Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.50 n.2 Texcoco Feb./Mar. 2016
Crop science
Rooting of juvenile cuttings of Bertholletia excelsa under different concentrations of indolebutyric acid
1 Universidade Federal Rural da Amazônia-UFRA, Av. Tancredo Neves No 250, Terra Firme, CEP: 66.077-530, Belém, Pará, Brasil. (iracema3c@gmail.com) (francisco.oliveira@ufra.edu. br).
2 Embrapa Amazônia Oriental, Travessa Enéas Pinheiro, N° 100, Montese, CEP: 66095100, Belém, Pará, Brasil. (osmar.lameira@embrapa.br).
3 Embrapa Florestas, Estrada da Ribeira, km 11, bairro Guaraituba, CEP: 83.411-000, Colombo, Paraná, Brasil. (ivar.wendling@ embrapa.br).
Recalcitrant behavior and slow and irregular germination are limiting factors for the production of Bertholletia excelsa seedling production, through the conventional process. The objective of this study was to evaluate the rooting of juvenile cuttings of B. excelsa associated to the application of indolebutyric acid (IBA) in subirrigation propagator. The experimental design was completely random with a factorial arrangement of 3x3x2 of treatments: three doses (0, 1000 and 3000 mg L-1) of IBA as growth regulator; three types of cuttings (apical, middle, basal); and two immersion times (1 s and 60 s). The data were analyzed with ANOVA and the treatment means were compared with the SNK test (p≤0.05). At 180 d the number and mean length of roots, percentage of rooting, presence of callus, and survival of the cuttings were evaluated. The results show that the middle and basal cuttings immersed for 1 s in 1000 mg L-1 of IBA had the highest percentages of rooting, 58.3 % and 41.7 %, respectively. The cuttings only presented, in average, the formation of a single root. The highest average root lengths were obtained with middle cuttings submerged for 1 s in 3000 mg L-1 of IBA (7.0 cm) and 1000 mg L-1 of IBA (6.4 cm). Thus, the IBA concentrations stimulate the surge of roots in different types of B. excelsa cuttings, the middle segment immersed in 1000 mg L-1 for 1 s is the most appropriate to increase the percentage of rooting, and the process of cuttings with B. excelsa juvenile material can be adopted for the vegetative propagation of the species.
Keywords: Vegetative propagation; Amazon species; Bertholletia excelsa; growth regulator; indolebutyric acid; juvenile cuttings
El comportamiento recalcitrante y la germinación lenta e irregular son factores limitantes en la producción de plántulas de Bertholletia excelsa mediante el proceso convencional. El objetivo de este estudio fue evaluar el enraizamiento de estacas juveniles de B. excelsa asociadas a la aplicación de ácido indolbutírico (AIB) en propagador de subirrigación. El diseño experimental fue completamente al azar con un arreglo factorial 3x3x2 de tratamientos: tres dosis (0, 1000 y 3000 mg L-1) de AIB como regulador de crecimiento; tres tipos de estacas (apical, media y basal); y dos tiempos de inmersión (1 s y 60 s). Los datos se analizaron con un ANDEVA y las medias de los tratamientos se compararon con la prueba de SNK (p≤0.05). A los 180 d se evaluó el número y la longitud media de las raíces, el porcentaje de enraizamiento, la presencia de callo y la supervivencia de las estacas. Los resultados muestran que las estacas medias y basales sumergidas 1 s a 1000 mg L-1 de AIB tuvieron los porcentajes mayores de enraizamiento, 58.3 % y 41.7 %, respectivamente. Las estacas sólo presentaron, en promedio, la formación de una raíz única. Las longitudes medias mayores de raíces se obtuvieron en las estacas medias sumergidas 1 s en 3000 mg L-1 de AIB (7.0 cm) y 1000 mg L-1 de AIB (6.4 cm). Así, las concentraciones de AIB estimulan el surgimiento de raíces en diferentes tipos de estacas de B. excelsa, el segmento medio inmerso en 1000 mg L-1 por 1 s es el más indicado para aumentar el porcentaje de enraizamiento, y el proceso de estacas con material juvenil de B. excelsa puede adoptarse para la propagación vegetativa de la especie.
