Introduction
Human health and life on this planet is a gift of natural environment. However, day by day upgraded technologies and industrialization have led to human life to many environmental impacts [1]. Environmental pollution [2] includes air, water, soil and noise pollution. Mostly it is caused by poisonous chemicals such as phenols, azo dyes, heavy metal ions, organic dyes and nitro compounds etc. which are discharged via wastage of industrial activities [3-6]. Synthetic pigments, azo dyes, nitro compounds and phenols are widely used in printing, paper industry, cosmetic, ceramics, pharmaceuticals, textile, and food processing industries [7]. When the concentrations of these hazardous chemicals range from 1-10mg/liter, which is much higher concentration for environment, acute toxicity on aquatic life, terrestrial organism and wildlife has been observed [8]. It has attracted attention of environmentalist and several chemists also. Environment pollution specially water pollution is an emerging threat of great concern in today’s content just because of effluent containing harmful organic pollutant are receiving water stream directly [9]. These toxic chemicals accumulate in soil in major quantities and in long term use, it can cause toxic effects on the crop productivity and ultimately health of human lives. The oxidation of these pollutants using chemical, biological and conventional treatment is very slow or ineffective [10], limitations of these methods are given below in the Table 1.
In the present study the oxidation of 2-aminophenol by HCF (III) ions in aqueous alkaline medium has been studied. HCF (III) is an easily available and cheap oxidant which acts through electron transfer reaction [16-17]. The product of this oxidative reaction gives a coupling product of 2-aminophenol and named as 2-aminophenoxazin-3-one (2-AHP). The oxidation of 2-aminophenol was monitored by UV-Vis spectroscopy and the rate constants were determined. The temperature variation of the overall rate constant was studied at pH 9 within the range 298-313 K and the corresponding activation energy was evaluated. The rate constant, activation energy, enthalpy, entropy, pre-exponential factor and free energy at 298 K are calculated. 2-AHP is related to the naturally occurring compound questiomycin A, an antineoplastic agent used clinically for the diagnoses in certain types of cancers [18-19].
Experimental
Materials and Methods
Instruments and Reagents
2- aminophenol was purchased from CDH (Central Drug House (P) Ltd, New Delhi, India). Analytical grade chemicals and reagents were used for present work. The digital pH meter (Systronics μ-pH System 361) was used to measure the pH of reaction mixture. The λ max of reaction mixture has been written down using Systronics Double-beam spectrophotometer -2203 in the range of 220-800 nm. The Fourier transform infrared (FT-IR) spectra were examined with FTIR (Perkin Elemer) in the range of 4000-450 cm-1 using the KBr cell technique. The HPLC chromatogram and GC-MS spectra were measured by GC-MS spectrometer model number QP2010 Plus. Double distilled water was used during all the experiments. All solutions were freshly prepared before use.
Experimental Procedure
For all oxidation experiments, 100 mL iodometric flask were used to make 2-aminophenol solution (0.0002 M) equipped with magnetic stirrers. pH of all solutions was adjusted to the appropriate value of using KH2PO4 and NaOH solution and measured using pH meter. All experiments of kinetics were carried out at optimum pH (9.0) and at constant temperature (25 ± 0.1 °C). The proper quantities of 2-aminophenol and oxidant were mixed in a 250 mL iodine flask. The reaction was initiated by adding required amount of 2-aminophenol and hexacynoferrate(III) ion into the reaction mixture. The reaction course has been monitored spectrophotometrically by double beam (Systronic 2203) spectrophotometer, at λmax of the reaction mixture i.e. 417.8 nm which remains same for about 90 minutes. Thus, the kinetic study has been made till 60 minutes only. Samples were taken out from the iodometric flask periodically using a pipette. The absorbance of the reaction mixture reduces as the reaction proceeds, with time representing a linear relation between absorbance and [2-aminophenol]. The initial rates (da/dt)i were estimated after 5 min from the start of the reaction by using the plane mirror method drawing a graph between absorbance (a) versus time (t). Each experiment was performed twice under identical conditions. The solvent extraction method is used for product extraction using dichloromethane and identified by FTIR and GC-MS. To extract the product of 2-aminophenol solvent extraction was done by the dichloromethane solvent.
