Introduction
Harmful algal blooms (HABs) are a leading environmental problem worldwide. Some of the microalgae responsible for HABs produce toxins that are bioaccumulated and biomagnified through marine food webs, which can result in severe poisoning and the deaths of humans, marine mammals, shorebirds, fish, crustaceans, and other organisms (Anderson 2009; Pérez-Morales et al. 2014, 2017; McPartlin et al. 2017). Global climate change and anthropogenic eutrophication processes have gradually affected marine ecosystems, favoring the expansion of HABs, increasing their frequency and duration, and causing HABs to occur in new locations. In addition, new phytoplankton species identified in HABs have created diverse impacts with a wide range of toxic consequences (Pérez-Morales et al. 2015, Glibert and Burkholder 2018, Glibert 2020).
Beaches the world over are in high demand for recreational purposes, which exposes people and animals to HABs and their toxins directly by contact, ingestion, or respiration or indirectly by consumption of contaminated foods (Pérez-Morales and Band-Schmidt 2011, Vidal et al. 2017). As such, the World Health Organization (WHO 2021) updated the Guidelines for Recreational Water Quality in the northern hemisphere in 2021, emphasizing the importance of monitoring HABs and cyanobacteria in particular. Therefore, continual monitoring of coastal waters is essential for understanding the composition and dynamics of phytoplankton populations and predicting the potential impacts of HABs (Hallegraeff 2010, Gowen et al. 2012).
In Mexico, NMX-AA-120-SCFI-2016 (DOF 2016), a national standard that establishes classification criteria for beach quality in the 17 coastal states of the country, is based on monitoring acute febrile gastrointestinal and respiratory diseases due to poor recreational water quality because of the potential consequences to human health (WHO 2003). This standard establishes a monitoring approach based on the presence of fecal Streptococcus or Enterococcus species (200 most probable number [MPN] per 100 mL), which are widely used indicators of seawater contamination and the associated health risks due to recreational use (DOF 2016). It should be noted that this standard does not consider continual HABs monitoring as a relevant source of information to characterize beach safety, even when potentially harmful phytoplankton pose a potential risk to human health.
In Mexico, the government authority responsible for recording HABs events and issuing health alerts is the Federal Commission for the Protection against Sanitary Risks (COFEPRIS, for its acronym in Spanish), which delegates the responsibility of establishing the sampling frequency to identify marine biotoxins and phytoplankton cells in natural marine and aquaculture zones to the state health authorities, without taking into account beaches for recreational use. In this sense, the Working Guidelines for Phytoplankton Sampling and Marine Biotoxin Detection is limited to a small number of species, including 14 dinoflagellates and only one genus of diatoms, without considering the most useful physicochemical variables such as salinity and temperature. The latest revised version of this document was published in September 2016 and does not consider cyanobacteria (https://www.gob.mx/cofepris/acciones-y-programas/lineamientos-of-the-red-tide-project).
In recent years, various HABs have been reported in coastal Mexican waters, particularly in the state of Campeche. From 2005 to 2021, a total of 45 HABs were recorded along the coast, with abundances ranging from 8.0 × 105 cell·L-1 (Poot-Delgado 2016; Poot-Delgado et al. 2018, 2021a, b; Poot-Delgado and Okolodkov 2020). In the past decade, cyanobacteria of the genera Anabaena, Cylindrospermopsis, and Trichodesmium have been recorded along the Campeche coast, ranging from 104 to 106 cell·L-1 (Poot-Delgado et al. 2018, 2021b). Various species of these genera are potentially harmful, highlighting the importance and urgency of paying particular attention to cyanobacteria and including them in marine water quality monitoring efforts.
Given the increase, distribution, and diversity of HABs-forming phytoplankton species along the Campeche coast and to follow-up with the Guidelines for Recreational Water Quality, this study reports the most recent changes in the composition and abundance of phytoplankton species, focusing on those that are harmful. In addition, the most useful physicochemical variables that likely influence the presence of these species were analyzed. This study offers a set of criteria to classify the environmental health of beaches and the seasons in which the risks to public health are low.
Materials and methods
From March to October 2019, monthly sampling was conducted at 6 recreational beaches on the Campeche coast: Punta Xen (19°10′51.48″ N, 90°54′13.49″ W), Villamar (19°17′41.44″ N, 90°46′15.87″ W), Boca del Río (19°21′42.02″ N, 90°43′4.12″ W), Payucan (19°39′36.60″ N, 90°42′15.43″ W), Sombrerón (19°42′15.93″ N, 90°40′53.86″ W), and Playa Bonita (19°47′40.21″ N, 90°37′18.53″ W; Fig. 1). At each site, water samples were collected in triplicate at approximately 1 m depth.
