Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Hidrobiológica
Print version ISSN 0188-8897
Hidrobiológica vol.11 n.2 Ciudad de México Dec. 2001
Artículos
Demography of genets of clonal red seaweeds: current limitations and proposed solutions using genetic markers from experimental populations
Ricardo Scrosati
University of British Columbia, Department of Botany, Vancouver, British Columbia V6T 1Z4, Canada. Email: scrosati@axion.net
Recibido: 18 de enero de 2001
Aceptado: 24 de julio de 2001
Abstract
For clonal red seaweeds, the genet is defined as the thallus that develops from a single spore. The demography of genets for these organisms is poorly known. This results from the inability of visually detecting genets when ramets (fronds) are abundant, when separate thalli actually represent fragments of one original genet and when holdfast coalescence occurs between neighboring genets. The use of genetic markers, such as allozymes and certain DNA regions, has allowed for the identification of genets in natural populations of clonal terrestrial plants. However, this may not work for natural populations of clonal red seaweeds, because several genets may be produced by asexual spores from the same individual, rendering those genets unidentifiable with certain genetic markers. A useful approach could be to study experimental populations under controlled laboratory conditions. The position and shape of genets could be monitored as they develop from genetically distinct spores, selected as such on purpose. Once the visual identification of genets becomes impossible due to the increasing density of ramets, mapping the different ramet genotypes should allow for the continuous identification of genets. Some genetic variation could arise within genets through somatic mutation and genetic transposition. Rates of these processes are largely unknown for clonal seaweeds, so they should be first estimated to determine their potential effects on demographic studies based on genetic markers.
Key words: clonal; demography; Gelidiales; genet; genetic markers; Gigartinales; Gracilariales; ramet; Rhodophyta.
Resumen
Para algas rojas clonales, defino el genet como el talo que se desarrolla a partir de una espora. La demografía de genets de estos organismos está muy poco estudiada. Esto se debe a nuestra incapacidad para diferenciar genets visualmente la densidad de ramets (frondas) es alta, cuando vemos talos distintos que, en realidad, son fragmentos de un mismo genet original y cuando algunos genets vecinos se fusionan. El mapeo del genotipo de ramets, usando marcadores genéticos como aloenzimas y ADN, ha permitido identificar a los genets de plantas clonales terrestres en poblaciones naturales. Sin embargo, esto no serviría para poblaciones naturales de algas rojas clonales, pues varios genets pueden derivar de un mismo individuo original mediante esporas asexuales, lo que determina que esos genets hijos sean genéticamente iguales para ciertos marcadores. La demografía de genets de algas rojas clonales podría estudiarse mejor en poblaciones experimentales de laboratorio. Por ejemplo, se podrían seleccionar esporas genéticamente distintas y monitorear la posición de los genets resultantes mientras crecen. Cuando su identificación visual se tornase imposible, al aumentar la densidad de ramets, el mapeo del genotipo de los ramets debería permitir identificar la posición de los genets y, por lo tanto, estudiar su demografía. Algún grado de variación genética podría ocurrir en algunos genets por mutación somática o por transposición genética. La frecuencia de estos fenómenos es generalmente desconocida para algas rojas, por lo que debería primeramente estimarse para determinar su posible efecto en estudios demográficos basados en marcadores genéticos.
Palabras clave: clonal; demografía; Gelidiales; genet; Gigartinales; Gracilariales; marcadores genéticos; ramet; Rhodophyta.
Introduction
Clonal plants are those that vegetatively produce similar functional units (e.g., shoots) that are potentially able to live on their own if physically separated from the parent plant. Such vegetative units are ramets, whereas the entire plant is termed genet (Harper and White, 1974). The demography of genets of clonal plants is less understood than those of nonclonal plants. Genets of clonal plants are difficult, and frequently impossible, to identify in the field, mainly because of their frequent fragmentation and of the difficulty in determining de association of ramets with their parent genet when they are produced by rhizomes or by intermingled stolons. These limitations seem to explain why research on plant demography has been principally done with nonclonal plants (Eriksson, 1993). Additionaly, these problems apparently resulted in demographic studies on clonal plants being mostly done at the ramet level (de Kroon, 1993; Hara, 1994; Peterson and Jones, 1997; Suzuki and Hutchings, 1997).