Palabras clave: Propagación vegetativa; especies amazónicas; Bertholletia excelsa; regulador de crecimiento; ácido indolbutírico; estaca juvenil
Introduction
Brazil nut (Bertholletia excelsa H.B.K.) is an abundant species in the Amazon region and its fruits are harvested almost exclusively in natural forests, where several communities are benefited with its production (Tonini et al., 2008). This species is key for the conservation and development of the Amazon region, due to its multiple uses by the communities that exploit it, is a source of employment and income for rural and urban workers in this region, and because of the use of its wood (Tonini, 2011). In northern Brazil, B. excelsa is a native species with great potential for reforestation (Tonini and Arco-Verde, 2005), and has a combination of favorable aspects such as wood of excellent quality, quick growth, abundant fructification; also, fruit collection causes a low environmental impact (Tonini et al., 2008).
Seed propagation in B. excelsa is limited by a quick loss of its viability, caused by its recalcitrant behavior, slow and irregular germination of up to six months, and difficulties to root. In addition, its fruits are susceptible to attacks by predators before their maturation (Figueiredo and Carvalho, 2002). To obtain a quick germination, it is necessary to remove the tegument, and in most cases mechanical damage is produced on the seed. Given these limitations, vegetative propagation through cuttings and the application of growth regulators, especially the synthetic auxin indolebutyric acid (IBA), is a promising technique for most species. IBA is a photostable substance, non-toxic, of localized action, and less sensitive to biological degradation (Husen, 2012). Its use is recommended to stimulate rooting in a large number of plants (Bortoline, 2008), and low and high concentrations are used, as are types and sizes of cuttings (Vernier and Cardoso, 2013). According to Xavier et al. (2009), the degree of success of vegetative propagation also depends on the season, physiological conditions of the plant, concentration of growth regulators, meteorological conditions, location, size and type of propagule, means for rooting, fungicides, and the influence of the substrate on rooting.
For some trees like Eucalyptus, the technique for propagation with cuttings is well-established, with dominion in the production of seedlings of high quality and low cost. In Schizolobium amazonicum (Rosa and Pinheiro, 2001), Calophyllum brasiliensis (Silva et al., 2010) and Ilex paraguariensis (Brondani et al., 2008), there are propagation protocols, but not for the majority of forest species. Studies by Pinheiro (1967) and Moraes et al. (2008) are the only ones with B. excelsa cuttings, and the results were not efficient for the development of protocols, which is why information is insufficient for the application of this technique.
Therefore, the objective of this study was to evaluate the rooting of juvenile cuttings of B. excelsa associated to the application of IBA in subirrigation propagators.
Materials and Methods
The study was performed in the forest nursery of Embrapa Amazonia Oriental, in the city of Belém, capital of the state of Pará, Brazil, using seeds bought from producers. For the plant production, the seeds of B. excelsa were submerged in water for 24 h and sown in plastic bags of 17 cm x 28 cm, which contained a mixture of substrate of 60 % black soil, 30 % chicken dung, and 10 % sand, and they were placed in a nursery under intermittent artificial irrigation.
When the plants reached 45 cm of height, they were selected in cuttings 10 cm long, cut obliquely at the base, containing at least two buds and two pairs of leaves reduced to half of their original size, with the goal of avoiding excessive transpiration. The cuts were separated into three groups: apical, middle and basal position. With the aim of maintaining the vigor and turgidity of the plant material, immediately after the collection, the cuttings were washed with water and placed in a thermal box with water.
For the rooting of the cuttings, they were placed for six months in a subirrigation type chamber propagator, with a metallic structure wrapped in polyethylene plastic that made it waterproof. The base was covered with four beds: 1) sand (6-10 cm); 2) gravel (10-15 cm); 3) rocks (5 cm); and 4) substrate (5 cm), made up of sterile sand, according to the Leakey et al. (1990) model, and adapted by Longman (1993). The propagator was inside a greenhouse with internal luminosity reduced to 50 % of the natural light by a middle shadow located on the external superior part of the structure, with relative moisture of 90 % and average temperature of 27 °C. During the period of permanence of the cuttings in the propagator, phytosanitary control was performed through a spray of Benomyl (1 g L-1) every 15 d.