Surface seawater samples were collected in 1-L plastic bottles, from which a 100-mL aliquot was taken to determine phytoplankton abundance. Aliquot samples were fixed with a neutral Lugol solution and subsequently preserved by adding 37% neutralized formalin to a final concentration of 4% (Andersen and Throndsen 2004). Phytoplankton cells were counted after sedimentation in 10-mL cylinders following the methods of Reguera et al. (2016). In addition, horizontal trawls were conducted manually with a conical net (mesh size of 20 μm), with each tow lasting 5 min. The material collected for phytoplankton identification was placed in plastic bottles and fixed following the same procedure employed in the quantitative analysis. The phytoplankton in the net samples were identified under a light microscope to ensure accuracy. The physicochemical variables of water temperature, salinity, and pH were measured in situ using an HI9828 multiparameter probe and HI769828 sensor (Hanna Instruments, Woonsocket, RI, USA).
The Utermöhl technique (Utermöhl 1958)) was used to quantify phytoplankton cells in 10 cm3 of sample using an inverted series 450 SI-PH microscope (IROSCOPE, Mexico City, Mexico) with incorporated phase-contrast objectives (10×/0.25 Ph1 ADL and LD 25×/0.30 Ph1). Due to their small size, nanoflagellates (<20 µm) were not identified down to the species level. Abundance values are expressed as cell·L-1. Fixed phytoplankton samples were identified at the species level with a BA210 compound microscope (Motic, Xiamen, China) with incorporated planachromatic objectives (5×/0.10, 10×/0.25, 20×/0.40, 40×/0.65, and 100×/1.25). Potentially harmful phytoplankton species were identified based on specialized literature (Hallegraeff et al. 2004, Licea et al. 2004, Lundholm et al. 2009, UNESCO 2009, Lassus et al. 2016, Licea et al. 2016, Steidinger and Meave del Castillo 2018). In addition, stains and treatments were used to identify genera of diatoms (Parsons et al. 2012), dinoflagellates (Hermosilla 1973), and cyanobacteria (Kruk et al. 2009) by light microscopy.
Significant differences (95% confidence level) in physicochemical variables and phytoplankton abundance among sampling months and stations were evaluated by a Kruskal-Wallis test and box-and-whisker plots, with a non-parametric one-way analysis of variance (ANOVA; Daniel 1993, Boyer et al. 2000). The F-ratio (2 in this case) is the quotient between the between-group and within-group estimations. Statistical tests were performed in Statgraphics Centurion XV v. 18.2.06. The data were plotted in Statistica 7 and Excel 365.
The water temperature, salinity, and pH data were evaluated through a canonical correspondence analysis (CCA) using a matrix of environmental factors and species abundance (Ter Braak 1986) to assess the effects on potentially harmful and bloom-forming species. Data were transformed to Log10 (data + 1) prior to the analysis because (1) the data were not normally distributed and (2) large differences in magnitude were present between the biological and physicochemical data. The significance of the axes of the CCA was tested using a Monte Carlo analysis with 499 permutations. The calculation routine was performed in CANOCO v. 4.5.