Although limiting a demographic study to the ramet level may seem enough to understand the population dynamics of clonal plants, there is essential information that results only from genet demography. The ecological and evolutionary dynamics of clonal plants depend on the interactions between their hierarchical levels of organization (Eriksson and Jerling, 1990; Vuorisalo et al., 1997). For example, demographic rates of ramets may depend on the position of ramets within a genet, on their distance to a neighboring genet, on the dynamics of a disease within a genet, or on genet age or size. Genet identification is also important in making inferences about the evolution of foraging and reproductive strategies of clonal plants, especially when selection pressures act mainly on genets.
Seaweeds are important components of several coastal marine ecosystems. Many species are nonclonal, such as some kelps and fucoids (Phaeophyceae -brown algae -), and their individuals are usually easy to identify. Their basic density-dependent patterns are similar to those for nonclonal terrestrial plants (Black, 1974; Chapman and Goudey, 1983; Dean et al., 1989; Reed, 1990; Ang and DeWreede, 1992; Creed, 1995; Flores-Moya et al., 1997; Creed et al., 1998; Arenas and Fernández, 2000), with some special circumstances, such as the recruitment of intertidal fucoids (Ang and DeWreede, 1992). There are also several clonal seaweeds, which may be found among the Rhodophyta (red algae), the Chlorophyta (green algae), and the Phaeophyceae. This paper will focus on clonal red seaweeds, many of which are dominant in their communities and have economic value as well.
The genet of clonal red seaweeds is interpreted here as the entire thallus that develops from a single spore, whether a carpospore or a tetraspore. The word "genet" was originally used for clonal terrestrial plants to refer to the genetic individual that develops from a seed and that produces a number of ramets (Harper and White, 1974; Kays and Harper, 1974). Recent definitions of the genet refer specifically to the zygote as its initial cell (de Kroon and van Groenendael, 1997). The term "genetic individual" has been coined to mean that all of the parts of the genet are genetically identical. However, somatic mutations can occur during the growth of the genet (Klekowski, 1997), so such interpretation should be abandoned. On the other hand, considering the zygote as the only initial cell of the genet is perhaps too limiting. The main purpose of having a definition of genet is to make the distinction between individuals that develop from a single reproductive cell and individuals that result from the vegetative fragmentation (clonal fragments, sensu Eriksson and Jerling, 1990) of a single original individual. Thus, the genet may be better defined as the free-living individual that develops from one zygote, parthenogenetic gamete, or spore and that produces ramets during growth. In this way, the definition of genet can be applied appropriately to the equivalent structure found in clonal seaweeds, bryophytes, and vascular plants. For more details on this discussion, see Scrosati (2002).
In many species of clonal red seaweeds, the genet is composed of a crustose (e.g., Gigartinales) or stoloniferous (e.g., Gelidiales) holdfast and of several fronds that vary greatly in form across species. Fronds can be considered as ramets, because of their capacity for independent life when separated from the parent thallus, provided that they remain attached to the substrate. Such separation could result from rock dislodgment, partial bleaching and subsequent death of certain regions of a genet, or partial herbivory on a genet. Despite the ecological and economic importance of these seaweeds, their genets have rarely been the subject of demographic studies, exceptions including those on the genus Mazzaella, in the Gigartinales (May, 1986; Dyck and DeWree-de, 1995; Scrosati, 1998). As is the case for clonal terrestrial plants, for these seaweeds more is known about ramet demography, although for only a few species. There are important similarities with many clonal terrestrial plants, such as the general lack of self-thinning among growing ramets of seasonal herbs (Suzuki and Hutchings, 1997), which was observed for the seaweeds Gelidium sesquipedale (Clemente) Bornet et Thuret (Gelidiales; Santos, 1995), Mazzaella cornucopiae (Postels et Ruprecht) Hommersand (Scrosati and DeWreede, 1997), Chondrus crispus Stackhouse (Gigartinales), and Pterocladiella capillacea (Gmelin) Santelices et Hommersand (Gelidiales; Scrosati and Servière-Zaragoza, 2000).
Current limitations
Why has genet demography been so poorly investigated for clonal red seaweeds? There seem to be three reasons. The first one is the difficulty of identifying neighboring genets when fronds are dense, up to 21 cm-2 for Mazzaella cornucopiae (Scrosati and Servière-Zaragoza 2000) and up 20 22 cm-2 for Pterocladiella capillacea (Scrosati, 2000) for example. High frond densities generally prevent the accurate detection of boundaries of crustose holdfasts and especially of stoloniferous ones, which often intermingle. The second reason is that a genet may break up into clonal fragments (sensu Eriksson and Jerling, 1990), each one composed of two or more ramets. Even if such clonal fragments are distant one cannot tell whether they represent one or more genets without knowing their history (Fig. 1). The third reason is holdfast coalescence: for some members of the Gigartinales and the Gracilariales, holdfast of young, neighboring genets may coalesce during growth (Tveter and Mathieson, 1976; Tveter-Gallagher and Mathieson, 1980; Maggs and Cheney, 1980; Santelices et al., 1996, 1999). At the adult stage, it is generally impossible to recognize the chimeric nature of a thallus after coalescence or in other words, that such a thallus is composed of different genotypes.