The experimental design was completely random with a factorial arrangement (3x3x2) of treatments: IBA (0, 1000 and 3000 mg L-1), types of cuttings (apical, middle and basal) and immersion times (1 s and 1 min). The IBA was dissolved in sodium hydroxide (NaOH) 1 N, was diluted in distilled water (50:50 % in v/v), and the base of the cuttings was immersed in the solution. Each treatment had three experimental units of 12 cuttings.
The measurements were performed in intervals of 30 d during 180 d, and the variables were a percentage of the cuttings rooted, the number and length of the roots, the presence of calluses and the percentage of mortality. The mean values of the percentage of rooting were transformed with the square root function of the arcsine for each observation and the average number of roots transformed into
The cuttings with roots were transferred to plastic trays with 24 cells, which contained black soil and vermiculite in 1:1 (v/v) proportion and placed in a greenhouse with intermittent irrigation for the growth of the aerial part. The seedlings formed were transferred to black polyethylene plastic bags of 14 cm x 20 cm with black soil and cow manure in the proportion of 1:1 (v/v), for the evaluation of the adaptation in the nursery and future planting in the field.
Statistical analysis
With the data, ANOVA was performed, regression, and the measurements were compared with the SNKα test (p≤0.05), using SISVAR 5.3 (Ferreira, 2010), because it uses the distribution of the Tukey test. The Keuls method is relatively easy to apply and considers the total number of treatments used in the experiment, and is a multiple amplitude test that evaluates the significance of a set of differences, making a balance between Errors of Type I and II.
Results and Discussion
Rooted cuttings, presence of calluses and mortality
The percentages of rooting (from 8 % to 58 %) were relatively low; however, the types of cuttings (Pr>F 0.002), IBA concentrations (Pr>F 0.002), immersion times (Pr>F 0.026) and the interactions between these factors (Pr>F 0.000) showed significant differences (p<0.05) (Tables 1 and 2). The positive interaction with the same factors was verified by Dias et al. (2012a) with vegetative propagation for mini cuttings of Anadenanther amacrocarpa and different IBA concentrations.
Table 1 Effect of the concentration and immersion time in indolebutyric acid (IBA) and the position of the cutting with the percentage of cuttings rooted (EE), callus formation (C), mortality (M) and the mean values of the number of roots (NR) and root length (LR) in Bertholletia excelsa.
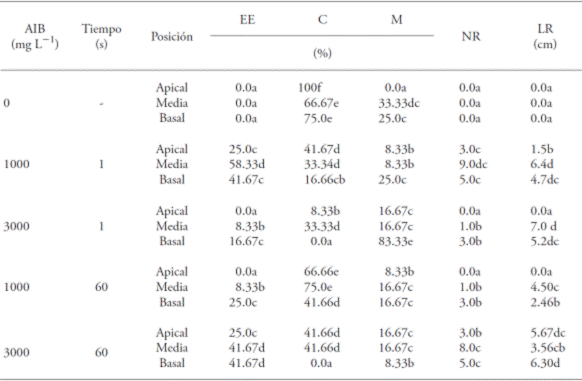
Values with different letters in a column are statistically different (p≤0.05).
Table 2 Effect of the concentration and immersion time in indolebutyric acid (IBA) and the position of the cutting, on the mean values of the root number (NR) and root length (LR) in Bertholletia excelsa juvenile cuttings.
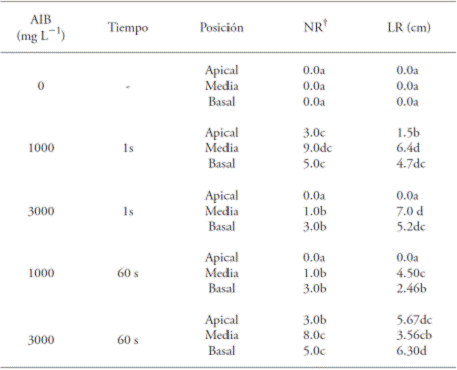
The mean values with a different letter in a column are different (SNK test; p≤0.05); † Data transformed to
Wendling and Xavier (2005) obtained 80-100 % of rooting when using concentrations of 500 to 1000 mg L-1 of IBA in cuttings of Eucalyptus clones. According to Santos et al. (2011), in addition to the effect of the growth regulator, the specific characteristics of each species also affect the rooting of cuttings, as was observed in their study with 20 forest species. These results highlight that the rooting of cuttings presented differentiated behavior and that for the start of rooting, intrinsic factors related to the plant itself influenced as well as extrinsic ones linked to environmental conditions (Hartmann et al., 2002; Pio et al., 2003).