Results
Physicochemical variables
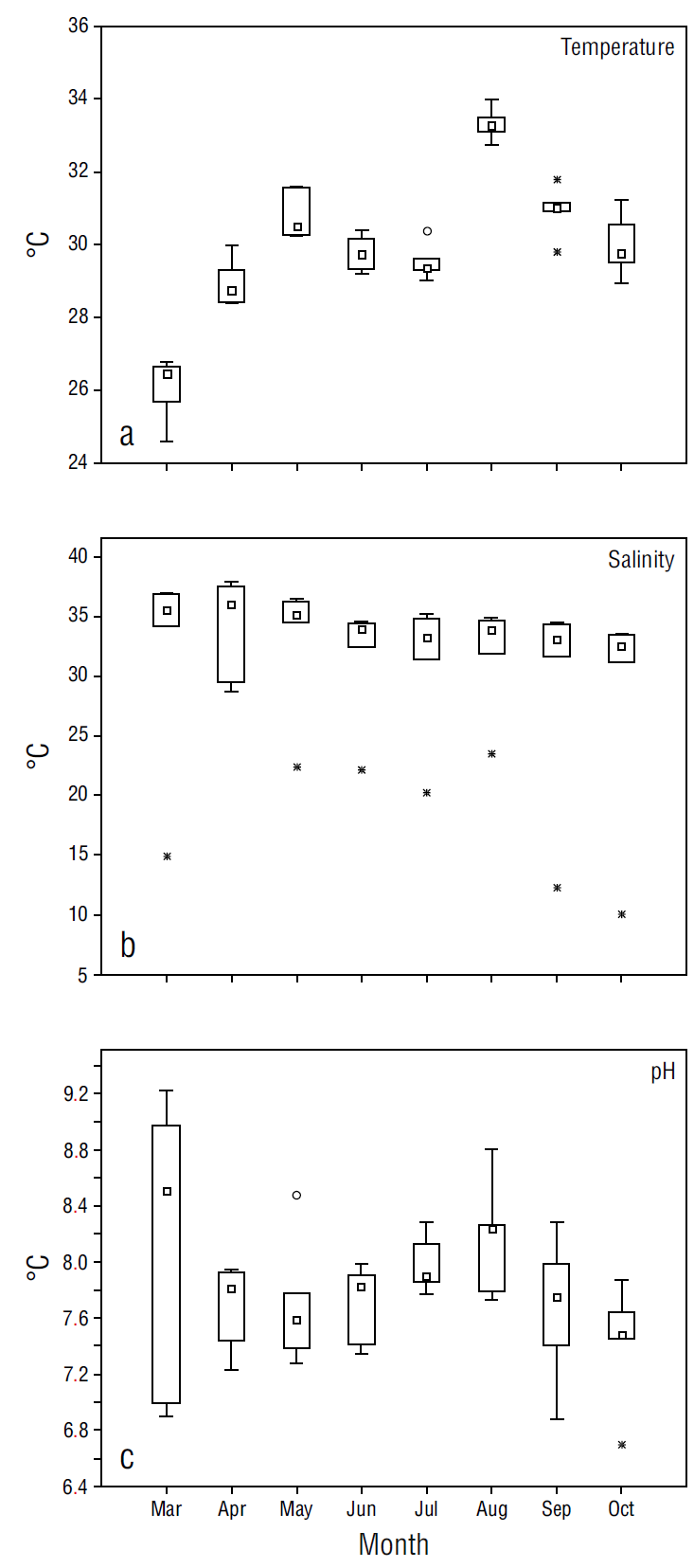
The temperature at the Punta Xen station exhibited a minimum value of 24.6 °C and a maximum value of 34 ± 2.1 °C (Table 1), thus showing seasonal variation. There were no significant differences in temperature among stations (F = 0.23, P > 0.05). Salinity showed a minimum value of 10.1 ± 6.3 at Boca del Rio and a maximum of 38.0 ± 1.9 at Playa Bonita (Table 1). The difference in salinity between these stations was significant (F = 35.94, P < 0.05). The pH showed a maximum value of 9.2 ± 0.6 at Payucan and a minimum of 6.7 ± 0.4 at Boca del Rio (Table 1). Significant differences in pH were identified among stations (F = 4.11, P < 0.05).
Table 1 Summary statistics (by sampling station) of the environmental variables from March to October 2019 in the recreational beaches of Campeche, southeastern Gulf of Mexico (range, mean, and standard deviation).
| Station | T (°C) | Salinity | pH |
| Playa Bonita | 26.8 - 33.5 | 32.6 - 38.0 | 7.3 - 8.8 |
| 30.0 ± 1.9 | 34.8 ± 1.9 | 7.7 ± 0.5 | |
| Sombrerón | 26.5 - 33.1 | 33.6 - 37.5 | 7.6 - 9.0 |
| 29.7 ± 1.9 | 35.4 ± 1.4 | 8.1 ± 0.4 | |
| Payucan | 26.7 - 32.7 | 33.3 - 36.8 | 7.5 - 9.2 |
| 29.7 ± 1.8 | 35.0 ± 1.1 | 8.1 ± 0.6 | |
| Boca del Rio | 26.4 - 33.2 | 10.1 - 28.7 | 6.7 - 7.8 |
| 29.5 ± 1.9 | 19.3 ± 6.3 | 7.3 ± 0.4 | |
| Villamar | 25.7 - 33.4 | 29.5 - 34.8 | 7.5 - 8.8 |
| 30.5 ± 2.2 | 32.2 ± 1.8 | 8.1 ± 0.4 | |
| Punta Xen | 24.6 - 34.0 | 31.8 - 36.3 | 6.9 - 8.2 |
| 30.0 ± 2.7 | 33.9 ± 1.4 | 7.7 ± 0.4 | |
| Differences among station | F = 0.23, P > 0.005 | F = 35.94, P < 0.005 | F = 4.11, P < 0.005 |
The physicochemical variables displayed temporal variations from March to October in the 6 stations of the central coast of Campeche. The minimum temperatures were recorded in March while the maximum temperatures were recorded in August (Fig. 2), with significant differences between these months (F = 62.52, P < 0.05). Salinity was constant throughout the study period and no significant differences were observed (F = 0.41, P > 0.05). For its part, pH showed wide variation, ranging from 7 to 9 in March However, it remained almost constant during the remaining months (Fig. 2), with no significant differences (F = 1.72, P > 0.05).
Composition of the phytoplankton community
The variation in the abundance of the main phytoplankton groups for the sampling sites by season is shown in Table 2. Phytoflagellates (nanophytoplankton <20 µm) exhibited a minimum abundance of 6.6 × 103 cell·L-1 at Villamar and a maximum abundance of 1.6 × 106 cell·L-1 at Boca del Rio; no significant differences were observed among stations (F = 0.89, P > 0.05). As a secondary component, the main microphytoplankton groups observed were diatoms, dinoflagellates, and cyanobacteria, with abundance values ranging from 103 to 106 cell·L-1. For diatoms, maximum values of 3.8 × 106 cell·L-1 were recorded at Villamar and minimum values of up to 3.0 × 103 cell·L-1 were observed at Sombrerón; no significant differences were observed among these stations (F = 0.99, P > 0.05).
