Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.52 n.2 Texcoco Feb./Mar. 2018
Biotechnology
Obtaining aromatic compounds through lignin oxidation with laccase immobilized in alginate
1Instituto Politécnico Nacional, UPIIG, Avenida Mineral de Valenciana No. 200 Fraccionamiento Industrial Puerto Interior, Silao de Victoria, Guanajuato México, C.P.
2Politécnico Colombiano Jaime Isaza Cadavid. Cra. 48, No.7-151, Medellín-Colombia.
Lignin is an abundant biopolymer in nature; because of its polymeric structure, it is a potential source of aromatic compounds with high added value in the chemical, food and pharmaceutical industries. Lignin can be used with chemical and biological procedures that depolymerize it in a gradual and selective manner. Peroxidases and laccases are enzymes used as oxidizing agents in processes of oxidative depolymerization; however, the use of H2O2 as secondary electron acceptor to increase the oxidizing effect of immobilized laccase is not reported so far. The objective of this study was to evaluate the catalytic capacity of laccase (1.25 mUI) immobilized in alginate pearls, with a particle diameter (Dp) of 3 mm, in order to obtain aromatic compounds from lignin oxidation. The reactions were kept with agitation and controlled temperature, the pH varied and H2O2 was used as secondary electron acceptor. The experimental design was factorial 3 x 4: three pH values (5.8, 6.5 and 8.6) and four concentrations of H2O2 (25, 50, 75 and 100 mM); the trials were performed in triplicate. ANDEVA was carried out with the data as well as means comparison tests (Tukey, p ≤ 0.05). The highest conversion of lignin to aromatic compounds was obtained with a pH of 8.6: benzoic acid (63.5 %), vanillic acid (25 %) and vanillin (11.5 %). The immobilizing support for the enzyme was disintegrated and dissolved in the medium in the reactions catalyzed with a pH over 7.0. The results allow suggesting that the degree of lignin depolymerization with immobilized laccase depends directly on the concentration of H2O2 and the medium’s pH.
Key words: laccase; lignin; enzyme degradation; immobilization; benzoic acid.
La lignina es un biopolímero abundante en la naturaleza, por su estructura polimérica es una fuente potencial de compuestos aromáticos con alto valor añadido en la industria química, alimentaria y farmacéutica. La lignina puede aprovecharse con procedimientos químicos y biológicos que la despolimerizan de forma gradual y selectiva. Las peroxidasas y lacasas son enzimas usadas como agentes oxidantes en procesos de despolimerización oxidativa; pero el uso de H2O2 como aceptor secundario de electrones para incrementar el efecto oxidante de lacasa inmovilizada no se ha reportado. El objetivo de este estudio fue evaluar la capacidad catalítica de lacasa inmovilizada (1.25 mUI) en perlas de alginato, con diámetro de partícula (Dp) de 3 mm, para obtener compuestos aromáticos por oxidación de lignina. Las reacciones se mantuvieron con agitación y temperatura controlada, el pH se varió y como aceptor secundario de electrones se utilizó H2O2. El diseño experimental fue factorial 3 x 4: tres valores de pH (5.8, 6.5 y 8.6) y cuatro concentraciones de H2O2 (25, 50, 75 y 100 mM); las pruebas se realizaron por triplicado. Con los datos se realizó ANDEVA y pruebas de comparación de medias (Tukey, p ≤ 0.05). Con pH de 8.6 se obtuvo la mayor conversión de lignina a compuestos aromáticos: ácido benzoico (63.5 %), ácido vainillínico (25 %) y vainillina (11.5 %). El soporte de inmovilización de la enzima se desintegró y se disolvió en el medio en las reacciones catalizadas con pH superior a 7.0. Los resultados permiten sugerir que el grado de despolimerización de lignina con lacasa inmovilizada depende directamente de la concentración de H2O2 y del pH del medio.