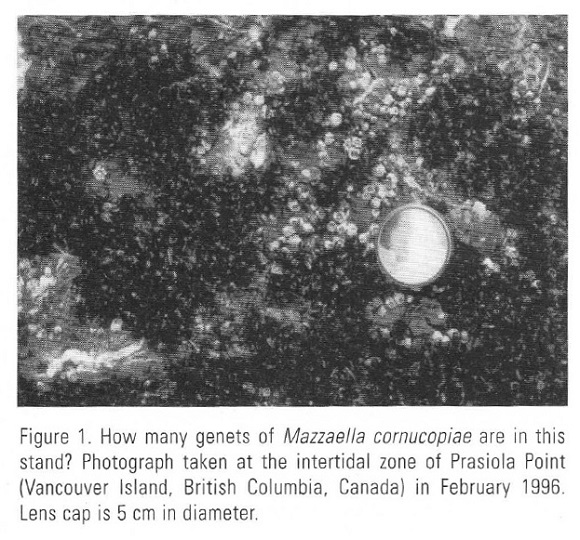
The extent of holdfast coalescence may be significant in natural populations (Santelices et al., 1999). The demography of genets of coalescent clonal seaweeds may differ from that of nonclonal seaweeds and even clonal terrestrial plants, which normally do not coalesce. Would coalescing genets of clonal seaweeds grow according to density-dependent patterns, as nonclonal plants generally do? Would a physical encounter between growing genets always result in coalescence? Or would it result in competition, depending on the life-history phase or genet size, age, or genotype? To answer these questions, it is necessary to develop techniques to identify genets through time.
Incorporating genetic markers
The basic technique, which has been used for the genus Mazzaella (May, 1986; Dyck and DeWreede, 1995; Scrosati, 1998), is the periodic mapping of genets while they are visually identifiable. For dense stands, additional techniques are needes. One approach might be to determine the genetic identity of ramets from a given area and thus, to gain the ability to identify genets and clonal fragments. From mosses to flowering plants, allozyme electrophoresis, the RAPD (random amplified polymorphic DNA) technique, and DNA finger-printing have been applied to samples of ramets to distinguish different neighboring genets (Ellstrand and Roose, 1987; Schaal et al., 1991; Widén et al., 1994; Cronberg, 1996; Harada et al., 1997; McLellan et al., 1997; Mayes et al., 1998; Reusch et al., 1998; Sydes and Peakall, 1998; Tyson et al., 1998; Bush and Mulcahy, 1999; Bushakra et al., 1999; Davis et al., 1999; Kudoh et al., 1999; Reusch et al., 1999; Sawada, 1999; Suzuki et al., 1999; Jónsdóttir et al., 2000; Pappert et al., 2000; Reusch et al. 2000). The level of resolution of these molecular techniques depends on the intraspecific variability of the enzyme system or DNA region selected for the study (Avise 1994; Parker et al., 1998). At first glance, the analysis of the spatial distribution of ramet genotypes with a high-resolution molecular technique would appear to be enough to identify the genets of a clonal seaweed population. However, there are certain restrictive factors.
