Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 n.4 Texcoco May./Jun. 2016
Articles
Comparison of vertical and horizontal resistance against late blight of potato in Toluca
1 Programa nacional de papa. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). Conjunto SEDAGRO, Metepec Estado de México. C. P. 52140. México. (machgg2@yahoo.com.mx; flores.roman@inifap.gob.mx).
One of the main problems of potato cultivation in Mexico and worldwide is late blight (Phytophthora infestans). During three years evaluations under field conditions Toluca Valley, the center of origin of P. infestans were to compare 10 genotypes of the population A vertical resistance (combination of higher R genes and minor genes) and 10 genotypes population B with horizontal resistance (no R genes). A population was generated by INIFAP in Mexico and Peru B population by the International Potato Center. The results showed that the population A was significantly higher in the 5 variables that were used to evaluate the resistance [relative area under the curve of infection, slope (slope of the linear regression: time vs. % leaf infection), % of foliar lesions covered by sporangia, number of days after emergence of the plants when the first foliar infection and tuber yield was observed]. These results indicate that minor resistance genes of the population B operate less efficiently than when combined with R genes, as in the population A.
Keywords: Solanum tuberosum; Phytophthora infestans; major and minor genes
Uno de los principales problemas del cultivo de la papa en México y a nivel mundial es el tizón tardío (Phytophthora infestans). Durante 3 años se hicieron evaluaciones bajo las condiciones de campo del Valle de Toluca, el centro de origen de P. infestans, para comparar 10 genotipos de la población A con resistencia vertical (combinación de genes mayores R y genes menores) y 10 genotipos de la población B con resistencia horizontal (sin genes R). La población A fue generada por el INIFAP en México y la población B en Perú por el Centro Internacional de la Papa. Los resultados demostraron que la población A fue significativamente superior en las 5 variables que se utilizaron para evaluar la resistencia [área relativa bajo la curva de infección, pendiente (pendiente de la regresión lineal: tiempo vs. % de infección foliar), % de las lesiones foliares cubiertas por esporangios, número de días después de la emergencia de las plantas cuando se observó la primera infección foliar y el rendimiento de tubérculos]. Estos resultados indican que los genes menores de resistencia de la población B operan con menor eficiencia que cuando están combinados con genes R, como ocurre en la población A.
Palabras clave: Solanum tuberosum; Phytophthora infestans; genes mayores y menores
Introduction
Late blight, caused by oomycete Phytophthora infestans Sulc., is one of the main problems for potato production in the world (Haverkort et al., 2009). In Mexico, central and northeast are the regions most affected by the disease, where at least two applications of fungicides are made per week during the period of greatest incidence of the disease (Rubio et al., 2000).
Since the devastation of potato fields by late blight occurred in Ireland in 1845, efforts to generate resistant strains intensified. Initially, breeding programs of several countries used the largest (R genes) from the wild species Mexican Solanum demissum as a source of resistance, however, many varieties whose vertical resistance was based on R genes became susceptible soon genes, so it was thought that should generate varieties with horizontal resistance genes based on smaller, which is expected to get a lasting resistance (Turkensteen, 1993; Landeo et al., 1995).
The vertical resistance is also known as qualitative resistance, specific resistance or resistance and horizontal overall resistance as quantitative resistance, overall strength, resistance or partial field resistance. However, at present it is known that the R genes are involved in all types of resistance so it is trying to develop varieties with a combination of several major and minor genes, to which has been called build pyramids of genes (Adillah et al., 2010; Kim et al., 2012; Zhu et al., 2013). This strategy is currently being developed with the help of genetic engineering, but some older varieties that have a combination of major and minor genes, which were generated by traditional methods in Mexico and other countries, have shown to have a lasting resistance (Grünwald et al., 2002; Rietman et al., 2012).
Since Van der Plank (1963) described the concepts of horizontal and vertical resistance, there has been a constant interest in differentiating the horizontal and vertical resistance. The slope of the curve of infection, the development stage of the plants where the first lesions appear, the presence of sporangia in the lesions, and the hypersensitivity reaction in the leaves are some of the parameters that have been used to differentiate the two types of resistance. Of these parameters, the first two have been calculated from the relative area under the curve of infection and have served to classify a large number of clones according to their type of resistance (Marhadour et al., 2013). In previous work carried out under controlled conditions in growth chambers, the response were compared to inoculation with P. infestans isolation in genotypes with and without R genes (Rubio et al., 2005; Rubio et al., 2006). In these works the same mechanisms of resistance in both groups of clones were observed, however, these results must be validated under natural field infection in a place where there is the greatest biodiversity of races of P. infestans, as is the Valley Toluca, which is considered the center of origin of this oomycete (Goodwin et al., 1992; Grünwald et al., 2001).