Palabras clave: lacasa; lignina; degradación enzimática; inmovilización; ácido benzoico
Introduction
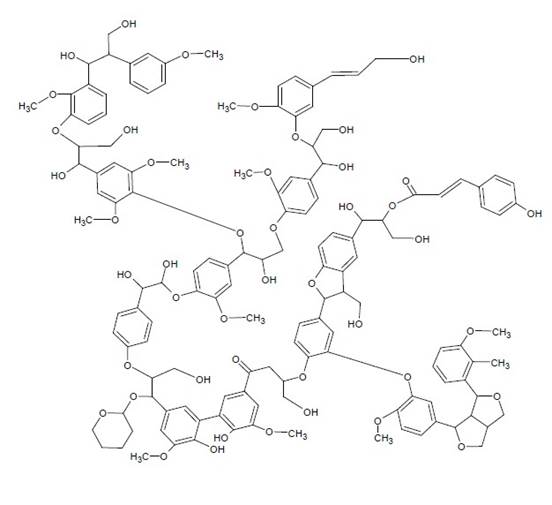
Lignin is one of the most abundant biopolymeres in plants, it is a phenolic macromolecule, and is united covalently to cellulose and other polysaccharides of the cell wall, such as hemicelluloses and pectins (Johansson et al., 2014). The chemical structure of lignin (Figure 1) consists primarily of three phenylpropanoid units: coniferyl, cumaric and sinapyl alcohol (Bai et al., 2014; Jin et al., 2014). Because of its polymeric nature, it is a potential source of aromatic compounds with added value, which can be used in the food industry for the production of vanillin and vanillic acid (Nagar et al., 2010; Menon and Rao, 2011; Eudes et al., 2014; Volokitina et al., 2015); and in the pharmaceutical industry for the chemical synthesis of guaiacol and catechol (Collinson and Thielemans, 2010; Menon and Rao, 2011).
In order to transform lignin, chemical and biological procedures were developed and depolymerize it in a selective and gradual manner. Among these there is the use of inorganic catalyzers, such as MnO2, Zr (CH3CO2), Co (CH3CO2) and Mn (CH3CO2) (Collinson and Thielemans, 2010; Doherty and Mousavioun, 2010; Zhang et al., 2014); however, heterogeneous catalysis tends to generate other products that must be purified for their use. The degradation of lignin was also performed with Trametes versicolor, Ceriporiopsis subvermispora, Cyanthus stercoreus and Phlebia radiata (Dávila and Vázquez-Duhalt, 2006), which produce enzymes, primarily laccases and peroxidases, which catalyze the separation of bonds between the subunits of lignin and cause their gradual depolymerization (Collinson and Thielemans, 2010).
Laccases (benzenediol:oxygen oxidoreductases E.C. 1.10.3.2) catalyze the oxidation of phenolic compounds and aromatic amines; they use molecular oxygen as an acceptor of electrons, and also oxidize metoxyphenolic acids (Forte et al., 2010; Kim et al., 2011; Jin et al., 2014), decarboxylize and degrade their methoxy groups from demetylation or demetoxylation (Solomon et al., 1996; Dávila and Vázquez-Duhalt, 2006; Moilanen et al., 2011; Polak and Jarosz, 2012; Rodrigues et al., 2012; Pang et al., 2015). Some laccases use electron transporters for their catalytic action, and others do not (Arana et al., 2002; Ganachaud et al., 2008; Forte et al., 2010; Kim et al., 2011; Lange et al., 2013). The objective of this study was to determine if H2O2, as mediator in electron transport, favors the catalytic capacity of immobilized laccase in the depolymerization of lignin. The hypothesis was that H2O2, as secondary acceptor of electrons, increases the oxidizing effect of laccase immobilized in alginate, in phenolic and non-phenolic lignin compounds.
Materials and Methods
Materials
The compounds like benzoic acid, vanillic acid, ρ-cumaric acid, ferulic acid, hydroquinone and vanillin (Sigma-Aldrich), laccase from Trametes versicolor (EC 1.10.3.2, 1.25 mIU, CAS 80498-15-3) and lignin (CAS 8068-05-1), and the others used in the study were of analytic grade or chromatographic grade.
Preparation and activation of the alginate pearls
The sodium alginate pearls, with diameter of 3 mm, were prepared with the method by Pal and Khanum (2011). Two grams of the compound were dissolved in 100 mL of hot deionized water, and to form the pearls the solution was poured by dripping in 0.2 M CaCl2 at 4 °C. To harden them, the pearls were stored for 24 h in 0.02 M CaCl2 at 4 °C. Then, the pearls were separated by filtering the CaCl2 solution and washed with deionized water in a proportion of 10 times the volume of CaCl2. To ensure their activation, the pearls were transferred to a solution of glutaraldehyde at 9 % (v / v) and citrate buffer (pH 5.0) and were kept in agitation for 90 min. The pearls were stored in glutaraldehyde at 9 % (v / v) with citrate buffer at 4 °C for 24 h.