As part of the life cycle of most red seaweeds, many carpospores are produced within a female gametophyte, each carpospore potentially giving rise to a tetrasporophyte (Hawkes, 1990; Hommersand and Fredericq, 1990). Carpospores are produced by a special reproductive phase referred to as the carposporophyte (gonimocarp, sensu van den Hoek et al., 1995), which develops withing the female gametophyte after repeated mitotic events from a single zygote. Therefore, all of the tetrasporophytes that result from carpospores from a single carposporophyte would be genetically identical. As a consequence, even the most refined molecular technique will not be able to distinguish among these tetrasporophytes. This may result in the underestimation of genet density if these tetrasporophytes were wrongly intepreted as clonal fragments from one original genet. This problem may also occur when gametophytes are produced by other gametophytes directly through carpospores. Some gametophytes of Mastocarpus papillatus (C. Agardh) Kützing (Gigartinales) are thought to produce carpospores through parthenogenesis from a single cell (Polanshek and West, 1977; Zupan and West, 1988) and some gametophytes of Mastocarpus stellatus (Stackhouse) Guiry are thought to produce carpospores from a zygote originated through self-fertilization, lacking male-determining alleles (Maggs, 1988). When coming from the same carposporophyte, both kinds of carpospores will likely produce genetically identical gametophytes, which may ultimatelly also result in unrealistically low numbers of genets identified. Additionally, a given tetrasporophyte may produce tetraspores that are genetically identical for certain markers even after meiotic of genet (each resulting gametophyte) density. The opposite situation, that is, the overestimation of genet density, could occur when clonal fragments of a genet are wrongly interpreted as sibling tetrasporophytes or parthenogenetically derived gametophytes. The possible under- or overestimation of genet density may also weaken evolutionary considerations about reproductive strategies, since the relative importance of recruitment and clonal fragmentation would be incorrectly assessed.
Additional factors that may limit the accuracy of genet identification through spatio-temporal mapping of ramet genotypes are somatic mutation, mitotic recombination, apomeiosis, and genetic transposition. These processes might induce genetic variation within a genet and result in an overestimation of genet density (not to be confused with overestimating genetic diversity, which would not occur) after sampling genetically different ramets from such a genet. Genetic mosaicism has been detected for a few species of clonal terrestrial plants (Gill et al., 1995).
Mutations are thought to have little effect on allele frequencies in each generation of eukaryotic organisms (Gill et al., 1995; Slatkin et al., 1995). Spontaneous mutation rates are between 10-4 and 10-6 mutations per gene per generation (Hartl, 1988). However, mutation rates depend on the physicochemical properties of the DNA, among other factors (Cock-burn, 1991). For example, in special areas of the genome termed VNTR (variable number of tandem repeats), mutation rates are particularly high, up to 10-2 per generation (Slatkin et al., 1995). For seaweeds, mutation rates are largely unknown, although there is evidence suggesting that rates vary across taxonomic groups (Russell, 1986).
Mitotic recombination is common in Gracilaria tikvahiae McLachlan (Gracilariales) under laboratory conditions and is thought to occur in other red seaweeds as well (van der Meer and Todd, 1977; van der Meer, 1981). Using genetic markers for thallus color, recombinant areas within thalli of G. tikvahiae were identified as patches of different color on the surface of fertile areas, although never on juvenile fronds. Mitotic recombination is apparently uncommon in other red seaweeds: for example, the patterns of recombination described for G. tikvahiae were not detected in Chondrus crispus (van der Meer, 1981). For terrestrial plants, rates of mitotic recombination have been estimated between 10-4 and 10-3 per cell (Gill et al., 1995).
Apomeiosis was observed in laboratory cultures of Gracilaria tikvahiae, for which cytokinetic failure during tetraspore formation resulted in bi- and tetranucleate spores that could produce genets with both male and female fronds (van der Meer, 1977). Rates of natural occurrence of apomeiosis are unknown for red seaweeds.
Genetic transposition has been proposed, but not yet established, as an explanation for the dynamics of unstable color mutations for three members of the Gracilariales (van der Meer and Zhang, 1988). Nevertheless, the spontaneous reversion of color reported for parts of the same genet represents another case of genetic variability within genets.
Experimental populations
The problems discussed above may discourage demographic studies on clonal algal genets when the history of a stand is unknown which is the case for most natural populations. However, genet demography may be studied under controlled conditions. For example, several aquaria could be seeded with spores that are all genetically different. By manipulating spore settlement densities, abiotic variables, and the proportion between carpospores and tetraspores, demographic patterns for genets could be studied by periodically mapping their shape and the position of ramet genotypes as genets grow. Mapping holdfast shape will be obviously easier for seaweeds that grow in hard substrates (e.g., Chondrus and Mazzaella) than for those that grow in sand (e.g., Gracilaria). Also, it will be easier for crustose holdfast (e.g., Chondrus and Mazzaella) than for stoloniferous ones (e.g., Gelidium and Pterocladiella).
The continuous spatial mapping of ramet genotypes will enable one to determine if contact areas between neighboring genets remain static after contact or if overgrowth occurs. Possible coalescence between crustose holdfasts could be detected through microscopic observations on holdfast samples from contact areas. For coalescing genets, genetic sampling of ramets should be continuous to investigate if one genotype progressively prevails over the other within the chimeric thallus. For coalescing genets of Gracilaria chilensis Bird, McLachlan et Oliveira, different parts of a single frond may display the different original genotypes (Santelices et al., 1996). Thus, it may be necessary to analyze more than one part per frond around coalescent areas to ensure an accurate genotype mapping. For species whose holdfasts do not coalesce, but intermingle, the periodical mapping of ramet genotypes should enable one to determine patterns of intermingling.