Most of the potato varieties released in Mexico by INIFAP possess a combination of major and minor genes that confer resistance to P. infestans (Flores and Cadena, 1996; Grünwald et al., 2002; Rubio et al., 2005). His evaluation was carried out under field conditions in the Valley of Toluca, which has allowed select some cultivars with durable resistance, however, have not been compared with genotypes that have only minor genes. The aim of this work is to make this comparison under field conditions in the Valley of Toluca, which is expected to contribute to the understanding of the two types of resistance and support the generation of potato varieties that have lasting resistance against blight late. This knowledge is of great importance at the present time, in which several researchers have issued controversial opinions that favor the use of pyramids of major genes, minor genes or a combination of both (Darsow, 2014).
Materials and methods
For 3 consecutive years (2008-2010) field experiments were established in the Experimental Site of the National Institute of Forestry, Agriculture and Livestock (INIFAP) in Metepec, located in the Valley of Toluca. In each agricultural cycle 10 clones free of R (horizontal resistance) genes versus 10 genotypes with genes R (vertical resistance) they compared pope. The first were generated by the International Potato Center (CIP) and are part of the group known as Population B, whose horizontal resistance against P. infestans comes from free advanced materials R genes, which in turn were derived from genotypes higher (R) and minor genes (Landeo, 1995). Clones of the population B used in the present study had already been assessed in previous years in the Experimental Site Metepec INIFAP and were selected for their resistance against late blight, for its performance and overall for its good adaptation to the conditions field in which (unpublished data) were sown. In this selection process they were eliminated from this study clones that cycle very late maturity, this was done to avoid bias that may cause the relationship between the level of resistance to the duration of the vegetative cycle genotypes Pope (Bradshaw et al., 2004; Darsow, 2014).
The genotypes of the population A were generated by INIFAP and its resistance is based on the combination of major and minor genes. Among this population there are 6 varieties that were previously released and 4 clones were selected for their good adaptation to the conditions under which potatoes are grown in the Valley of Toluca. In these materials, resistance against late blight comes from the wild species Solanum demissum, which is native to Mexico (Fores et al., 1996; Grunwald et al., 2002).
The period of crop development was during the period of rain in the months of june to september, so no irrigation was applied. The experimental design was randomized blocks with 10 repetitions. In each repetition plant each of the 20 genotypes resistant to late blight (10 population A and 10 of the population B) 1 plant a susceptible variety (Alpha) intercalated every 3 plants resistant genotypes was planted. This was done to ensure uniform late blight in the experimental batch distribution. Row spacing was 92 cm and 30 cm between plants. During the growing season no fungicide or the plants were inoculated was applied, so that infection with P. infestans plant occurred naturally. On each floor the date on which appeared the first injury caused by late blight and later weekly observations on the progress of the disease as a percentage of infected leaf area were observed. The percentage of injuries covered by spores was visually estimated during the most favorable time for sporulation, which was mainly associated with the period of greatest frequency and amount of rainfall. In some years they were made up to 3 sporulation assessments due to uncertainty about when the maximum happen, but only the data that reported the highest values were used.
Each of the 5 variables analyzed in this study [on area under the curve of infection (ARBCI), slope (slope of the linear regression: time vs (%) of infection), (%) of leaf lesions covered by sporangia, number of days after emergence of the plants when the first leaf infection and tuber yield (g/plant)] was observed were statistically analyzed using PROC GLM de SAS v9.2. In the analysis of variance (ANOVA) was tested whether there was a difference between genotypes (10 populations A, 10 of the population B and the susceptible variety Alpha), between repetitions in space (10 repetitions in each experiment) and between repetitions time (three years). Statistical analysis of such experiments is described in Steel and Torrie (1980). The ANVA reported significant differences between genotypes, so we proceeded to compare individual means by Tukey test and means between populations using a test of contrasts.