Determination of enzymatic activity
The activity of laccase was determined by oxidation of ABTS 2,2´-azino-bis (3-ethylbenzothiazoline)-6-sulfonate in acetate buffer (0.1 M and pH 5) at 50 °C. In a test tube, 2.85 mL of the 0.5 mM ABTS solution and 0.15 mL of enzyme were mixed in an acetate buffer, and kept for 1 h at 30 °C. The reaction was stopped by ice bath and the absorbance was read at 420 nm in a UV-Vis spectrophotometer (Jenway, model 6715). One unit of laccase (mIU mL-1) was defined as 1 lmol of ABTS oxidized per minute (Childs and Bardsley, 1975).
Immobilization of laccase
The pearls were separated by filtering of the glutaraldehyde solution with citrate buffer, and washed with deionized water. Then, they were placed in a solution of laccase (1.25 mIU mL-1), with vigorous agitation for 1.5 h at room temperature. The alginate pearls with the immobilized enzyme were stored at 4 °C until their use (She et al., 2010).
Biocatalytic activation of the enzyme
The alginate pearls with immobilized enzyme were transferred to phosphate buffer, at the desired pH (5.8, 6.5 and 8.6), and lignin solution was added (80 g), previously solubilized at room temperature, in 100 mL of deionized water, for each treatment. Immediately the solution of H2O2 was added, in the concentrations established, the enzymatic reaction began at 25 °C and agitation (150 rpm) in an orbital agitator (ZHCHENE, ZHWY-200D model). The reaction was kept for 24 h; every 1.5 h a sample was taken, and the absorbance was determined for each sample in the UV-Vis spectrophotometer (Janshekar et al., 1981).
Catalytic activity controls
Two controls were included in each experimental process. These, according to what was established in the experimental design, without immobilized laccase. The control without H2O2 was kept under the conditions of reaction established in the experimental design.
Quantification of H2O2 consumption
The solution 0.1 M of KMnO4 was heated up to boiling. Simultaneously, an aliquot of 0.25 mL of oxygenated water was transferred to a volumetric flask of 5 mL and was gauged with deionized water. Then, an aliquot of 1.25 mL of the oxygenated water solution was transferred to a precipitate cup, 0.15 mL of H2SO4 6 M and 1.25 mL of deionized water were added, and this was heated at 60 °C. The volumetric titling of the H2O2 solution with the standardized permanganate solution was continued until the appearance of a permanent pink color (Sant, 1956).
Thin layer chromatography
For the preparative separation by thin layer chromatography (TLC) of the reaction products, silica gel plates (0.25 mm of thickness and 5 × 10 cm) were used as stationary phase and indicator of UV254 fluorescence (Merck AG, Darmstadt, Germany); before using, they were heated (activated) at 40 °C, for 1 h. The mobile phase was water: methanol (50 / 50, v / v) chromatographic degree. Fifty (L of samples and standard solutions of benzoic acid, vanillic acid, ρ-cumaric acid, ferulic acid, hydroquinone and vanillin were applied in different individual lanes of the plate. Before eluting, the chamber was saturated for 15 min, at the end of the elution the plates were kept at 25 - 30 °C until complete evaporation of the solvent, then they were observed under ultraviolet light, and the trajectory of the samples was detected through fluorescence. The retention factor was calculated with the following relation:
Retention factor (Fr) = distance travelled by the standard or the sample / distance travelled by the solvent.
Quantification of reaction products
The products of the lignin depolymerization reaction were quantified with calibration curves of standards of benzoic, vanillic, ρ-cumaric and ferulic acids, and hydroquinone and vanillin, in solutions of up to 2.5 mM. The maximum absorbance of each compound was previously determined in UV-Vis spectrophotometer (Jenway, 6715), between 250 and 350 nm.
Experimental design and statistical analysis
The experimental design was factorial 3 x 4: three levels of pH (5.8, 6.5 and 8.6) and four levels of H2O2 concentration (25, 50, 75 and 100 mM); the tests were carried out by triplicate. The results were analyzed with ANDEVA and the Tukey means comparison test (p ≤ 0.05), with MINITAB 16 (Minitab Inc. State College, Pennsylvania).