Table 2 Abundance (cell·L-1) by station of the main phytoplankton groups from March to October 2019 in the recreational beaches of Campeche, southeastern Gulf of Mexico (mean, range, and standard deviation).
| Station | Nanoflagellates | Diatoms | Dinoflagellates | Cyanobacteria |
| Playa Bonita | 4.1 × 104 ± 1.9 × 104 | 3.1 × 104 ± 4.8 × 104 | 1.8 × 104 ± 3.0 × 104 | 2.6 × 103 ± 5.3 × 103 |
| 9.6 × 103 - 7.4 × 104 | 4.3 × 103 - 1.4 × 105 | 1.0 × 103 - 9.1 × 104 | 692-1.5 × 104 | |
| Sombrerón | 4.0 × 104 ± 1.5 × 104 | 4.0 × 104 ± 4.7 × 104 | 3.7 × 103 ± 2.6 × 103 | 3.4 × 104 ± 9.1 × 104 |
| 1.9 × 104 - 6.0 × 104 | 3.0 × 103 - 1.2 × 105 | 1.3 × 103 - 9.4 × 103 | 769-2.6 × 105 | |
| Payucan | 3.8 × 104 ± 1.9 × 104 | 8.3 × 104 ± 8.1 × 104 | 3.2 × 103 ± 2.6 × 103 | 8.0 × 103 ± 1.2 × 104 |
| 6.8 × 104 - 7.1 × 104 | 4.6 × 103 - 2.1 × 105 | 4.6 × 102 - 8.1 × 103 | 769-3.5 × 104 | |
| Boca del Rio | 2.3 × 105 ± 5.7 × 105 | 1.1 × 104 ± 1.3 × 105 | 2.9 × 104 ± 7.1 × 104 | 3.2 × 103 ± 4.1 × 103 |
| 1.1 × 104 - 1.6 × 106 | 6.9 × 103 - 3.1 × 105 | 77-2.0 × 105 | 769-1.2 × 104 | |
| Villamar | 5.0 × 104 ± 4.7 × 104 | 5.4 × 105 ± 1.3 × 106 | 4.8 × 103 ± 3.6 × 105 | 8.4 × 103 ± 9.0 × 103 |
| 6.6 × 103 - 1.5 × 105 | 7.4 × 103 - 3.8 × 106 | 1.2 × 103 - 1.0 × 106 | 538-2.6 × 104 | |
| Punta Xen | 3.9 × 104 ± 3.4 × 104 | 9.9 × 104 ± 1.0 × 105 | 1.7 × 105 ± 3.6 × 105 | 6.4 × 103 ± 6.1 × 103 |
| 7.5 × 103 - 9.3 × 104 | 1.2 × 104 - 3.2 × 105 | 1.1 × 103 - 1.0 × 106 | 692-1.6 × 104 | |
| Differences among station | F = 0.89, P > 0.005 | F = 0.99, P > 0.005 | F = 1.45, P > 0.005 | F = 0.79, P > 0.005 |
The maximum dinoflagellate abundance of 1.0 × 106 cell·L-1 was recorded at Villamar while the minimum abundance of around 103 cell·L-1 was recorded at Punta Xen. Although dinoflagellate abundance varied widely, no significant differences were observed among these stations (F = 1.45, P > 0.05). Cyanobacteria showed low abundance ranging from absence to 2.6 × 105 cell·L-1, with no significant differences among stations (F = 0.79, P > 0.05).
The responses of the largest phytoplankton groups and physicochemical variables were mainly explained by the first 2 axes of the CCA (axis 1: 78.8%, axis 2: 21.2%, total 100%). The correlation among the main phytoplankton groups and physicochemical variables was low (r ≈ 0.5), indicating that the relationship between the taxa and variables considered in the analysis was not significant, at least for the study period. No interactions among canonical axes were statistically significant (P > 0.05, Monte Carlo, Table 3).
Table 3 Eigenvalues and percentage of the total variance explained by the temporal canonical correspondence analysis (CCA) of the main phytoplankton groups from March to October 2019 in the recreational beaches of Campeche, southeastern Gulf of Mexico.
| Axes | Eigenvalues | Species-environmental correlations | Cumulative percentage variation |
| 1 | 0.002 | 0.169 | 78.8 |
| 2 | 0.001 | 0.281 | 100.0 |
| 3 | 0.085 | 0.000 | 0.0 |
| 4 | 0.008 | 0.000 | 0.0 |
| Test of significance of first canonical axis | F = 1.173 | P = 0.750 | |
| Test of significance of all canonical axes | F = 0.500 | P = 0.704 | |
The temporal variation in the abundance of the main phytoplankton groups recorded from March to October is shown in Figure 3. Phytoflagellates (nanophytoplankton <20 µm) showed a peak abundance of 106 cell·L-1 in April at Boca del Río, although their abundance was highly variable throughout the period (Fig. 3a). Diatoms showed a maximum abundance of around 106 cell·L-1 in March at Playa Bonita, followed by abundance values of 105 cell·L-1 at Boca del Río in April, May, and July. At Punta Xen, several abundance peaks of 105 cell·L-1 occurred in May and October (Fig. 3b). No significant differences were observed among months (F = 0.97, P > 0.05).