Results and discussion
The analysis of variance showed that almost all sources of variation (genotype, repetition and year) were statistically significant (p≤ 0.05) in the dependent variables ARBCI, slope (%) of sporulation, first foliar infection and tuber yield, with except repetitions effect and performance years. These results indicate that climatic differences between years and the differences between genotypes had influence on the variables used to evaluate the resistance against late blight in plants.
The progress of the disease during the development of plants in the three years of study is presented in Figure 1, which can be seen in the years 2008 and 2010 the percentage of infected leaf area was higher than in 2009. The total amount of rainfall that occurred during the development of plants (june-september) was 657, 684 and 669 mm in 2008, 2009 and 2010 respectively. The differences in total precipitation over the 3 years were small, but in 2009 there was a period of low rainfall between days 49 and 63 after the plants emerged, which made infection was lower this year. In Figure 1 you can also see a large difference of genotypes that have some type of resistance (populations A and B) with respect to the Alpha range, which is susceptible. However, the difference between A and B populations is very small but significant at the end of the development cycle of plants, especially in years with greater severity of late blight (2008 and 2010).
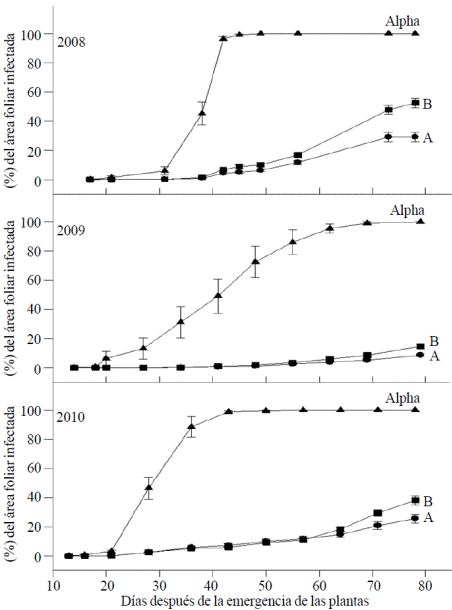
Figure 1 Progress of foliar infection by P. infestans in potato genotypesof populations A, Band the Alpha variety in field trials conducted in the Toluca Valley for 3 years.
These curves of disease progression are related to the way operating the two types of resistance. According to the observations of various researchers (Van der Plank, 1963; Nelson, 1978; Marhadour et al., 2013), it has established that horizontal resistance, based on minor genes, reflected in a low slope of the curve without infection present a sharp increase indicating that the resistance has been overcome, as in the genotypes having only genes R. the shape of the curves of disease development (Figure 1) suggest that the resistance of both populations is not complete and that the defense mechanisms in both populations are partially overcome, but population a are more effective than population B to decrease the development of the disease. These differences are due to the resistance of the population B is based on minor genes (Landeo, 1997) and population B there is a combination of major and minor genes (Flores and Cadena, 1996; Grünwald et al., 2002; Rubio et al., 2005). This fact is supported by the significant differences between the means of ARBCI, the slope and the time when the first foliar infection seen in the A and B populations (Table 1).
Table 1 Relative area under the curve of infection (ARBCI), slope (slope of the linear regression: time vs % infection),% of leaf lesions covered by sporangia, number of days after emergence of the plants when he observed the first foliar infection and tuber yield (g/plant) of 10 genotypes of the population a, 10 genotypes of population B and a susceptible variety (Alpha). The numbers are averages of 3 years and 10 repetitions per year.
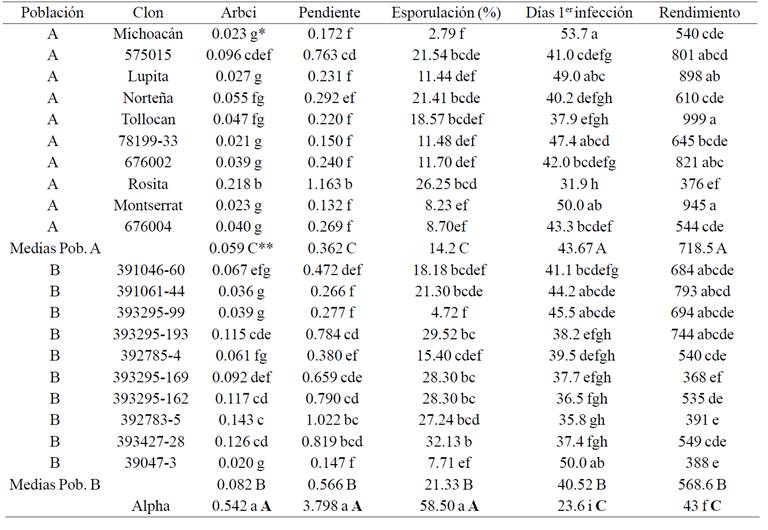
*Prueba deTukey para comparar las medias de los genotipos. Medias con diferentes letras minúsculas indican diferencias significativas (p≤ 0.05). **Prueba de contrastes para comparar las medias entre poblaciones y con la variedad Alpha. Medias con diferentes letras mayúsculas indican diferencias significativas (p≤ 0.05).