Results and Discussion
The depolymerization of lignin increased proportionally with the pH of the medium (Figures 2 to 4). With a pH higher than 7.0 the immobilization support was disintegrated and this allowed the laccase to be solubilized in the medium and to increase its activity (Figure 4); thus, at a pH of 8.6 the concentration of lignin derivatives was higher than at pH 5.8 and 6.5. In the latter, the enzyme remained immobilized. The higher yields of conversion were for benzoic acid 63.5 %, vanillic acid 25 %, and vanillin 11.5 %, and were obtained at pH 8.6. The yield of vanillin is comparable at 8.5 and 11.5 % reported by Crestini et al. (2006), with methyltrioxorhenium (MeReO3) as catalyzer and H2O2 as oxygen donator; however, it exceeded the 1.5 % reported by Zheng et al. (2014), with zeolite as catalyzer. This allows suggesting that the depolymerization of lignin with laccase is feasible. In the control reactions lignin oxidation was not observed with any pH or concentration of H2O2, which is why the oxidative power of the H2O2 decreased in these reactions, probably as a result of the high concentration of H2O2. According to Rodríguez et al. (2008), this compound in excess captures hydroxyl radicals, which decreases their oxidative capacity; in addition, the speed of degradation of the H2O2 is also low, compared to some complex substances, which is why its use in combination with other oxidants is recommended.
The disintegration of the immobilization support allowed for the enzyme to be solubilized and the effect of the H2O2 concentration was scarcely significant (Figure 5), since the concentration of benzoic acid, main derivative of lignin in the reactions catalyzed with laccase remained practically constant, independent of the H2O2 concentration. In contrast, in the reactions where the enzyme remained immobilized the higher production of lignin derivatives depended on the H2O2 concentration; thus, at pH of 5.8 the highest production was obtained with 75 mM de H2O2 (Figure 6) and at pH of 6.5 with 50 mM (Figure 7). This is because the oxidative capacity of the H2O2 depends on pH, temperature, concentration and reaction time. Therefore, it is convenient to establish the optimal dose of H2O2. This confirmed that the medium’s pH is able to regulate the delignification through oxidation. In this regard, Ruuttunem and Vuorinen (2005) evaluated different catalyzers in the same reaction and determined that the relative reactivity of the catalyzers with the phenolic groups in lignin increased when the pH decreased.

Figure 5 Effect of H2O2 concentration on the production of benzoic acid at pH of 8.6, with soluble enzyme.
Although the higher yields of lignin conversion were obtained with solubilized enzyme, and that the H2O2 concentration did not affect the catalytic activity of the enzyme significantly, it is possible to infer synergetic activity of laccase with H2O2, since a direct correlation was observed in the reactions with the immobilized enzyme between the pH of the reaction and the concentration of H2O2 used. That is, at higher pH the concentration of H2O2 necessary to mediate the lignin catalysis will be lower. Therefore, the H2O2 concentration necessary to mediate the reaction at pH of 8.6 can be lower than 25 mM H2O2. It should also be considered that the immobilized enzymes show lower catalytic activity than the soluble enzyme and this allows explaining partially the fact that in the reactions at pH of 8.6 the production of lignin derivatives was higher.
The consumption of H2O2 indicated that 89.4 % of the H2O2 50 mM was consumed at a pH of 5.8, in the liquid medium; and was different from the 93.5 % consumed when the concentration was 75 mM (Table 1). In the reactions at a pH of 6.5 with H2O2 50 mM, the consumption of H2O2 (95.2 %) was higher than in the reactions with 75 mM (82.3 %). Thus, the higher consumption of H2O2, in the reactions catalyzed by laccase immobilized in alginate pearls, coincided with the higher conversion of lignin in each pH. This agreed with the report by Solomon et al. (1996) and Boukari et al. (2011) that the H2O2 facilitated the extraction and conversion of lignin from lignocellulosic materials. Besides, it was observed that this oxidizing agent did not provoke enzymatic inhibition and that in all the reactions the consumption of H2O2 was significant.
Table 1 Difference between the H2O2 used in the lignin hydrolysis reaction and the one quantified from titling with potassium permanganate.
| pH | H2O2 (mM) | Moles añadidos de H2O2 | Moles finales de H2O2 |
| 5.8 | 50 | 8.12 x 10-5 | 8.63 x 10-6 |
| 75 | 1.62 x 10-4 | 1.06 x 10-5 | |
| 6.5 | 50 | 8.12 x 10-5 | 3.93 x 10-6 |
| 75 | 1.62 x 10-4 | 2.86 x 10-5 |
The factors analyzed and their interaction were significant (p = 0.02) for the factor pH, 0.035 for the factor concentration of H2O2 and 0.046 for the interaction pH x H2O2.
