1. Introduction
Argon plasmas have a wide range of applications in both research and industry. Metal vapour, particularly iron vapour, can penetrate the Argon plasma and alter its properties in a number of these applications. Tungsten inert gas welding, metal inert gas welding, plasma spraying of metallic particles, metal ladle heating, scrap metal heating, and tundish heating are only a few examples of applications1-4.
Thermal plasmas created by plasma torches and electrical arcs are being used in a growing number of industrial applications. This method has numerous applications in metallurgy, the steel industry, and the large field of materials research. The development of these techniques and their improvement require time consuming and expensive practical tests. Thermal plasma radiative transfer is studied in order to develop physical and/or numerical models of these approaches, which are characterized by high temperature energy exchanges. Radiation is therefore an essential term in the energy balance which has to be taken into account in any model5.
N.E.C (Net Emission Coefficient) is useful in determining the amount of radiant energy lost from a plasma. In a number of plasma analyses (see e.g.,6-8). N.E.C are basic representations of the radiative source term, however given the options of completing a full radiative source term analysis9 or using the method of partial characteristics10 N.E.C are useful. Some comparisons of results determined by using N.E.C and other methods are given by J. J. Lowke (1974)11.
An estimated approach of accounting for radiative transmission of energy in thermal plasmas is computationally convenient of N.E.C of radiation. Because the energy balance equation at the arc center is driven by a balance between radiation losses and ohmic heating, these coefficients significantly dictate computed values of central arc temperatures. Even though the method cannot predict the absorption in the plasma regions with steep temperature gradients, it is widely used in computational flow dynamics (see e.g. S. Ailas et al.6 and S. Ailas12).
A. Gleizes et al. 19938 calculate the net emission coefficient using the line escape factor approach (H. W. Drawin and F. Emard 197313) for the stronger lines, while assuming the weaker lines to beoptically thin. Both of these assumptions are good for many conditions, but certain situations exist where inaccuracies can result. A large number of lines are used for all atomic and ionic species. Our study deals with the calculation of the N.E.C for isothermal and homogeneous plasmas, for instance, in the articles by14-17.
To eliminate, B. Liani et al. (1997)18 have studied the evaluation of the N.E.C in total radiation which escapes from a plasma mixture of CH4-H2. Only in this research18, the contribution of the diatomic molecular bands was neglected so that the results are valid for temperature T ≥ 5000 K.
The N.E.C is then calculated in a classical way for thermal plasmas, i.e. considering an isothermal plasma sphere of radius Rp. The results are presented for atmospheric thermal plasmas in a temperature range between 5000 and 30 000 K, and various gas proportions in the mixture.
The first part of this work is devoted to a brief description of the considered chemical species and to the method used to obtain the equilibrium composition of the plasma. The second part presents the calculation of the radiative properties with the N.E.C method, has been devoted to calculate the radiative properties of the plasma including the determination of absorption coefficients of the N.E.C which represents the total power radiated by the plasma. The contributions have been treated separately in the calculation: atomic emission self-absorbed lines and not self-absorbedn lines, continuum (radiative attachment, radiative recombination and radius).
2. Plasma composition
The first step consists in calculating the equilibrium composition of the plasma versus the temperature and the pressure, only the following gaseous species are taken into account: electrons, Ar, He, Ar+, He+, Ar2+, He2+ and Ar3+.
The numerical method used to calculate the equilibrium composition of the plasma is based upon the mass action law and on the basic chemical concept defined by S. Askri et al.19. This law enables the generation of as many equations as there are independent chemical processes existing in a plasma. This law is written as follows for a specific reaction20:
with N the total number of chemical species considered in this reaction, vi the corresponding stoichiometric coefficients, n
i and
where
Finally, the charged species create a Coulombian field, which modifies the plasma’s state by creating an interaction potential. When the population number densities of charged particles are important at high temperatures, this impact is critical. The interactions between neutral particles also indicate a modification of the perfect gas law at low temperatures and pressures higher than atmospheric pressure. Therefore, the Debye−Hückel and the Viriel corrections24 have been considered.
3. Theoretical background for N.E.C in spherical symmetry
The calculation of the N.E.C is based upon simplifying assumptions concerning the plasma geometry, the plasma is supposed to be homogeneous, spherical and isothermal25-27. The local thermodynamic equilibrium hypothesis is also considered. Of course, in most cases, the geometry of a thermal plasma is cylindrical and not spherical but it has been demonstrated that the N.E.C at the centre of a sphere is close to the one obtained on the axis of an infinite cylinder28. The N.E.C is defined by the difference between the power radiated by a unit volume and the radiation proceeding from the other regions of the plasma and absorbed in this unit volume.
For a spherical geometry, the N.E.C (in Wm-3sr-1) is written as follows (more details of the implementation in the N.E.C can be found in29,30):
where λ is the wavelength, Bv is the Planck function31, Rp is the plasma radius and k’v is the monochromatic absorption coefficient corrected for the effects of induced emission and correlated with the local emission coefficient εv by the Kirchhoff law 31:
The monochromatic absorption coefficient:
where h and kB are the Planck and Boltzmann constants, c is the light velocity and kv is the total absorption coefficient.