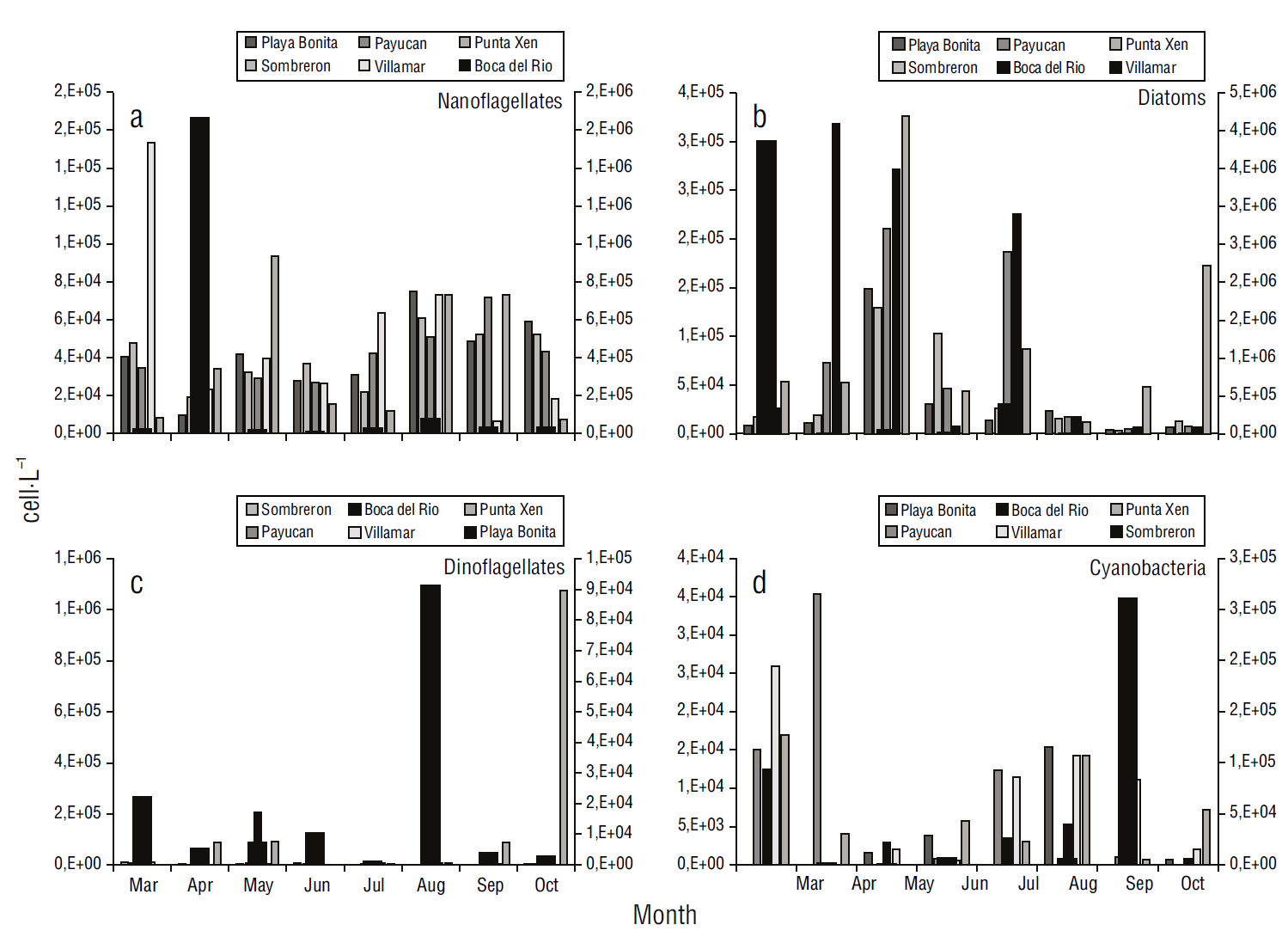
Figure 3 Temporal variation (2019) in abundances (cell·L-1) of the major phytoplankton groups in the recreational beaches of Campeche, southeastern Gulf of Mexico. The second axis indicates the highest abundances.
At Playa Bonita and Boca del Río, maximum dinoflagellate abundance (106 cell·L-1) was recorded in August, although dinoflagellate abundance was less than 105 cell·L-1 in March. In addition, dinoflagellate abundance at Punta Xen was 104 cell·L-1 (Fig. 3c). No significant differences were observed among months (F = 0.85, P > 0.05). Cyanobacteria abundance was extraordinarily high at Sombrerón (105 cell·L-1) while remaining low at all other stations, with values of 103 to 104 cell·L-1 throughout the study period (Fig. 3d). No significant differences were observed among months (F = 0.90, P > 0.05).
Potentially harmful phytoplankton and cyanobacteria
Potentially harmful phytoplankton and cyanobacteria were identified each month at the 6 sampling stations throughout the study period. Diatoms, particularly Pseudo-nitzschia, included several unidentified species and were present in almost all stations except Punta Xen. The maximum abundance of Pseudo-nitzschia (5.5 ×104 cell·L-1) was recorded in Payucan in May. In contrast, the abundance of Pseudo-nitzschia was orders of magnitude less at all other stations from March to October, except in September (Fig. 4).