The low percentage of root formation is explained because the cuttings responded differently to the action of the hormone; some need to remain in contact with the growth regulating solution for longer and others root sporadically or they do not root. This difference is due to the physiological variations between cuttings from the same plant, although they may be juvenile; in addition, the process can be inhibited based on the concentration of the growth regulator. These results suggest that juvenile plant material is not always favorable for the rooting process of woody species from seeds, as found with B. excelsa cuttings. Fachinello et al. (2005) point out that despite the capacity for rooting of woody species, the potential of a cutting to form roots varies with the species and type of crop due to the interaction of factors such as manipulation of the plant, good nutritional status, period of recollection and age of the plant, length and diameter of cuttings, presence of leaves and buds, hormonal treatment, illumination, temperature and relative moisture.
The use of IBA increased the number of roots per cutting, and promotes a greater percentage of rooting according to Endres et al. (2007). The same was observed by Gratieri-Sossella et al. (2008), in studies with Brazilwood (Caesalpinia echinata) and ceibo (Erythrina crista-galli), respectively.
In absence of IBA the formation of calluses varied from 66 to 100 %, especially in the apical position (Table 1; Figure 1). The presence of calluses in the sections of cuttings occurred independently from the presence of the growth regulator or position of the cut. Hernández et al. (2013), with regard to the vegetative propagation with cuttings of Cariniana estrellensis, report that the presence of callus is produced with or without the presence of IBA, both in apical and in intermediate cuttings.
Fachinello et al. (2005) point out that the callus is formed from a mass of parenchyma and disorganized cells, as a result of the lesions of the tissues of the xylem and phloem during the preparation of the cutting. The natural auxin produced in the leaves and buds moves towards the inferior part of the cuttings stimulating the rooting activity and accumulating in the base of the cutting, together with sugars and other nutrients (Janick, 1996), acting as inhibiting substances in the initiation of the preformed adventitious roots and potentiating the formation of calluses at the base of the cuttings (Hartmann and Kester, 2011).
Iritani et al. (1986) and Caldwell et al. (1988) affirm that the roots can appear directly from caulinar tissue or solely through the callus. The formation of calluses must be due to the presence of equilibrium of the auxin applied (IBA) in the presence of an endogenous cytokinin and, according to Hartmann and Kester (2011), this equilibrium can also occur when cytokinin is applied with the endogenous auxin. Thus, the presence of calluses in B. excelsa cuttings would indicate that if the cuttings remain for a longer period in the rooting environment, there would be an increase in the percentage of rooted cuttings. However, the roots that arise from differentiation of callus cells rarely have vascular connections with the cuttings, so propagation from these is not recommended (George et al., 2008).
With regard to mortality, the cuttings in the basal position, immersed 1 s with 3000 mg L-1 of IBA presented higher percentage (83.3 %) in comparison to other types of position and immersion time, suggesting that there was phytotoxicity at that concentration. Among treatments, only the apical cuttings in absence of IBA did not present mortality (Table 1). Bastos et al. (2004) studied the rooting of cuttings from starfruit (Averrhoa carambola) and suggest that there was phytotoxicity under different IBA concentrations and immersed 10 s, and that the survival capacity of the apical cuttings in absence of the growth regulator was higher than those exposed to IBA.
Number and length of roots
The cuttings presented, in average, the formation of only one root (Figure 2). The treatment that caused a higher number of roots was the one with concentration of 3000 mg L-1 of IBA (Table 1).
The middle cuttings submerged 1 s at 1000 mg L-1 of IBA showed a higher percentage of rooting (58.3 %), followed by the basal cuttings at the same time of immersion and the same concentration (41.7 %) (Table 1). However, the latter did not differ from the middle and basal cuttings, immersed 1 min in 3000 mg L-1 of IBA. In absence of IBA, for the three types of cuttings used, there was no root formation, as well as in the apical cuts submerged for 1 s in a concentration of 3000 mg L-1 of IBA and immersed for 1 min at 1000 mg L-1 of IBA (Table 1).