3.1. Emission of the continuum
In thermal plasmas, the continuum radiation is produced by four radiative processes6. To calculate the variation of the molecular absorption coefficient with the temperature, it is assumed that the absorption cross section does not depend on temperature. As excited electronic molecular states become populated when the temperature increases, the continuum absorption coefficient is thus underestimated with this simplifying assumption. It is given by12:
where Nmol being the number density of the molecule and σabs indicate photo-absorption cross section12.
3.2. Net emission of lines
The N.E.C of the lines is calculated with the assumption there are two types of lines defined by their self-absorption coefficients. If the transition of the line involves a low level close to the fundamental, it will be considered to be self-absorbed, this term means that the radiation emitted by the atom is reabsorbed in the plasma (photo-excitation mechanism). The others lines will be assumed to be light, crossing the medium without being absorbed (as if it were optically thin) and having a close leakage factor of 1. To make this selection which saves considerable time in calculations, we referred to the energy levels given by the literature18,32.
3.2.1. Net emission of not self-absorbed lines
These lines are low-intensity and poorly absorbed in plasma. This helps to simplify Eq. (3) by removing the terms of self-absorption such as the leakage factor and/or exponential term. Thus the net emission coefficient of lines not self-absorbed is written as follows (more details see the references7):
3.2.2. Net emission of self-absorbed lines
For these lines, the net emission calculation takes into account the absorption effects in plasma through the leakage factor. In general, highly absorbed lines are the resonance lines (transitions leading to the fundamental level). The coefficient net emission of self absorbed lines is therefore obtained by the following relation (more details see the references7):
where Λr(α,τ0) is the leakage factor13 which depends on the optical thickness τ0 center of the line and its total enlargement through the coefficient α.
As mentioned that the total net emission factor is the sum of the three contributions: the net emission due to the atomic continuum, the emission of self-absorbed lines and not self-absorbed lines32:
4. Results and discussions
It should be mentioned for calculating the partition functions of different species, we used values from the literature reference energies: JANAF tables21, Internal partition function of atomic species:33, Internal partition function of diatomic species: JANAF data21 and22, Continuum radiation: NIST database34 and35, Lines radiation: NIST database34 and36-38.
4.1. Equilibrium composition
In Fig. 1, we plot the plasma composition obtained for equilibrium composition of a 50%Ar-50%He mixture as a function of the temperature in the range between 5000 and 30 000 K, shown for four selected pressure values: (a) p = 1 atm, (b) p = 4 atm, (c) p = 10 atm and (d) p = 100 atm. The first phase is characterized by dominance of the species Ar, He in a temperature range ranging from 5000- 15 000 k, with the gradual appearance of species Ar+, He+ and Ar+. Because of their low ionization potential, the species Ar+ are more dominant by relation to the ion He+. When the temperature is between 15 000 - 30 000 K, we note that the elections are the dominant. On the other hand, in the 1c) (i.e. p = 10 atm, for example) we plotted the evolution of densities (higher pressure value), we note that species densities are significantly proportional to plasma pressure.
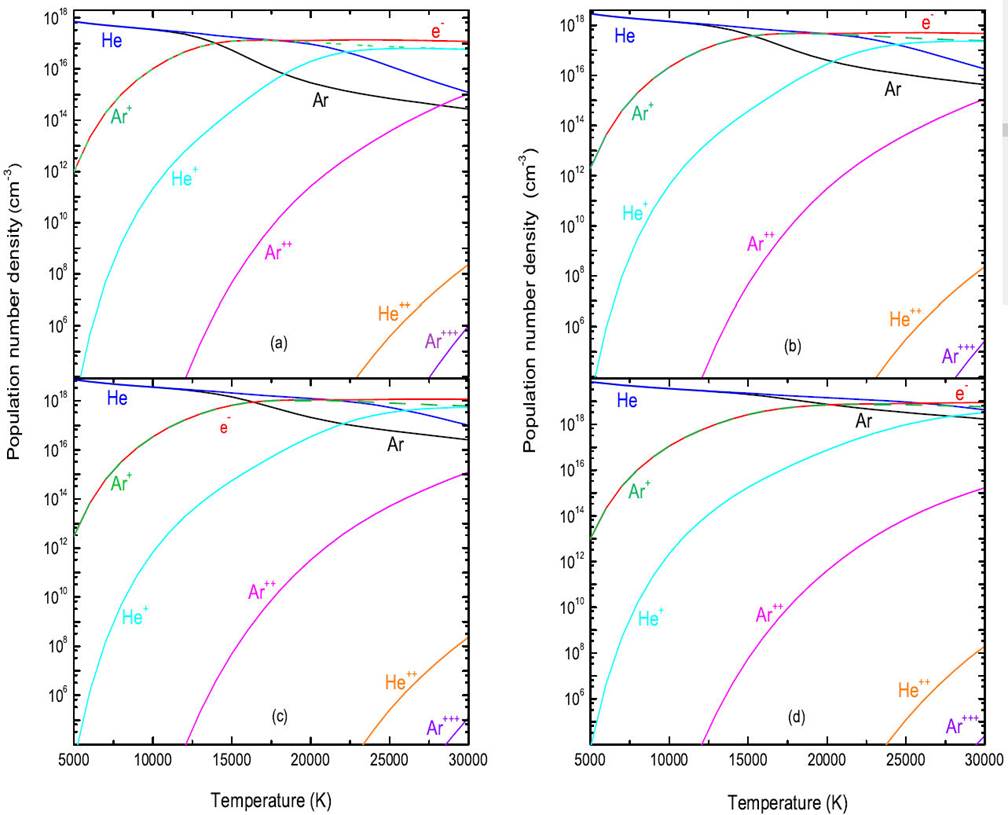
Figure 1 Equilibrium composition of 50%Ar-50%He mixture as a function of the temperature, shown for four selected pressure values: a) p = 1 atm, b) p = 4 atm, c) p = 10 atm and d) p = 100 atm.