From another point of view, much like the disease of A and B (Figure 1) populations develops it indicates the difficulty of separating the horizontal and vertical resistance due to the association between factors controlling resistance. In this connection, Stewart et al. (2003) demonstrated that there is a residual effect of the resistance R after the genes have been overcome by compatible races of P. infestans.
Based on the association between R and QTLs genes, which are attributed the vertical and horizontal resistance respectively has been proposed that horizontal resistance can be an expression of several genes including alleles R genes, which have a complementary effect on the activation of defense mechanisms (Gebhardt and Valkonene, 2001). In this scheme, vertical resistance is the result of efficient coordination between R genes and those genes involved in secondary resistance. This proposal is supported by the theory on the number of sensors needed to initiate the reactions of resistance, which is based on the different effectiveness shown by the R1 and R2 genes to trigger hypersensitivity reactions (Vleeshouwers et al., 2000).
In this case, an increase in receptors may increase the sensitivity to detect and control the pathogen, therefore allelic variation can regulate receptors and consequently affect the plant defense response. On the side of the pathogen, Vleeshouwers et al. (2011) have described the mechanisms P. infestans used to the substances secreted by invading its host, they are not detected by proteins that are produced in the plant by effect of R. genes Based on these resistance mechanisms it is assumed that the clones of the population a used in this study, have several major and minor resistance genes and whose additive effect makes them more resistant clones of the population B, where supposedly there is no R genes, but in which their alleles may exist that reacted similarly but less effective than the genotypes of the population A.
Since the analysis of variance indicated significant differences among genotypes, the next step was comparing her stockings. Table 1 shows the mean of the 5 variables are presented by genotype and population. In this picture we can see that according to the relative area under the curve of infection (ARBCI) and slope, all clones of both populations are more resistant to late blight the variety Alpha. In both populations (A and B) there is a wide range of resistance levels, being the genotypes 78199- 33, Montserrat, Michoacan, Lupita, 676,002, 676,004, Tollocan and Norteña the toughest of the population A. In population B the more resistant clones are: 3947-3, 391061-44 and 393295-99. The comparison of the mean population 5 variables (Table 1) allows for a wide superiority of genotypes that have some type of resistance (populations A and B) on the Alpha variety, which is susceptible. However, the differences between the A and B populations are narrow, but significant and indicate strength and performance of the genotypes of the population to the population genotypes B. The highest yield of the population A is associated with lower infection in the foliage, so there is a greater amount of photosynthate that are produced in the aerial part of the plant and transported to tubers.
The greatest resistance population A cannot be attributed to the length of the growing cycle of plants, since in the two populations were no genotypes that are considered very late. It is generally known that the higher the duration of the vegetative cycle of the plant is increased resistance against late blight of potato (Bradshaw et al., 2004; Darsow, 2014). This relationship is clearer in genotypes with horizontal resistance, however, recent molecular studies have shown that there may be some QTLs that confer resistance, but are not linked to genes conferring late maturity of plants (Danan et al., 2011). Unfortunately there have been no studies to define the R genes and QTLs possessing materials to the population used in this study. This information, along with modern genetic engineering techniques, could serve to make the process of generation and selection of genotypes with high level of resistance not be overcome in the short term more efficient.
In the present study found that the population B sporulation highest percentage of the population A (Table 1). The lower sporulation observed in population A can be explained considering that the resistance is based on a combination of major and minor genes; therefore its resistance effect is greater than when only have minor genes. This explanation is based on the concepts of Van der Plank (1963), who mentioned that genotypes with vertical resistance show an initial reduction of sporulation due to the action of the R genes, but once the resistance is overcome, the amount inoculum increases rapidly. In contrast, with horizontal resistance genotypes are characterized by a reduction of sporulation throughout its cycle due to the joint action of several minor genes (Niederhauser, 1961; James and Fry, 1983). Therefore, by combining both types of resistance a smaller population sporulation is to be expected in A.