If a new allelic combination arises through mitotic recombination in a ramet from contact areas between neighboring genets, knowledge of the original genotypes may enable one to associate the ramet to its parent if neighboring genets are genetically different enough. The production of male and female fronds within a genet resulting from an apomeiotic spore should not compromise genet identification either if the original genotypes are known. Somatic mutation might still complicate genet identification if it occurs in ramets arising in contact areas, especially if one analyzes rapidly evolving DNA areas, such as microsatellites, located in VNTR loci (Avise, 1994; Jarne and Lagoda, 1995). Genetic transposition might also be a problem if it occurs in contact areas. Genetic differentiation within a genet occurs during the growth of Gracilaria chilensis, as detected with RAPD markers, although the nature of such changes is still unknown (Meneses et al., 1999). Rates of somatic mutation and of genetic transposition should be estimated for red seaweeds, so their potential effects on the accuracy of genet identification using the methods suggested here can be assessed.
In conclusion, this paper proposes that determining the changing shpae of genets and the position of ramet genotypes from experimental populations with known initial genotypes is a potentially useful method to upgrade our understanding of genet demography for clonal red seaweeds. Rates of genetic change within genets should be previously estimated to design adequate experiments.
Acknowledgements
I am grateful to Dr. Susan L. Williams and to two anonymous reviewers, for their valuable comments. Support from the Northwest Biological Research Center (CIBNOR; La Paz, Baja California Sur, México), through research grant RM-7, is also acknowledged. The Mexican Science and Technology Council (CONACYT) provided additional funds for the completion of this study through the "Cátedra Patrimonial de Excelencia" and the "Sistema Nacional de Investigadores" (SNI) programs.
References
ANG, P. O. and E. DE WREEDE, 1992. Density-dependence in a population of Fucus distichus. Marine Ecology Progress Series 90: 169-181. [ Links ]
ARENAS, F. and C. FERNÁNDEZ, 2000. Size structure and dynamics in a population of Sargassum muticum (Phaeophyceae). Journal of Phycology 36: 1012-1020. [ Links ]
AVISE, J. C., 1994. Molecular markers, natural history and evolution. Chapman & Hall, New York. 511 p. [ Links ]
BLACK, R., 1974. Some biological interactions affecting intertidal populations of the kelp Egregia laevigata. Marine Biology 28: 189-198. [ Links ]
BUSH, S. P. and D. L. MULCAHY, 1999. The effects of regeneration by fragmentation upon clonal diversity in the tropical forest shrup Poikilacanthus macranthus: random amplified polymorphic DNA (RAPD) results. Molecular Ecology 8: 865-870. [ Links ]
BUSHAKRA, J. M., S. A. HODGES, J. B. COOPER and D. D. KASKA, 1999. The extent of clonality and genetic diversity in the Santa Cruz Island ironwood, Lyonothamnus floribundus. Molecular Ecology 8: 471-475. [ Links ]
CHAPMAN, A. R. O. and C. L. GOUDEY, 1983. Demographic study of the macrothallus of Leathesia difformis (Phaeophyta) in Nova Scotia. Canadian Journal of Botany 61: 319-323. [ Links ]
COCKBURN, A., 1991. An introduction to evolutionary ecology. Blackwell Scientific Publications, Oxford. 370 p. [ Links ]
CREED, J. C., 1995. Spatial dynamics of a Himanthalia elongata (Fucales, Phaeophyta) population. Journal of Phycology 31: 851-859. [ Links ]
CREED, J. C., J. M. KAIN (JONES) and T. A. NORTON, 1998. An experimental evaluation of density and plant size in two large brown seaweeds. Journal of Phycology 34: 39-52. [ Links ]