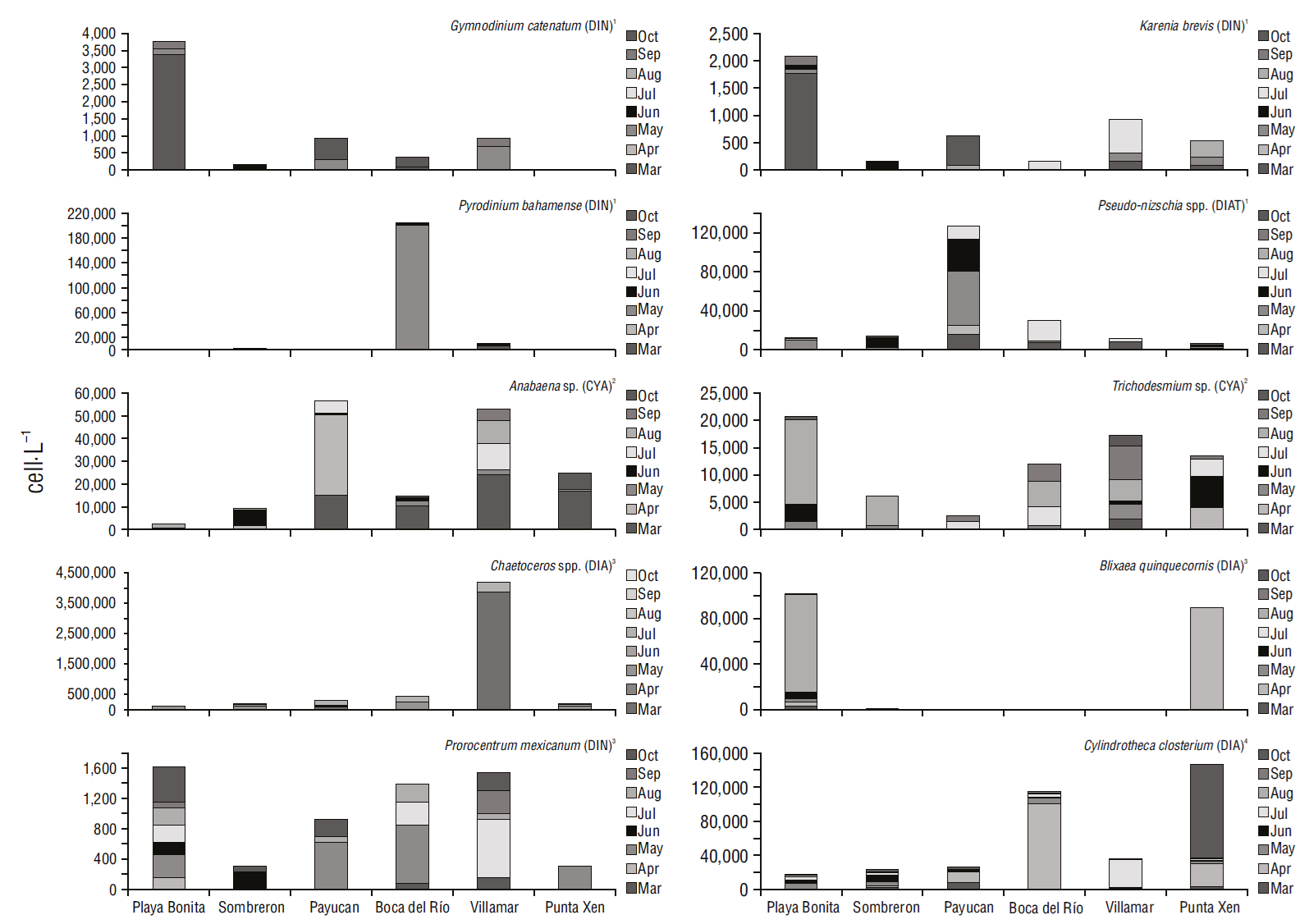
Figure 4 Abundance (cell·L-1) of potentially harmful and bloom-forming species recorded from March to October 2019 in the recreational beaches of Campeche, southeastern Gulf of Mexico. Taxonomic groups: diatoms (DIA), dinoflagellates (DIN), and cyanobacteria (CYA).
Cylindrotheca closterium showed abundances of 1.0 × 105 cell·L-1 in Boca del Rio and Villamar in April and October (Fig. 4). In contrast, C. closterium abundance was lower in the remaining stations in all months (103 to 104 cell·L-1). However, the maximum abundance of Chaetoceros (3.8 × 106 cell·L-1) was recorded at Villamar in March, followed by abundance values of 2.5 × 105 cell·L-1 in May at Boca del Río and 3.1 × 105 cell·L-1 in July at Villamar (Fig. 4).
With respect to dinoflagellates, Blixaea quinquecornis showed peak abundance in April at Punta Xen (8.9 × 104 cell·L-1) and later in August at Playa Bonita (8.9 × 104 cell·L-1; Fig. 4). Gymnodinium catenatum and Karenia brevis were rare, exhibiting maximum abundance in March at Playa Bonita (3.3 × 103 and 1.7 × 103 cell·L-1, respectively) and minimum abundance in October at Payucan (6.1 × 102 and 5.3 × 102 cell·L-1, respectively; Fig. 4). Pyrodinium bahamense showed maximum abundance in May at Boca del Río (2.0 × 105 cell·L-1) and was absent in Playa Bonita, Payucan, and Punta Xen.