Additional observations made during plant development indicate that all genotypes in this study had at some stage of their development hypersensitivity reactions (RH), which consists of air small necrotic appearing on the leaves as a result of programmed death cells to prevent the spread of infection. These results are consistent with the work of Gees and Hohl (1988), who showed that the RH can occur either in potato plants with vertical and horizontal resistance. Other researchers (Vleeshowers et al., 2000) found that the RH is not unique genotypes with R genes but may also occur in wild potato species with various levels of resistance to late blight, however, in this case, speed and effectiveness of RH to control infection was associated with resistance levels of different genotypes. Therefore, the RH initially by Van der Plank (1963) for distinguishing valid horizontal and vertical resistance cannot be considered in all cases.
It is important to note that some parents of the population B come from genotypes R genes (Landeo, 1997). According to this researcher, the breeding process to select free genotypes of R genes was based on the absence of the RH when clones were inoculated with race 0 of P. infestans. However, it has been shown that some genotypes without R genes may show RH with race 0 (Gees and Hohl, 1988; Vleeshowers et al., 2000). Furthermore, it is unknown what could be the reaction of plants possessing genes R unknown. The uncertainty of the test to separate genotypes with and without R genes using the reaction to race 0 of P. infestans, the similar behavior of the curves of development of disease A and B in this study population and the association between genes R and QTLs, opening the possibility that the clones of the population B have genes R.
The results in this work performed under field conditions, consistent with previous studies conducted under controlled conditions in growth chambers (Rubio et al., 2005; Rubio et al., 2006). In these studies, two varieties of the population A and two B clones inoculated with a race of P. infestans were compared, and the results indicated greater resistance in the genotypes of the population B. It is important to note that the tests conducted in this work with more genotypes and repeated for 3 years under field conditions in the Toluca Valley, give greater certainty to the results. The great biodiversity of P. infestans races found in the Toluca Valley reassures the evaluation process of resistance. The results of Blackburn et al. (2007) show that the use of complex races of P. infestans distinguish genotypes with horizontal and vertical resistance. In our study, the materials were subjected to field conditions in a place where there is great biodiversity of races of P. infestans (Goodwin et al., 1992; Grünwald et al., 2001).
The durability of resistance can only be evaluated when a variety is planted for several years in a place where there is great biodiversity of P. infestans races, as is the Valley of Toluca. The durability of the resistance of the varieties of the population A (Tollocan, Montserrat, Northern, Michoacan and Rosita) used in this study has been previously demonstrated under the conditions of the Toluca Valley (Grunwald et al., 2002). This confirms that the process of genetic improvement of potato program INIFAP has been, so far, an adequate system to select genotypes resistant and stable.
Conclusion
The results of this study demonstrate that potato genotypes of the population A (vertical resistance) have greater resistance than population B (horizontal resistance). The population showed superior resistance evaluated by five variables: relative area under the curve of infection (ARBCI), slope (slope of the linear regression: time vs % infection), % of leaf lesions covered by sporangia, number of days after emergence of the plants when the first leaf infection and tuber yield was observed. The combination of major and minor population A genes and genotypes has created high level of resistance, which can be long-lasting. The selection of materials under natural conditions of infection in the Valley of Toluca, where there is the greatest biodiversity of races of P. infestans, has been an appropriate strategy to select varieties resistant to late blight.