Results and Discussion
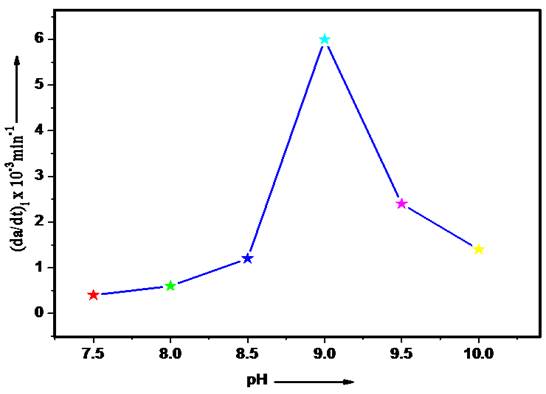
Effect of pH
The most important parameter, which affects the oxidation of organic compounds, is pH. The study was carried out by varying pH from 7.5-10 to find the optimum pH. The presented results in Fig. 1 show that the rate of reaction increases on increasing pH initially but after pH 9 it starts decreasing. Thus, the optimum pH for the oxidation of 2-aminophenol by HCF (III) ions is 9.
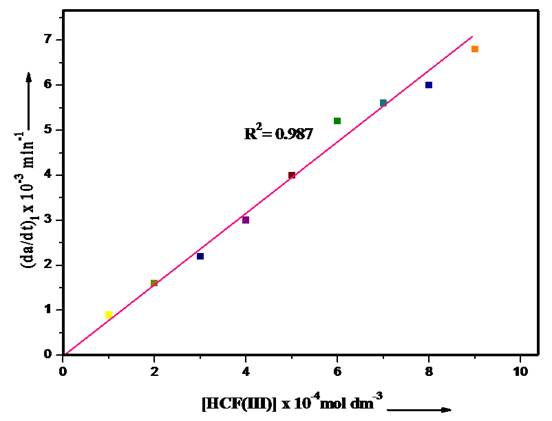
Effect of HCF (III) ion concentration
Data in Fig. 2 illustrate the influence of [oxidant] on the observed rate of the oxidation of 2- aminophenol. To study the impact of oxidant [HCF(III)] on the oxidation of 2-aminophenol, the [HCF(III)] was varied from 1×10−4 - 9×10−4 mol/dm3, keeping the [2-aminophenol], temperature, and pH constant. The results shown in the form of Fig. 2 reveal that with the increase in [oxidant], the rate of oxidation of 2-aminophenol increases linearly showing that it follows first-order kinetic model with respect to [HCF(III)].
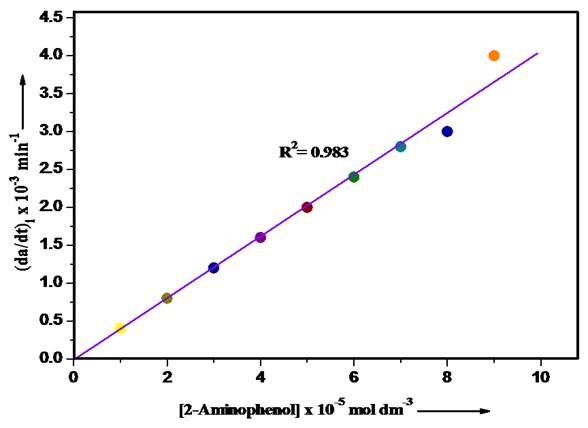
Effect of 2-aminophenol concentration
The impact of [2-aminophenol] on the rate of reaction was examined by varying its molar concentration from 1 × 10−5 - 9 × 10-5 mol/dm3. A straight line plot between reaction rate and [2-aminophenol] and value of R2 (0.983) shows that reaction follows first-order kinetics with respect to [2-aminophenol] (Fig. 3).