CRONBERG, N., 1996. Clonal structure and fertility in a sympatric population on the peat mosses Sphagnum rubellum and Sphagnum capillifolium. Canadian Journal of Botany 74: 1375-1385. [ Links ]
DAVIS, J. L., D. L. CHILDERS and D. N. KUHN, 1999. Clonal variation in a Florida Bay Thalassia testudinum meadow: molecular genetic assessment of population structure. Marine Ecology Progress Series 186: 127-136. [ Links ]
DEAN, T. A., K. THIES and S. L. LAGOS, 1989. Survival of juvenile giant kelp: the effects of demographic factors, competitors, and grazers. Ecology 70: 483-495. [ Links ]
DE KROON, H., 1993. Competition between shoots in stands of clonal plants. Plant Species Biology 8: 85-94. [ Links ]
DE KROON, H. and J. VAN GROENENDAEL 1997. The ecology and evolution of clonal plants. Backhuys Publishers, Leiden. 453 p. [ Links ]
DYCK, L. J. and R. E. DE WREEDE, 1995. Patterns of seasonal demographic change in the alternate isomorphic stages of Mazzaella splendens (Gigartinales, Rhodophyta). Phycologia 34: 390-395. [ Links ]
ELLSTRAND, N. C. and M. L. ROOSE, 1987. Patterns of genotypic diversity in clonal plant species. American Journal of Botany 74: 123-131. [ Links ]
ERIKSSON, O., 1993. Dynamics of genets in clonal plants. Trends in Ecology and Evolution 8: 313-316. [ Links ]
ERIKSSON, O. and L. JERLING, 1990. Hierarchical selection and risk spreading in clonal plants. pp. 79-94. In: J VAN GROENENDAEL and H. DE KROON (Eds.). Clonal growth in plants: regulation and function. SPB Academic Publishers. [ Links ]
FLORES-MOYA, A., J. A. FERNÁNDEZ and F. X. NIELL 1997. Growth pattern, reproduction and self-thinning in seaweeds: a re-evaluation in reply to Scrosati. Journal of Phycology 33: 1080-1081. [ Links ]
GILL, D. E., L. CHAO, S. L. PERKINS and J. B. WOLF, 1995. Genetic mosaicism in plant and clonal animals. Annual Review of Ecology and Systematics 26: 423-444. [ Links ]
HARA, T., 1994. Growth and competition in clonal plants - Persistence of shoot populations and species diversity. Folia Geobotanica & Phytotaxonomica 29: 181-201. [ Links ]
HARADA, Y., S. KAWANO and Y. IWASA 1997. Probability of clonal identity: inferring the relative success of sexual versus clonal reproduction from spatial genetic patterns. Journal of Ecology 85: 591-600. [ Links ]
HARPER, J. L. and J. WHITE, 1974. The demography of plants. Annual Review of Ecology and Systematics 5: 419-463. [ Links ]
HARTL, D. L., 1988. A primer of population genetics. Second edition. Sinauer Associates, Suderland. 305 p. [ Links ]
HAWKES, M. W., 1990. Reproductive strategies, pp. 455-476. In: K. M. COLE and R. G. SHEATH (Eds.). Biology of the red algae. Cambridge University Press. [ Links ]
HOMMERSAND, M. H. and S. FREDERICQ, 1990. Sexual reproduction and cystocarp development. pp. 305-345. In: K. M. COLE and R. G. SHEATH (Eds.). Biology of the red algae. Cambridge University Press. [ Links ]
JARNE, P. and P. J. L. LAGODA, 1995. Microsatellites, from molecules to populations and back. Trends in Ecology and Evolution 11: 424-429. [ Links ]
JÓNSDÓTTIR, I. S., M. AUGNER, T. FAGERSTRÖM, H. PERSSON and A. STENSTRÖM, 2000. Genet age in marginal populations of two clonal Carex species in the Siberian Arctic. Ecography 23: 402-412. [ Links ]
KAYS, S. and J. L. HARPER, 1974. The regulation of plant and tiller density in a grass sward. Journal of Ecology 62: 97-105. [ Links ]
KLEKOWSKI, E. J., 1997. Somatic mutation theory of clonality pp. 227-241. In: H. DE KROON and J. VAN GROENENDAEL (Eds.). The ecology and evolution of clonal plants. Backhuys Publishers. [ Links ]
KUDOH, H., H. SHIBAIKE, H. TAKASU, D. F. WHIGHAM and S. KAWANGO, 1999. Genet structure and determinants of clonal structure in a temperate deciduous woodland herb, Uvularia perfoliata. Journal of Ecology 87: 244-257. [ Links ]
MAGGS, C. A., 1988. Intraspecific life history variability in the Florideophycidae (Rhodophyta). Botanica Marina 31: 465-490. [ Links ]
