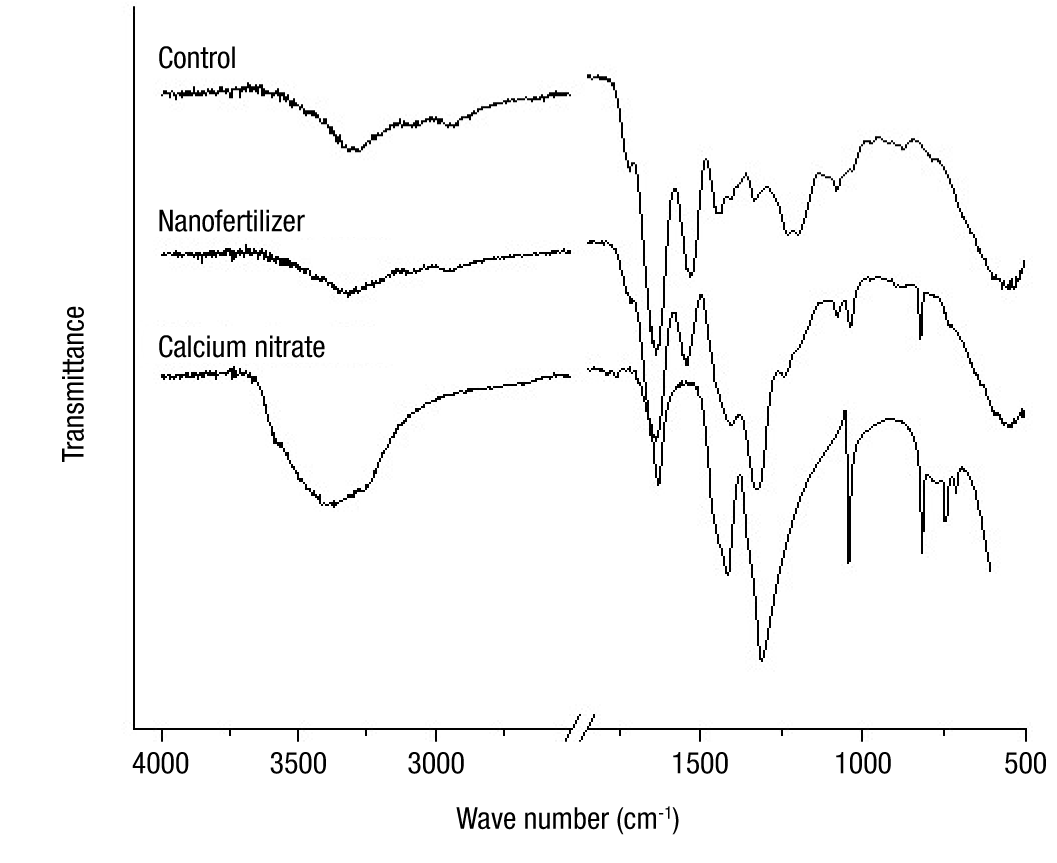
Figure 2 FTIR spectra of the calcium source and of the gelatin nanoparticles with and without calcium loading.
The incorporation of calcium into the gelatin led to identifiable changes in the FTIR spectrum of the nano-fertilizer. The widening and shifting of the band at 1310 cm-1, and the marked decrease in the intensity of the band at 1415 cm-1 are visible. Additionally, a decrease in the intensity of the bands corresponding to protein amides I and II is observed. With these results it can be inferred that Ca2+ ions were adsorbed by the gelatin molecules by coordinating the ions with the carbonyl groups (C=O) of the amide.
Quantification of calcium and release tests
The quantification of calcium by ICP showed that the loaded nanoparticles contained 60.28 g·kg-1 of calcium, which is similar to the calculated theoretical value (67.20 g·kg-1), and the concentration in calcium nitrate was as expected (163.12 g·kg-1 of calcium). On the other hand, the unfilled particles showed a concentration of 0.876 g·kg-1 of calcium, which can be attributed to the gelatin, since a concentration of 0.840 g·kg-1 of calcium was obtained in that material.