Regarding cyanobacteria, Anabaena was observed in all stations with abundance values that ranged from 102 to 104 cell·L-1 throughout the study period, although peak abundance (3.5 × 104 cell·L-1) was recorded in April at Payucan. Trichodesmium showed a maximum abundance of 1.5 × 104 cell·L-1 at Playa Bonita in August, although it was also observed in all sampling stations and months (Fig. 4).
The response of potentially harmful phytoplankton and cyanobacteria to physicochemical variables was mainly explained by the first 2 axes of the CCA (axis 1: 46.4%, axis 2: 40.5%, total 86.9%). The correlation between potentially harmful phytoplankton and physicochemical variables was high (r ≈ 0.7), reflecting a significant relationship between the species and physicochemical variables included in the analysis. However, the canonical axes were not statistically significant (P > 0.05, Monte Carlo; Table 4).
Table 4 Eigenvalues and percentage of the total variance explained by the temporal canonical correspondence analysis (CCA) of potentially harmful phytoplankton and cyanobacteria from March to October 2019 in the recreational beaches of Campeche, southeastern Gulf of Mexico.
| Axes | Eigenvalues | Species-environmental correlations | Cumulative percentage variation |
| 1 | 0.025 | 0.490 | 46.4 |
| 2 | 0.022 | 0.393 | 86.9 |
| 3 | 0.007 | 0.210 | 100.0 |
| 4 | 0.215 | 0.000 | 0.0 |
| Test of significance of first canonical axis | F = 0.969 | P = 0.912 | |
| Test of significance of all canonical axes | F = 0.715 | P = 0.818 | |
Discussion
The environmental conditions (temperature, salinity, and pH) of the recreational beaches in this study are favorable for the presence of potentially harmful species. This conclusion is supported by the high correlation (r ≈ 0.7) of the CCA. Temperature, salinity, and pH varied seasonally and are likely influenced by the local hydrography and shallowness of the study area (Poot-Delgado et al. 2014, 2021a, b). According to Vargo (2009), temperature and salinity can determine the occurrence and distribution of HABs, as well as the presence of nutrients that regulate the growth rate, biomass, and duration of blooms. The central coastal area of Campeche receives untreated wastewater discharge from important cities in the region (Gracia et al. 2014), including Campeche City, Seybaplaya, and Champotón. Excessive nutrients overload systems beyond what they can support and recycle, generating constant eutrophication due to compounds with high nitrogen, phosphorus, and silica loads (Pérez-Morales et al. 2015).
In this study, the presence of the planktonic-benthic pennate diatom C. closterium is worth highlighting, as it is an indicator of eutrophication, which reinforces the hypothesis of the association between polluting anthropogenic contributions and HABs in the area. Cylindrotheca closterium blooms have been reported previously in Mexican seas, in the northwestern Pacific, the southeastern Gulf of Mexico, and the coastal waters of northern Yucatán (Poot-Delgado et al. 2016). In September 2016, a bloom of C. closterium (1.0 × 105 cell·L-1) was recorded in a coastal area receiving wastewater discharge from a shrimp farm near Sombrerón, Campeche (Poot-Delgado and Okoldkov 2020). It should be noted that C. closterium is present in large numbers throughout the year, although mainly from mid-May to mid-October in the coastal waters of northern Yucatán; thus, its seasonality remains undefined (Merino-Virgilio et al. 2019), which is consistent with our observations in the present study.
The genus Pseudo-nitzschia includes several species that produce a neurotoxin called domoic acid, which causes intoxication in humans who have consumed contaminated shellfish. This genus was present in almost all stations, including several unidentified species, with a maximum abundance of 5.5 × 104 cell·L-1 recorded in Payucan in May. Pseudo-nitzschia blooms on the Campeche coasts have occurred in 2005, 2008, 2011, 2017, and 2018, generally from June to September and from October to January, with abundance values ranging from 6.0 × 103 to 6.4 × 105 cell·L-1 (Poot-Delgado et al. 2021b). These records agree with what was shown in the present study, although our data are insufficient to define a monthly pattern.
In the southern coastal zone of the Gulf of Mexico, the dinoflagellate B. quinquecornis has been frequently observed in blooms and is widely distributed (Pérez-Morales et al. 2015, Aké-Castillo and Poot-Delgado 2016). Given its innocuousness for human health, it has not warranted a monitoring program by the Mexican Ministry of Health. Indeed, the production of toxins or harmful compounds has not been reported in B. quinquecornis, but like other non-toxic microalgae, it is still harmful to natural marine resources (Biswas et al. 2014). For example, in high densities, B. quinquecornis obstructs fish gills, causing death by suffocation (Alkawri et al. 2016). Furthermore, when a population of this microalgae dies, their remains decompose as they sink, oxidizing and depleting dissolved oxygen in the water column (Gárate-Lizárraga and Muñetón-Gómez 2008). These processes promote hypoxia and anoxia, resulting in the deaths of marine organisms during these events (Anderson et al. 2002). Therefore, B. quinquecornis poses a potential risk to the quality of recreational beaches.