MAGGS, C. A. and D. P. CHENEY, 1980. Competition studies of marine macroalgae in laboratory culture. Journal of Phycology 26: 18-24. [ Links ]
May, G., 1986. Life history variations in a predominantly gametophytic population of Iridaea cordata (Gigartinaceae, Rhodophyta). Journal of Phycology 22: 448-455. [ Links ]
MAYES, S. G., M. A. MCGINLEY and C. R. WERTH, 1998. Clonal population structure and genetic variation in sand-shinnery oak, Quercus havardii (Fagaceae). American Journal of Botany 85: 1609-1617. [ Links ]
MCLELLAN, A. J., D. PRATI, O. KALTZ and B. SCHMID, 1987. Structure and analysis of phenotypic and genetic variation in clonal plants. pp. 185-210. In: H. DE KROON and J. VAN GROENENDAEL (Eds.). The ecology and evolution of clonal plants. Backhuys Publishers. [ Links ]
MENESES, I., B. SANTELICES and P. SÁNCHEZ, 1999. Growth-related intraclonal genetic changes in Gracilaria chilensis (Gracilariales: Rhodophyta). Marine Biology 135: 391-397. [ Links ]
PAPPERT, R. A., J. L. HAMRICK and L. A. DONOVAN, 2000. Genetic variation in Pueraria lobata (Fabaceae), an introduced, clonal, invasive plant of the southeastern United States. American Journal of Botany 87: 1240-1245. [ Links ]
PARKER, P. G., A. A. SNOW, M. D. SHUG, G. C. BOOTON and P. A. FUERST, 1998. What molecules can tell us about populations: choosing and using a molecular marker. Ecology 79: 361-382. [ Links ]
PETERSON, C. J. and R. H. JONES, 1997. Clonality in woody plants: a review and comparison with clonal herbs. pp. 263-289. In: H. DE KROON and J. VAN GROENENDAEL (Eds.). The ecology and evolution of clonal plants. Backhuys Publishers. [ Links ]
POLANSHEK, A. R. and J. A. WEST 1977. Culture and hybridization studies on Gigartina papillata. Journal of Phycology 11: 434-439. [ Links ]
REED, D. C., 1990. An experimental evaluation of density dependence in a subtidal algal population. Ecology 71: 2286-2296. [ Links ]
REUSCH, T. B. H., C. BOSTRÖM, W. T. STAM and J. L. OLSEN, 1999. An ancient eelgrass clone in the Baltic. Marine Ecology Progress Series 183: 301-304. [ Links ]
REUSCH, T. B. H., W. T. STAM and J. L. OLSEN, 1998. Size and estimated age of genets in eelgrass, Zostera marina, assessed with microsatellite markers. Marine Biology 133: 519-525. [ Links ]
REUSCH T. B. H., W. T. STAM and J. L. OLSEN, 2000. A microsatellite-based estimation of clonal diversity and population subdivision in Zostera Marina, a marine flowering plant. Molecular Ecology 9: 127-140. [ Links ]
RUSSELL, G., 1986. Variation and natural selection in marine macroalgae. Oceanography and Marine Biology Annual Review 24: 309-377. [ Links ]
SANTELICES, B., J. A. CORREA, I. MENESES, D. AEDO and D. VARELA, 1996. Sporeling coalescence and intraclonal variation in Gracilaria chilensis (Gracilariales, Rhodophyta). Journal of Phycology 32: 313-322. [ Links ]
SANTELICES, B., J. A. CORREA, D. AEDO, V. FLORES, M. HORMAZÁBAL and P. SÁNCHEZ, 1999. Convergent biological processes in coalescing Rhodophyta. Journal of Phycology 35: 1127-1149. [ Links ]
SANTOS, R., 1995. Size structure and inequality in a commercial stand of the seaweed Gelidium sesquipedale. Marine Ecology Progress Series 119: 253-263. [ Links ]
SAWADA, H., 1999. Genetic variation in clonal traits of Trifolium repens and species interactions. Plant Species Biology 14: 19-28. [ Links ]
SCHAAL, B. A., S. L. O'KANE and S. H. ROGSTAD, 1991. DNA variation in plant populations. Trends in Ecology and Evolution 6: 329-333. [ Links ]
SCROSATI, R., 1998. Mechanisms of recolonization of the clonal intertidal alga Mazzaella cornucopiae (Rhodophyta, Gigartinaceae) after disturbances. Canadian Journal of Botany 76: 1717-1724. [ Links ]