Conclusions
The depolymerization of lignin in order to obtain aromatic compounds is possible with laccase immobilized in alginate pearls and H2O2 as a secondary acceptor of electrons, since the yields are comparable to those of other oxidative depolymerization processes; in addition, it has the advantage of being a safe and clean process. The concentration of H2O2 influences significantly the conversion yield and has a direct relationship with the pH of the reaction. The action of H2O2 is synergic with the laccase immobilized in lignin depolymerization. Although during the reaction practically all the H2O2 is reduced, and the enzymatic is not inhibited.
Literatura Citada
Arana, A., A. Téllez, T. González, and A. González 2002. Aspectos generales de la biodegradación de la madera: Aplicaciones industriales de las lacasas. BioTecnología 7:40-55. [ Links ]
Bai, X., K. H. Kim, R. C. Brown, E. Dalluge, C. Hutchinson, Y. J. Lee, and D. Dalluge. 2014. Formation of phenolic oligomers during fast pyrolysis of lignin. Fuel 128: 170-179. [ Links ]
Boukari, I., C. Rémond, M. O´Donohue, and B. Chabbert. 2011. Effect of lignin content on a GH11 endoxylanase acting on glucoronoarabioxylan-lignin nanocomposites. Carbohyd. Polym. 89: 423-431. [ Links ]
Childs, R. E., and W. G. Bardsley, 1975. The Steady-State kinetics of peroxidase with 2,2'-Azino-di-(3-ethylbenzthiazoline-6-sulphonic acid). Biochem. J. 145: 93-103. [ Links ]
Collinson, S., and W. Thielemans. 2010. The catalytic oxidation of biomass to new materials focusion on starch, cellulose and lignin. Coord. Chem. Rev. 254: 1854-1870. [ Links ]
Crestini, C., C. Caponi, D. S. Argyropoulos, and R. Saladino. 2006. Immobilized methyltrioxo rhenium (MTO)/H2O2 systems for the oxidation of lignin and lignin model compound. Bioorg. Med. Chem. 14: 5292-5302. [ Links ]
Dávila, G., and R. Vázquez-Duhalt. 2006. Enzimas lignolíticas fúngicas para fines ambientales. Mensaje Bioquím. XXX: 29-55. [ Links ]
Doherty, W., and O. P. Mousavioun. 2010. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crops Prod. 33: 259-276. [ Links ]
Eudes, A., Y. Liang, P. Mitra, and D. Loqué. 2014. Lignin bioengineering. Curr. Opin. Biotechnol. 26: 189-198 [ Links ]
Forte, S., J. Polak, D. Valensin, M. Taddei, R. Basosi, S. Vanhulle, A. Jarosz-Wilkolazka, and R Pogni. 2010. Synthesis and structural characterization of a novel phenoxazinone dye by use of a fungal laccase. J. Mol. Catal. B: Enz. 63: 116-120. [ Links ]
Ganachaud, C., V. Garfagnoli, T. Tron, and G. Iacazio. 2008. Trimerisation of indole through laccase catalysis. Tetrahedron Lett. 45: 2476-2478. [ Links ]
Janshekar, H., C. Brown, and A. Fiechter. 1981. Determination of biodegraded lignin by ultraviolet spectrophotometry. Anal. Chim. Acta. 130: 81-91 [ Links ]
Jin, S., Z. Xiao, C. Li, X. Chen, L. Wang, J. Xiang, W. Li, and C. Liang. 2014. Catalytic hydrodeoxygenation of anisole as lignin model compound over supported nickel catalysts. Catal. Today. 234: 125-132 [ Links ]
Johansson, K., T. Gillgren, S. Winestrand, L. Järnström, and L. Jönsson. 2014. Comparison of lignin derivatives as substrates for laccase-catalyzed scavenging of oxygen in coatings and films. J. Biol. Eng. 8:1-12 [ Links ]
Kim, S., S., C., P. Huh, J. Kumar, B. Kim, J. O. Lee, and F. Bruno. 2011. Polyoxometalate/laccase-mediated oxidative polymerization of catechol for textile dyeing. Green Chem. 9: 44-48. [ Links ]