In the regression equation, the total number of roots obtained through direct morphogenesis tended towards a linear increase in the number of roots produced by cuttings, as the concentration of the growth regulator increases (Figure 3).

Figure 3 Effect of eight concentrations of IBA (mg L-1) on the number of roots produced in Bertholletia excelsa cuttings. r=0.864.
Within each IBA concentration, the cuttings in the apical position had the lower percentage of rooting (Table 1). In this sense, Dias et al. (2012b) point out that the lower rates of rooting occur in apical cuttings, since they have the meristem, which is one of the principal places of natural auxin synthesis and, therefore, the exogenous application of synthetic growth regulators on the base is not needed; if it takes place, it causes a hormonal imbalance and inhibits the appearance of roots (Hartmann et al., 2002; Lopes et al., 2011). The greater mean root lengths were obtained in treatments with cuttings in the middle position, submerged for 1 s at 3000 mg L-1 of IBA (7.0 cm) and in 1000 mg L-1 of IBA (6.4 cm) without significant differences between them. The smallest mean root length (1.5 cm) was observed with the cuttings in the apical position, immersed for 1 s in the 1000 mg L-1 concentration of IBA (Table 2).
The linear regression showed a growing trend with an increase in the concentration of IBA, indicating that it would eventually have better results in higher concentrations. However, per cutting there was broad variation in the root length. This fact could be due to genetic factors, since each cutting presented unique characteristics and, therefore, the requirements for propagation also tended to be unique (Figure 4).
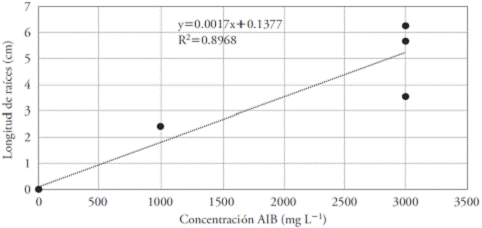
Figure 4 Effect of eight concentrations of IBA (mg L-1) on the root length in Bertholletia excelsa cuttings. R=0.947.
These results are similar to those found by Xavier et al. (2003), who found significant differences in the rooting of Cedrela fissilis cuttings. Hernández et al. (2012) in a study with Piptadenia gonoacantha obtained higher average values than in the middle and basal cuttings with the application of IBA (6000 mg L-1), so the three types of cuttings responded to the application of IBA.
According to the observations performed, after six months, the surviving cuttings were green and strengthened, suggesting a later initiation and elongation of adventitious roots. However, the success of this technique depends on the type of plant material, the species and its nutritional conditions, the type of growth regulator, the concentrations used and the immersion time. Due to the low percentage of rooting and time for rooting, it is necessary to make adjustments for the technique to be viable and to become an alternative for the production of seedlings throughout the year, especially in situations where the seed is a limiting input.
Conclusions
The use of IBA stimulated the growth of roots in different types of cuttings of Bertholletia excelsa; the concentration of 1000 mg L-1 stood out as the most appropriate to increase the percentage of rooting, submerging the base of the cutting in the IBA solution for one second and using the middle segment to obtain the cuttings.
The technique of cuttings may be adopted for the mass vegetative propagation of the species. However, the low rooting suggests that this process depends on multiple factors which must be considered to stimulate and optimize the later production of seedlings.