Literatura citada
Adillah, T. M. Y.; Hutten, R. C. B.; Visser, R. G. F. and Van Eck, H. J. 2010. The effect of pyramiding Phytophthora infestans resistance genes RPi-mcd1 and RPi-ber in potato. Theor. Appl. Genet. USA. 121(1):117-125. [ Links ]
Blackburn, S. R. M.; Stewart, H. E. and Bradshaw, J. E. 2007: Distinguishing major-gene from field resistance to late blight Phytophthora infestans of potato Solanum tuberosum and selecting for high levels of field resistance. Theor. Appl. Genet. 115(1):141-149. [ Links ]
Bradshaw, J. E.; Pande, B.; Bryan, G. J.; Hackett, C. A.; McLean, K.; Stewart, H. E. and Waugh, R. 2004. Interval mapping of quantitative trait loci for resistance to late blight [Phytophthora infestans (Mont.) de Bary], height and maturity in a tetraploid population of potato (Solanum tuberosum subsp. tuberosum). Genetics. 168(2):983-995. [ Links ]
Danan, S.; Veyrieras, J. B. and Lefebvre, V. 2011. Construction of a potato consensus map and QTL meta-analysis offer new insights into the genetic architecture of late blight resistance and plant maturity traits. BMC Plant Biol. 11(1):1-16. [ Links ]
Darsow, Ulrich. 2014. Pre-breeding and breeding of potatoes for quantitative resistance to Phytophthora infestans on foliage and tubers and for different utilization-problems, solutions and results. Julius Kühn-Institut. Germany. 312 p. [ Links ]
Flores, G. F. X. and Cadena, H. M. A. 1996. Evaluation of horizontal resistance and effects of R-genes in ten Mexican cultivars against potato late blight (Phytophthora infestans) under natural conditions in the central plateau of Mexico. Rev. Mex. Fitopatol. México. 102(1):97-102. [ Links ]
Gebhardt, C. and Valkonen, J. P. T. 2001. Organization of genes controlling disease resistance in the potato genome. Ann. Rev. Phytopathol. 398(1):79-102. [ Links ]
Gees, R. and Hohl, H. R. 1998. Cytological comparison of specific (R3) and general resistance to late blight in potato leaf tissue. Phytopathol. 78(3):350-357. [ Links ]
Goodwin, S.B.; Spielman, L. J.; Matuszak, J. M.; Bergeron, S. N. and Fry, W. E. 1992. Clonal diversity and genetic differentiation of Phytophthora infestans populations in northern and central Mexico. Phytopathol. 82(9):955-961. [ Links ]
Grünwald, N. J.; Cadena, H. M. A.; Rubio, C. O. A.; Rivera, P. A.; Niederhauser, J. S. and W. E. Fry. 2002. Potato cultivars from the Mexican national program: sources and durability of resistance against late blight. Phytopathol. 92(7):688-693. [ Links ]
Grünwald, N. J.; Flier, W. G.; Sturbaum, A. K.; Garay S. E.; Van Den Bosch, T. B. M.; Smart, C. D.; Matuszak, J. M.; Lozoya S. H.; Turkensteen, L. J. and Fry, W. E. 2001. Population structure of Phytophthora infestans in the Toluca valley region of central Mexico. Phytopathol. 91(9):882-890. [ Links ]
Haverkort, A.; Struik, P.; Visser, R. and Jacobsen, E. 2009. Applied biotechnology to combat late blight in potato caused by Phytophthora infestans. Potato Res. 52(3):249-264. [ Links ]
James, R.V. and Fry, W. E. 1983. Potential for Phytophthora infestans populations to adapt to potato cultivars with rate-reducing resistance. Phytopathol. 73(7):984-988. [ Links ]
Kim, H. J.; Lee, H. L.; Jo, K. R.; Mortazavian, S. M. M.; Huigen, D. J.; Evenhuis, B.; Kessel, G.; Visser, R. G. F.; Jacobsen, E. and Vossen, J. H. 2012. Presence of the potato late blight resistance gene RB does not promote adaptive parasitism of Phytophthora infestans. Theor. Appl. Genet. 124:923-935. [ Links ]
Landeo, J. A. 1997. Strategies on breeding for resistance to late blight in potato at CIP. In: Memorias del Simposium Internacional de la papa. INIFAP. Toluca, México. 9-17 p. [ Links ]
Landeo, J. A.; Gastelo, M.; Pinedo, H. and Flores, F. 1995. Breeding for horizontal resistance to late blight in potato free of R-genes. In: Dowley, L. J.; Bannon, E.; Cooke, L. R.; Keane, T. and O’Sullivan, E. (Eds.). Phytophthora infestans. Dublin Boole Press. Dublin, Germany. 268-274 pp. [ Links ]