Lange, H., S. Decina, and C. Crestini. 2013. Oxidative upgrade of lignin - Recent routes reviewed. Eur. Polym. J. 49:1151-1173. [ Links ]
Maijala, P., M. L. Mattinen, P. Nousiainen, J. Kontro, J. Asikkala, J. Sipila, and L. Viikari. 2012. Action of fungal laccases on lignin model compounds in organic solvents. J. Mol. Catal. B: Enz. 76: 59-67. [ Links ]
Menon, V., and M. Rao. 2011. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Comb. Sci. 38: 522-550. [ Links ]
Moilanen, U., M. Kelloc, S. Galkin, and L. Viikari. 2011. The laccase-catalyed modification of lignin for enzymatic hydrolisis. Enzyme Microb. Tech. 49: 492-498. [ Links ]
Nagar, S., A. Mittal, D. Kumar, L. Kumar, and V. K. Gupta. 2010. Immobilization of xylanase on glutaraldehyde activated aluminum oxide pellets for increasing digestibility of poultry feed. Process Biochem. 12: 5-6. [ Links ]
Pang, R., M. Li, and C. Zhang. 2015. Degradation of phenolic compounds by laccase immobilized on carbon nanomaterials: Diffusional limitation investigation. Talanta 131: 38-45. [ Links ]
Pal, A., and F. Khanum. 2011. Covalent immobilization of xylanase on glutaraldehyde activated alginate beads using response surface methodology: Characterization of immobilized enzyme. Process Biochem. 46: 1316-1322. [ Links ]
Polak, J., and W. A. Jarosz. 2012. Fungal laccases as green catalysts for dye synthesis. Process Biochem. 19: 20-33. [ Links ]
Rodríguez, T., D. Botelho, and E. Cleto. 2008. Tratamiento de efluentes industriales de naturaliza recalcitrante usando ozono, peróxido de hidrógeno y radiación ultravioleta. Ver. Fac. Ing. Univ. Antioquia. 46: 24-38. [ Links ]
Rodrigues, P., C. Esteves, C. Egidío, and R. A. Rodrigues. 2012. Oxidation of lignin from eucalyptus globules pulping liquors to produce syringaldehide and vanillin. Ind. Eng. Chem. Res. 52: 4421-4428. [ Links ]
Ruuttunem, K. and T. Vuorinen. 2005. Developing catalytic oxygen delignification for Kraft pulp: Kinetic study of lignin oxidation with polyoxometalate anions. Ind. Eng. Chem. Res. 44: 4284-4291. [ Links ]
Sant, B. R. 1956. Thiocyabate interference in the permanganimetry of hydrogen peroxide. Anal. Chim. Acta. 15: 413-414. [ Links ]
She, D., F. Xu, Z. Geng, R. Sun, J. G. Lloyd, and M. S. Baird. 2010. Physicochemical characterization of extracted lignin from sweet sorghum stem. Ind. Crops Prod. 32: 21-28. [ Links ]
Solomon, E., U. Sundaram, and T. E. Machonkin. 1996. Multicopper oxidases and oxygenases. Chem. Rev. 7: 2563-2606. [ Links ]
Volokitina, M., V. K. Bobrov, K. Piens, E. Eneyskaya, T. B. Tennikova, E. G. Vlakh, and A. A. Kulminskaya. 2015. Xylan degradation improved by a combination of monolithic columns bearing immobilized recombinant β-xylosidase from Aspergillus awamori X-100 and Grindamyl H121 β-xylanase. Biotechnol. J. 10: 210-221. [ Links ]
Zheng, A., Z. Zhao, S. Chang, Z. Huang, H. Wu, X. Wang, F. He, and H. Li. 2014. Effect of crystal size of ZSM-5 on the aromatic yield and selectivity from catalytic fast pyrolysis of biomass. J. Mol. Catal. A: Chem. 383-384: 23- 30. [ Links ]
Zhang, J., J. Teo, X. Chen, H. Asakura, T. Tanaka, K. Taramura, and N. Yan. 2014. A series of NiM (M = Ru, Rh, and Pd) bimetallic catalysts for effective lignin Hydrogenolysis in water. ACS Catal. 4: 1574−1583. [ Links ]
Received: October 01, 2016; Accepted: September 01, 2017











 text in
text in