Effect of ionic strength
The impact of ionic strength on the rate of oxidation of 2-aminophenol was studied by using various concentrations of KCl in the range of 0.30 to 0.45 M. The linear increase in rate with the ionic strength shows the primary salt effect i.e. involvement of same type of species in reaction mechanism.
Thermodynamic parameters
The calculation of thermodynamic parameters was performed after investigating the impact of temperature on reaction rates at 25, 30, 35, and 40 °C.
Thermodynamic parameters including enthalpy of activation (ΔH #), pre-exponential factor (A), activation energy (Ea), energy of formation (ΔF#) and entropy of activation (ΔS#) for the oxidation of 2-aminophenol by HCF(III) ions have been evaluated using Arrhenius equation and plot (Fig. 4). The data represents that the reaction was characterized by a more negative value of entropy (S#) of activation shows the formation of polar species during the reaction. Positive value of enthalpy of activation (ΔH #) shows reaction is endothermic because the enthalpy of product is greater than enthalpy of reactant. The Arrhenius equation is mentioned as follows:
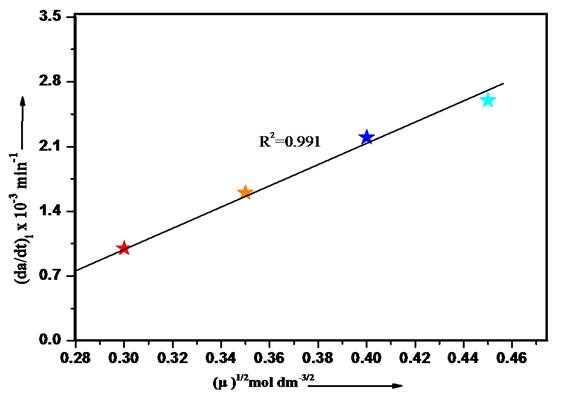
Fig. 4 Effect of ionic strength on the rate of oxidation of 2- Aminophenol. Temp. 25 °C, λmax 417.8nm, [HCF(III)] 3x10-4 mol/dm3, [2-AP] 3x10-5 mol/dm3.
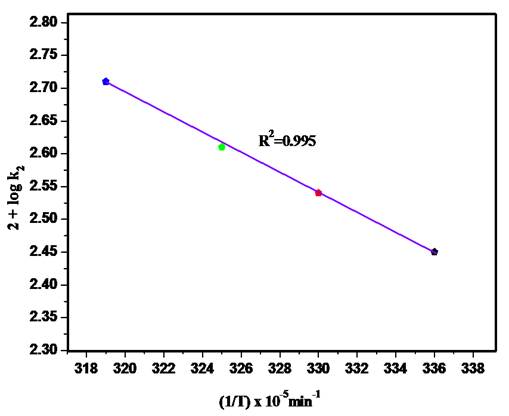
Fig. 5 Impact of temperature on the oxidation of 2- Aminophenol. pH 9, λmax 417.8, [HCF(III)] 3x10-4 mol/dm3, [2-AP] 3x10-5 mol/dm3.
Separation and identification of product
The product is extracted from reaction mixture by dichloromethane [20] after about 24 hours of reaction and then the extraction had been run in column of silica gel to obtain product. The red crystals separated were analyzed by preliminary test as melting point, element detection and functional group test. After separation the yield of product was about 60 %. The melting point 253 °C and functional groups -NH2 and ˃CO group indicates the formation of 2-AHP [21]. The product is further characterized by FTIR and GC-MS methods of analysis.