Literatura Citada
Bastos, D. C., A. B. G. Martins, E. J. Scaloppi Junior, I. Sarzi, e J. C. Fantinansi. 2004. Influencia do ácido indolbutírico no enraizamento de estacas apicias e basais de caramboleira (Averrhoa carambola L.) sob condições de nebulização intermitente. Rev. Bras. Frut. 26: 284-286. [ Links ]
Bortolini, M. F., D. M. de Lima, G. B. de Alcantara, F. P. Fanti, L.A Biasi, M. Quoirin, H. S. Koehler, K .C. Zuffella Toribas. 2008. Enraizamento de estacas de Ficus benjamina L. Scientia Agraria 4: 539-543. [ Links ]
Brondani, G., M. A. Araujo, I. Wendling, e D. Kratz. 2008. Enraizamento de miniestacas de erva-mate sob diferentes ambientes. Pes. Flor. Bra. 1: 29-38. [ Links ]
Caldwell, J. D., D. C. Coston, e K. H. Brock. 1988. Rooting of semihard wood “Hayward” kiwifruit cuttings. Hortscience 23: 714-717. [ Links ]
Dias, P. C., L. S. Oliveira, A. Xavier, e I. Wendling. 2012a. Estaquia e miniestaquia de espécies florestais lenhosas do Brasil. Pes. Flor. Bra. 32: 453-462. [ Links ]
Dias, P. C., A. Xavier, L. S. Oliveira, H. N. de Paiva, e A. C. G. de Correia. 2012b. Propagação vegetativa de progênies de meios-irmãos de angico-vermelho (Anadenanthera amacrocarpa (Benth) Brenan) por miniestaquia. Rev. Árvore 36: 389-399. [ Links ]
Endres, L., P. M. G. Marroquim, C. M. Santos, e N. N. F. Souza. 2007. Enraizamento de estacas de Pau-Brasil (Caesalpinia echinata Lam.) tratadas com ácido indolbutírico e ácido naftaleno acético. Ciên. Rural 37: 886-889. [ Links ]
Fachinello, J. C., A. Hoffmann, e J. C. Nachtigal. 2005. Propagação de Plantas Frutíferas. Embrapa Informações Tecnológicas. Brasília. 221 p. [ Links ]
Ferreira, D. F. 2010. SISVAR 5.3-Sistema de Análises Estatísticas. Lavras: UFLA, (Software estatístico). [ Links ]
Figueiredo, F. J. C. e C. J. R. de Carvalho. 2002. Aspectos fisiológicos de sementes de castanha-do-Brasil submetidas a condições de estresse: emergência e respiração. Belém: Embrapa Amazonia Oriental, Boletim de Pesquisa e Desenvolvimiento 05. 19 p. [ Links ]
George, E. F.; Hall, M. A.; De Klerk, G. J. 2008. Plant Propagation by Tissue Culture: volume 1. The Background. 3rd ed. Dordrecht: Springer. 479 p. [ Links ]
Gratieri-Sossella, A., C. Petry, e A. A. Nienow. 2008. Propagação da corticeira do banhado (Erythrina crista-galli L.) (Fabaceae) pelo processo de estaquia. Rev. Árvore 32: 163-171. [ Links ]
Hartmann, H. T., D. E. Kester, F. T. Davies Junior, e R. L. Geneve. 2002. Plant Propagation: Principles and Practices. 7th ed. Upper Saddle River, Prentice-Hall. 8809 p. [ Links ]
Hartmann, H. T. and D. E. Kester. 2011. Plant Propagation: Principles and Practices. 8th ed. Boston: Prentice-Hall. 915 p. [ Links ]
Hernández, W., A. Xavier, H. N. de Paiva, e I. Wendling. 2013. Propagação vegetativa do jequitibá-rosa (Cariniana estrellensis (Raddi) Kuntze) por estaquia. Rev. Árvore 37: 955-967. [ Links ]
Hernández, W., A. Xavier, H. N. de Paiva, eI. Wendling, 2012. Propagação vegetativa do pau-jacaré (Piptadenia gonoacantha (Mart.) Macbr.) por estaquia. Rev. Árvore 36: 813-823. [ Links ]
Husen, A. 2012. Changes of soluble sugars and enzymatic activities during adventitious rooting in cuttings of Grewia optiva as affected by age of donor plants and auxin treatments. Am. J. Plant. Physiol. 7: 1-16. [ Links ]
Iritani, C., R. V. Soares, e A. V. Gomes. 1986. Aspectos morfológicos da aplicação de reguladores de crescimento nas estacas de Ilex paraguariensis St. Hilaire. Acta Biol. Paranaense 15: 21-46. [ Links ]