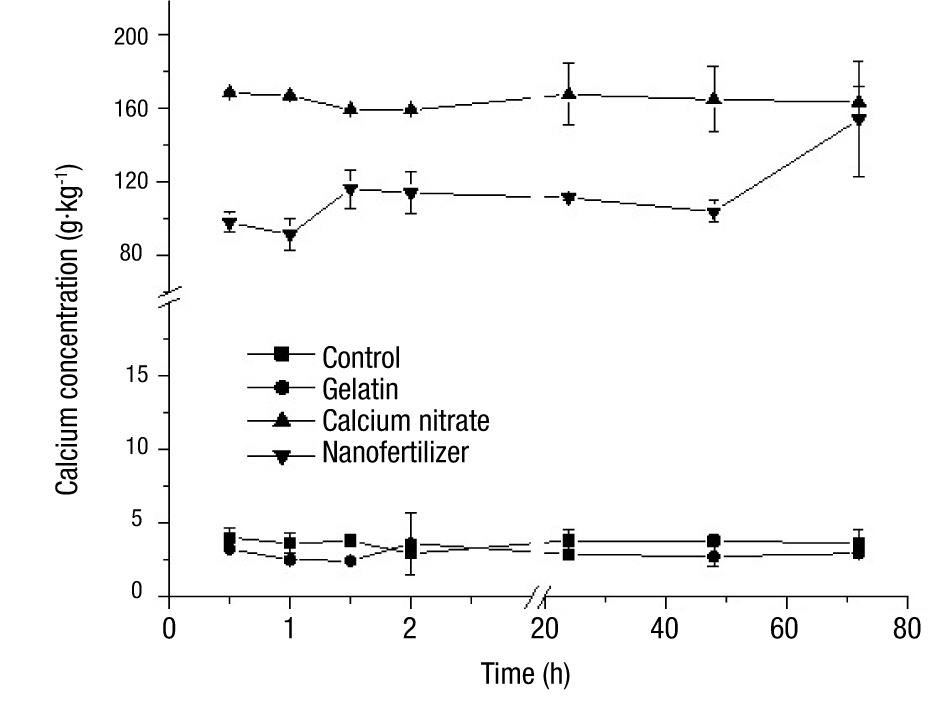
Figure 3 shows the calcium release curves of the nanoparticles, calcium nitrate and gelatin, through time. In this figure it can be seen that only the loaded particles showed an increase from 97.6 to 154.1 g·kg-1 in the calcium concentration as time went by. These values were significantly different (P < 0.05) from those obtained with calcium nitrate, with the exception of the 72-hour determination where this compound presented a value of 164.2 g·kg-1. This allows to assume that at this point the total release of the biopolymer matrix load was achieved. The other analyzed compounds presented a small variation in the concentration of calcium, obtaining average values of 3.7 g·kg-1 in the unfilled particles and 2.9 g·kg-1 in the gelatin.
Due to the observed behavior of calcium-loaded nanoparticles, they can be defined as a diffusion control system, more specifically as a nanosphere system, since the active agent is uniformly distributed throughout the polymer matrix and the release rate is determined by the load or concentration of the dispersed agent, the nature of the components and the shape of the particle (Lshizawa and Nakamatsu, 2002). Rai et al. (2015) mention that the preparation of nanometric formulations of nutrient inputs can have an effect on the efficiency of the fertilizer, causing a different behavior than a conventional fertilizer. This can be seen in Figure 3, where the concentration of calcium in solution showed a slight increase in the first hour and a half of agitation, and then remained almost constant until 48 h and increased again at 72 h, indicating that the polymer formulation helped to delay the release of calcium. This would imply that the calcium ions near the surface of the particles will be the ones that are first detached from the matrix, leaving cavities that become pathways through which the calcium from the interior can be released more quickly, which causes an increase of the element in the medium.
Conclusions
Calcium-loaded gelatin nanoparticles with spherical morphology, smooth microstructure and average size below 700 nm were obtained by spray-drying. FTIR spectra confirmed the establishment of electrostatic interactions between Ca2+ ions and the carbonyl groups of the biopolymer. The elaborated nanomaterials showed the characteristic of a controlled release system, since calcium was slowly released through time. In general, the results observed are promising and create a real possibility that the processed material may be used as a source of agricultural fertilizer.











 text in
text in