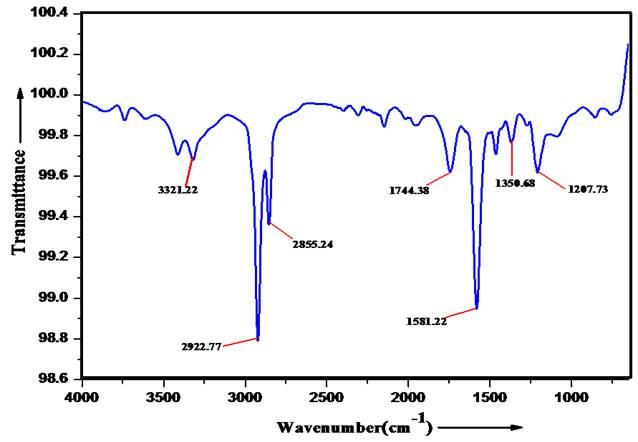
FT-IR (Fourier transform infrared) spectroscopy
The product is analyzed by FT-IR technique to find the existences of the functional groups in it. The IR spectra of product show bands in the region 3321.22, 2922.77, 2855.24, 1744.38, 1581.22, 1350.68 and 1207.73cm-1. The comparison of data with the literature data [22] suggests the presence of ether group (1207.73cm-1), amine group (1350.68 and stretching at 3321.22cm-1). The band frequency at 1581.22, 1744.38 and 2855.24 cm-1 correspond to =N-H, ˃CO and aromatic groups [23] respectively.
GC-MS
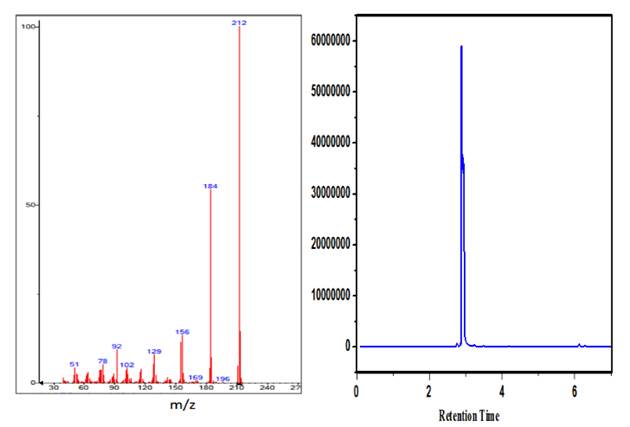
Gas chromatography-mass spectroscopy (GCMS) is a technique that merges the physical separation capabilities of gas chromatography with the mass analysis. Hence, in the present study m/z values have been used to investigate the product of the reaction. Single peak with retention time 3.0 min and m/z value 212 confirm the formation of 2-AHP as the main oxidation product.
Antibacterial Activity: Agar well diffusion method
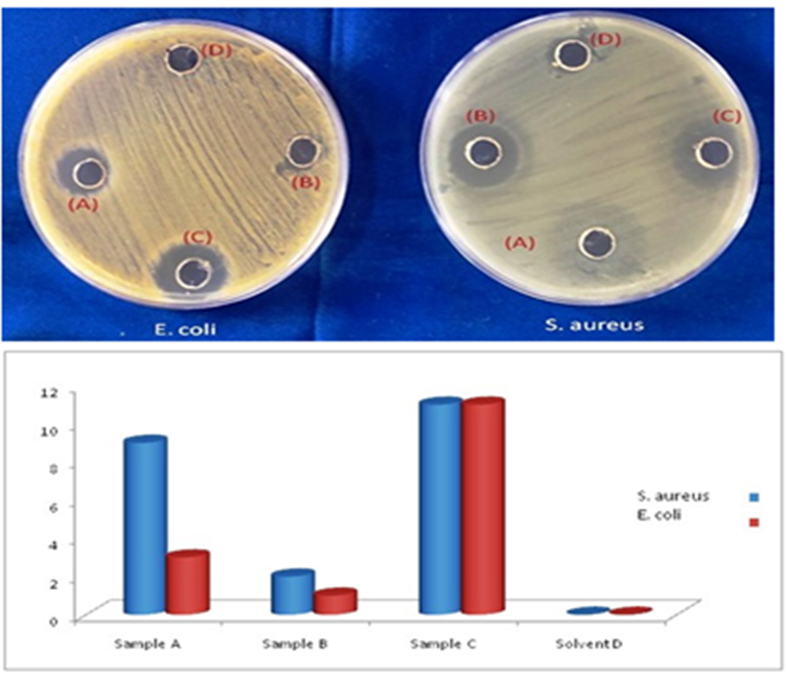
The gram positive (Staphylococcus aureus) and gram negative (E. Coli) bacteria are used to examine the antibacterial activity of 2-AHP. Activity of 2-AHP was evaluated qualitatively by agar well diffusion method in Muller Hinton agar medium. The concentration of 2-AHP was 100 (sample (A)) and 50 (sample (B)) µg/mL. The wells of diameter 6 mm were puncture with a sterile cork borer. The solution of product (2-AP) was used in two concentrations ((A) and (B)) and DMSO used as a solvent (D) and a positive control (C). A control of vancomycin was also used for positive control (C). After incubation of petriplate for 24 hours at 37 °C, then the zone inhibition was measured. The Fig. 9 inform that vancomycin (sample (C)) gives 17 mm, 100 µg/mL (sample (A)) 15 mm and 50 µg/mL (sample (B)) 8 mm zone of inhibition with S. aureus. In E. coli again vancomycin shows 17 mm, sample A 9 mm and sample B 7 mm zone of inhibition.