Janick, J. 1996. Orientação do crescimento da planta. In: Janick, J (ed.) A Ciência da Horticultura. 2 ed. Rio de Janeiro: USAID. pp: 202-237. [ Links ]
Leakey, R. R. B., J. F. T. Mesen, Z. Tchoundjeu, K. A. Longman, J. McP. Dick, A. Newton, A. Matin, J. Grace, R.C. I. Munro, P. N. Muthoka. 1990. Low-technology techniques for the vegetative propagation of tropical trees. Commonwealth For. Rev. 3: 247-257. [ Links ]
Longman, A. K. 1993. Rooting cuttings of tropical trees. In: FAO (ed). Tropical Trees: Propagation and Planting Manuals. London: Commonwealth Science Council 1. 64 p. [ Links ]
Lopes, V. R., C. de S. Mudry, M. M. Bettoni, e K. C. Zuffellato-Ribas. 2011. Enraizamento de estacas caulinares de Ficus benjamina L. sob diferentes concentrações de ácido indolbutírico. Sci. Agraria, Curitiba 12: 179-183. [ Links ]
Moraes, R. P., L. C. Garcia, e R. M. B. de Lima. 2008. Propagação vegetativa de Bertholletia excelsa H.B.K. por estaquia. In: IV Jornada de Iniciação Científica da Embrapa Amazônia Ocidental. Manaus.. Anais. Manaus: Embrapa Amazônia Ocidental. Documentos, 58. pp: 122-131. [ Links ]
Pio, R., J. D. Ramos, N. N. J. Chalfun, J. H. C. Coelho, T. C. A. Gontijo, e E. Carrijo. 2003. Enraizamento de estacas apicais de figueira tratadas com sacarose e ácido indolbutírico por imersão rápida. Rev. Bras. Agro. 9: 35-38. [ Links ]
Pimentel-Gomes, F. Curso de Estatística Experimental. 2009. 15 ed. Piracicaba: Fealq-Usp. 451 p. [ Links ]
Pinheiro, E. 1967. Propagação vegetativa da castanheira (Bertholletia excelsa H.B.K.): observações preliminares. Belém, PA: IPEAN. il. Publicação não convencional. Mimeografado. Biblioteca(s): Embrapa Amazônia Oriental. 13 p. [ Links ]
Rosa, L. S., e K. A. O. Pinheiro. 2001. Propagação vegetativa de estacas de paricá (Schizolobium amazonicum Huber ex. Ducke) obtidas de material juveniel e imersas em ácido indol-3-butírico. Rev. Ciên. Agra. 35: 79-88. [ Links ]
Santos, J. de P. dos., A. C. Davide, L. A. F. Teixeira, A. J. S. Melo, e L. A. de Melo. 2011. Enraizamento de estacas lenhosas de espécies florestais. Cerne 17: 293-301. [ Links ]
Silva, R. L., M. L. Oliveira, M. A. Monte, e A. Xavier. 2010. Propagação clonal de guanandi (Calophyllum brasiliense) por miniestaquia. Agro. Costarrincense 1: 99-104. [ Links ]
Tonini, H. 2011. Fenologia da castanheira-do-brasil (Bertholletia excelsa Humb. y Bonpl., Lecythidacea) no sul do estado de Roraima. Cerne 17: 123-131. [ Links ]
Tonini, H., e M. F. Arco-Verde. 2005. Morfologia da copa para avaliar o espaço vital de quatro espécies nativas da Amazônia. Pesp. Agro. Bras. 40: 633-638. [ Links ]
Tonini, H., P. Costa, e P. E. Kaminsky. 2008. Manejo de produtos florestais não madeireiros na Amazônia (Castanheira-doBrasil). Bol. de Pesq. Desenv. 2. 31p. [ Links ]
Vernier, R. M., S. B. Cardoso. 2013. Influência do ácido indolbutírico no enraizamento de estacas em espécies frutíferas e ornamentais Rev. Elet. Edu. Ciências. Garça (3):2 11-16. [ Links ]
Xavier, A., G. A. dos Santos, e M. L. de Oliveira. 2003. Enraizamento de estaca caulinar e foliar na propagação vegetativa de cedro-rosa (Cedrela fissilis Vell.). Rev. Árvore 27: 351-356. [ Links ]
Xavier, A., G. A. dos Santos, I. Wendling, eM. L. de Oliveira. 2009. Propagação vegetativa de cedro-rosa por miniestaquia. Rev. Árvore 27: 139-143. [ Links ]
Wendling, I., e A. Xavier. 2005. Influência do ácido indolbutírico e da miniestaquia seriada no enraizamento e vigor de miniestacas de clones de Eucalyptus grandis. Rev. Árvore 29: 921-930. [ Links ]
Received: February 2015; Accepted: November 2015











 text in
text in 