Marhadour, S.; Pellé, R.; Abiven, J. M.; Aurousseau, F.; Dubreuil, H.; Hingrat, Y. L. and Chauvin, J. E. 2013. Disease progress curve parameters help to characterise the types of resistance to late blight segregating in cultivated potato. Potato Res. 56(2):99-114. [ Links ]
Nelson, R. R. 1978. Genetics of horizontal resistance. Annual Rev. Phytopathol. 16(1):359-378. [ Links ]
Niederhauser, J. S. 1961. Genetic studies of Phytophthora infestans and Solanum species in relation to late-blight resistance in potato. Recent Adv. Bot. 1:491-497. [ Links ]
Rietman, H.; Bijsterbosch, G.; Cano, L. M.; Lee, H. R.; Vossen, J. H.; Jacobsen, E.; Visser, R. G. F.; Kamoun, S. and Vleeshouwers, V. G. A. A. 2012. Qualitative and quantitative late blight resistance in the potato cultivar sarpo mira is determined by the perception of five distinct RXLR effectors. Mol. Plant Microb Int. USA. 25(7):910-919. [ Links ]
Rubio, C. O. A.; Rangel, G. J. A.; Flores, L. R.; Magallanes, G. J. V.; Díaz, H. C.; Zavala, Q. T. E.; Rivera, P. A.; Cadena, H. M. A.; Rocha, R. R.; Ortíz, T. C.; López, D. H.; Díaz, V. M. y Paredes, T. A. 2000. Manual para la producción de papa en las sierras y valles altos del centro de México. Libro técnico No. 1. Campo Experimental Valle de Toluca, CIRCE-INIFAP. Toluca, Mexico. 61 p. [ Links ]
Rubio, C. O. A.; Douches, D. S.; Hammerschmidt, R.; Da Rocha, A. and Kirk, W. W. 2005. Effect of temperature and photoperiod on symptoms associated with resistance to Phytophthora infestans after leaf penetration in susceptible and resistant potato cultivars. Am. J. Potato Research. 82(2):139-146. [ Links ]
Rubio, C. O. A.; Douches, D. S.; Hammerschmidt, R.; Da Rocha, A. and Kirk, W. W. 2006. Effect of photoperiod and temperature on resistance against Phytophthora infestans in susceptible and resistant potato cultivars: effect on deposition of structural phenolics on the cell wall and resistance to penetration. Am. J. Potato Res. 83(4):325-334. [ Links ]
SAS Institute. 2012. SAS release 9.3 ed. SAS Institute, SAS. Cary, NC, USA. 320 p. [ Links ]
Steel, R. G. D. and Torrie, J. H. 1980. Principles and procedures of statistics, a biometrical approach. Second edition. McGraw- Hill, Inc. USA. 633 p. [ Links ]
Stewart, H. E.; Bradshaw, J. E. and Pande, B. 2003. The effect of the presence of R-genes for resistance to late blight (Phytophthora infestans) of potato (Solanum tuberosum) on the underlying level of field resistance. Plant Pathol. 52(2):193-198. [ Links ]
Turkensteen, L. J. 1993. Durable resistance of potatoes against Phytophthora infestans. In: Jacobs, T. and Parlevliet, J. E. (Eds.). Durability of disease resistance. Kluwer academic publishers. The Netherlands. 115-124 pp. [ Links ]
Van der Plank, J. E. 1963. Plant diseases: epidemics and control. Academic Press, New York. USA. 349 p. [ Links ]
Vleeshouwers, V. G. A. A.; Van Dooijeweert, W.; Govers, F.; Kamoun, S. and Colon, L. T. 2000. The hypersensitive response is associated with host and nonhost resistance to Phytophthora infestans. Planta. 210(6):853-864. [ Links ]
Vleeshouwers, V. G. A .A.; Raffaele, S.; Vossen, J. H.; Champouret, N.; Oliva, R.; Segretin, M. E.; Rietman, H.; Cano, L.M.; Lokossou, A.; Kessel, G.; Pel, M. A. and Kamoun, S. 2011. Understanding and exploiting late blight resistance in the age of effectors. Annual Rev. Phytopathol. 49:507-31. [ Links ]
Zhu, S.; Duwal, A.; Su, Q.; Vossen, J. H.; Visser, R. G. F. and Jacobsen, E. 2013. Vector integration in triple R gene transformants and the clustered inheritance of resistance against potato late blight. Transgenic Res. 22(2):315-325. [ Links ]
Received: February 2016; Accepted: May 2016











 text in
text in 