SCROSATI, R., 2000. The interspecific biomass-density relationship for terrestrial plants: where do clonal red seaweeds stand and why? Ecology Letters 3: 191-197. [ Links ]
SCROSATI, R., 2002. An updated definition of genet applicable to clonal seaweeds, bryophytes, and vascular plants. Basic and Applied Ecology 3, in press. [ Links ]
SCROSATI, R. and R. E. DE WREEDE, 1997. Dynamics of the biomass-density relationship and frond biomass inequality for Mazzaella cornucopiae (Gigartinaceae, Rhodophyta): implications for the understanding of frond interactions. Phycologia 36: 506-516. [ Links ]
SCROSATI, R. and E. SERVIERE-ZARAGOZA, 2000. Ramet dynamics for the clonal seaweed Pterocladiella capillacea (Rhodophyta, Gelidiales): a comparison with Mazzaella cornucopiae and with Chondrus crispus (Gigartinales). Journal of Phycology 36: 1061-1068. [ Links ]
SLATKIN, M., K. HINDAR and Y. MICHALAKIS, 1995. Processes of genetic diversification. pp. 213-225. In: V. H. HEYWOOD (Eds.). Global biodiversity assessment. Cambridge University Press. [ Links ]
SUZUKI, J. I., T. HERBEN, F. KRAHULEC and T. HARA, 1999. Size and spatial pattern of Festuca rubra genets in a mountain grassland: its relevance to genet establishment and dynamics. Journal of Ecology 87: 942-954. [ Links ]
SUZUKI, J. I. and M. J. HUTCHINGS, 1997. Interactions between shoots in clonal plants and the effects of stored resources on the structure of shoot populations. pp. 311-329. In: H. DE KROON and J. VAN GROENENDAEL (Eds.). The ecology and evolution of clonal plants. Backhuys Publishers. [ Links ]
SYDES, M. A. and R. PEAKALL, 1998. Extensive clonality in the endangered shrub Haloragodendron lucasii (Haloragaceae) revealed by allozymes and RAPDs. Molecular Ecology 7: 87-93. [ Links ]
TVETER, E. and A. C. MATHIESON, 1976. Sporeling coalescence in Chondrus crispus (Rhodophyceae). Journal of Phycology 12: 110-118. [ Links ]
TVETER-GALLAGHER, E. and A. C. MATHIESON, 1980. An electron microscopy study of sporeling coalescence in the red alga Chondrus crispus. Scanning Electron Microscopy 3: 571-579. [ Links ]
TYSON, M., R. E. VAILLANCOURT and J. B. REID, 1998. Determination of clone size and age in a mallee eucalypt using RAPDs. Australian Journal of Botany 46: 161-172. [ Links ]
VAN DEN HOEK, C., D. G. MANN and H. M. JAHNS, 1995. Algae. An introduction to phycology. Cambridge University Press, Cambridge. 623 p. [ Links ]
VAN DEN MEER, J. P., 1977. Genetics of Gracilaria sp. (Rhodophyceae, Gigartinales) II. The life history and genetic implications of cytokinetic failure during tetraspore formation. Phycologia 16: 367-371. [ Links ]
VAN DEN MEER, J. P., 1981. Genetics of Gracilaria tikvahiae (Rhodophyceae). VII. Further observations on mitotic recombination and the construction of polyploids. Canadian Journal of Botany 59: 787-792. [ Links ]
VAN DEN MEER, J. P. and E. R. TODD, 1977. Genetics of Gracilaria sp. (Rhodophyceae, Gigartinales). IV. Mitotic recombination and its relationship to mixed phases in the life history. Canadian Journal of Botany 55: 2810-2817. [ Links ]
VAN DEN MEER, J. P. and X. ZHANG, 1988. Similar unstable mutations in three species of Gracilaria (Rhodophyta). Journal of Phycology 24: 198-202. [ Links ]
VUORISALO, T., J. TUOMI, B. PEDERSEN and P. KÄÄR, 1997. Hierarchical selection in clonal plants. pp. 243-261. In: H. DE KROON and J. VAN GROENENDAEL (Eds.). The ecology and evolution of clonal plants. Backhuys Publishers. [ Links ]
WIDÉN, B., N. CRONBERG and M. WIDÉN, 1994. Genotypic diversity, molecular markers and spatial distribution of genets in clonal plants, a literature survey. Folia Geobotanica & Phytotaxonmica 29: 245-263. [ Links ]
ZUPAN, J. R. and J. A. WEST, 1988. Geographic variation in the life history of Mastocarpus papillatus (Rhodophyta). Journal of Phycology 24: 223-229. [ Links ]