Mechanism
Taking the above observation in view at lower concentration of reactants, the following rate expression may be suggested
Where kobs is observed rate constant. The observed k value was found to be 2.34 mol-1dm3s-1 and [S] represents concentration of substrate 2-AP.
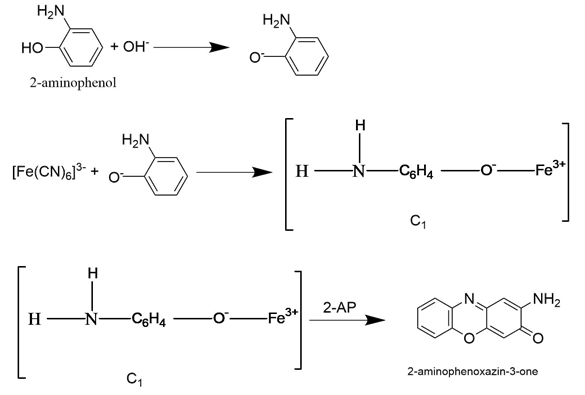
Thus, in order to explain the above said results, the following reaction mechanism can be proposed in alkaline medium
This anion forms a labile complex (C1) with ferricyanide ion (Scheme 1). The complex C1 dissociates into the final product and ferrocyanide ion through the fast step.

Scheme 1 In the above scheme it is based on the results and reported work [24]. It is assumed that in alkaline medium 2AP(S) forms anion [S-]with OH-.
Now from the step (ii), the rate of reaction would be given by:
from equation (i), assuming that water is in excess, and its concentration remains constant during the reaction, the concentration of anion [S-] is:
From equation 2 and 3:
since the concentration of OH- is constant, so a new constant k׀ is used at the place of a constant K[OH-]. The derived rate law equation (5) is similar to the experimental rate law (1) verifies the proposed mechanism at lower concentration of reactants.
Based on the experimental work and literature, following oxidation scheme is proposed:
The formation of product is confirmed by product analysis using GC-MS and FT-IR methods of analysis.
Conclusion
The oxidative degradation of 2-aminophenol was observed kinetic-spectrophotometrically by using hexacyanoferrate (III) ions in aqueous alkaline medium. The oxidative kinetics of 2-aminophenol follows 1st order kinetic model with respect to [HCF(III)] and [2-aminophenol].
The oxidized product 2-aminophenoxazin-3-one was identified by the FTIR and GC-MS methods of analysis. The product of oxidation (2-AHP) shows good antibacterial activity for S. aureus (gram positive) than E. coli (gram negative). The results present in Fig. 8 shows that zone of inhibition of b) ˂ a) i.e. lower the concentration of 2-AHP, lower is its inhibition power. Thus, the present method seems to be green and environmentally friendly for the oxidative conversion of 2-aminophenol to 2-AHP. The method is efficient, simpler, and cheaper due to the need of less amount of oxidant [HCF (III)] as compared to the other reported methods of oxidation (in Table 1). Thus, this study may be helpful for improvement in quality of wastewater of many industries and others.











 text new page (beta)
text new page (beta)