An aspect that deserves particular attention is the presence of species that may produce the phycotoxins reported in this study (see Table 4). These species were observed in low abundance, ranging from 102 to 105 cell·L-1. However, this does not preclude the potential occurrence of toxic HABs. In this regard, there is evidence of the presence of 2 of the most powerful paralyzing toxins (Saxitoxin [STX] and Neo-Saxitoxin [NeoSTX]), which are similar to those described in some wild isolates of P. bahamense from other latitudes or cultivated strains (Núñez-Vázquez et al. 2022). In previous studies, Poot-Delgado et al. (2014) and Poot-Delgado (2016) reported that the abundance of P. bahamense did not exceed 105 cell·L-1. However, given the records of paralyzing marine toxins, ciguatoxins, tetrodotoxin, and analog compounds, as well as domoic acid in fish collected from the Campeche coast (Núñez-Vázquez et al. 2022), the emerging problems and potential risks associated with potentially harmful phytoplankton species that are either rare or occur in low abundance, deserve to be highlighted.
Although human intoxication is mainly related to the ingestion of seafood contaminated with toxins produced by several species of HABs-forming microalgae, intoxication due to direct ingestion of seawater or inhalation cannot be ruled out. The health authorities have no records of such an event to date. However, this may be due to clinical symptoms being masked by other health conditions, leading to a misdiagnosis of intoxicated individuals based on symptoms similar to those of other diseases. This has happened with brevetoxin poisoning at different latitudes on the Atlantic coast, resulting in bias, poor estimates, or underreporting of HABs-related intoxication events (Pérez-Morales and Band-Schmidt 2011 and references therein).
Another indicator of the effect of anthropogenic stressors along the coasts in the study area is the high abundance of cyanobacteria, which has been accompanied by low salinities (Gómez-Figueroa et al. 2023). When coupled with high temperatures (>20 °C), these conditions may favor the growth of cyanobacteria and be closely related to eutrophication processes (Anderson et al. 2002). In addition, some cyanobacteria genera are potentially toxic to humans (Chorus and Bartram 1999), with their presence lowering the quality of recreational beaches.
Cyanobacteria of the genus Anabaena showed abundance values of 104 cell·L-1 in this study and were present in virtually all sampling stations. One of the main characteristics of Anabaena species is the production of chemicals with an earthy and musty odor (geosmin). Another genus of cyanobacteria observed in the present study is Trichodesmium, with values of 104 cell·L-1. Trichodesmium blooms cause skin irritation through direct contact and gastrointestinal disorders if water contaminated with these cyanobacteria is ingested (Wiśniewska et al. 2019), negatively affecting public health. In addition, a very potent neurotoxic chemical was isolated from a mixed culture of Trichodesmium thiebautii and Trichodesmium erythraeum collected from a bloom in the eastern Caribbean (Landsberg 2002). Although Karenia sp. were observed in very low abundance or were absent from most sampling stations, it should be noted that these species are targets in the monitoring programs conducted by health authorities. Indeed, Karenia spp. have been reported in variable abundance in different locations on the coasts of the Gulf of Mexico, from Tamaulipas to Yucatán, causing isolated HABs events (Aké-Castillo 2011, Pérez-Morales and Band-Schmidt 2011, Soto et al. 2012, Merino-Virgilio et al. 2013, Muciño-Márquez et al. 2015, Poot-Delgado et al. 2018).
It should be noted that the working document issued by the health authorities for phytoplankton sampling includes G. catenatum, Pseudo-nitzschia spp., and K. brevis in the list of sentinel species, with maximum allowable limits of 5.0 × 103, 5.0 × 105, and 5.0 × 103 cell·L-1, respectively. These maximum allowable limits for toxic phytoplankton in seawater lack sufficient scientific evidence and do not consider the scientific studies conducted on the coasts of Mexico. In addition, as the potentially harmful phytoplankton and cyanobacteria reported in this study are not listed in the document mentioned above, our findings reinforce the importance of an urgent update of NMX-AA-120-SCFI-2016, which establishes guidelines and the foundations of applicable working documents.
It is important to highlight that during the study period, no changes were observed in the color of seawater in the study beaches, which is probably due to the low cell densities of 102 to 103 cell·L-1, with isolated abundance values of 104 to 106 cell·L-1. This is a problem for health authorities because HABs cannot be visually identified to issue precautionary alerts. Finally, little has been investigated in this region on the effects of bioaerosols of microalgae and cyanobacteria transported by sea breeze, which may be responsible for several public health problems, including allergies, inflammatory responses, skin irritation, burning eyes, rhinitis, and multiple respiratory issues, as have been recorded in various studies conducted in the northern Gulf of Mexico, primarily in Florida in the United States (see the review conducted by Pérez-Morales and Band-Schmidt 2011).











 text in
text in