By examining these curves, we note the strong presence of Ar up to a temperature close to ∼ 15 000 K, where it begins to decrease, indeed, the species Ar+ and e- become majority at a temperature close to ∼ 15 000 K up to the temperature 30 000 K. It should also be noted that the ion Ar+ ensures the electrical neutrality of the plasma with the electrons in the temperature range 15 000-30 000 K, the Ar2+ species begins to appear from ∼ 12 000 K, the other by the Ar3+ species remains very low in the temperature range considered.
It is clear that the inclusion of the calculation of the composition of a plasma in thermodynamic equilibrium shows that the densities of the species present in the plasma are not independent, they are dependent on temperature and pressure. For the pressure, we can say that the densities of the species are appreciably proportional to the pressure of the plasma, and for the temperature, we see that the density of the atomic species will decrease (slightly) for large temperatures (due to ionization), however, the densities of the charged species will increase.
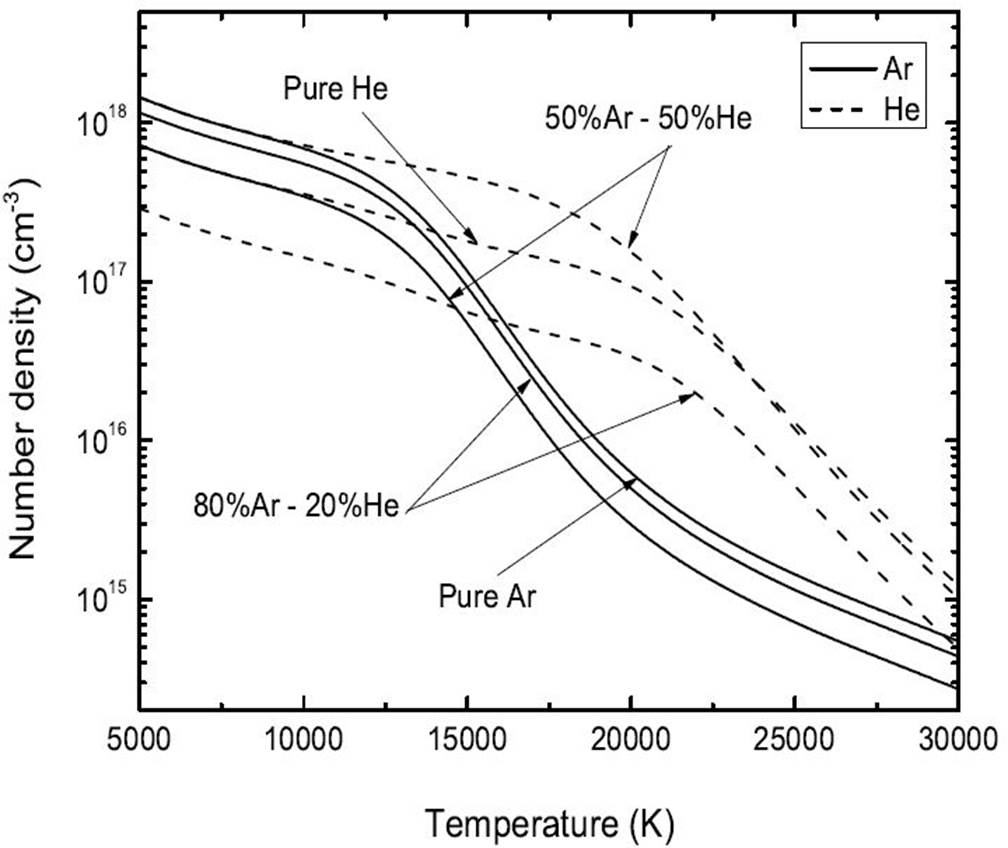
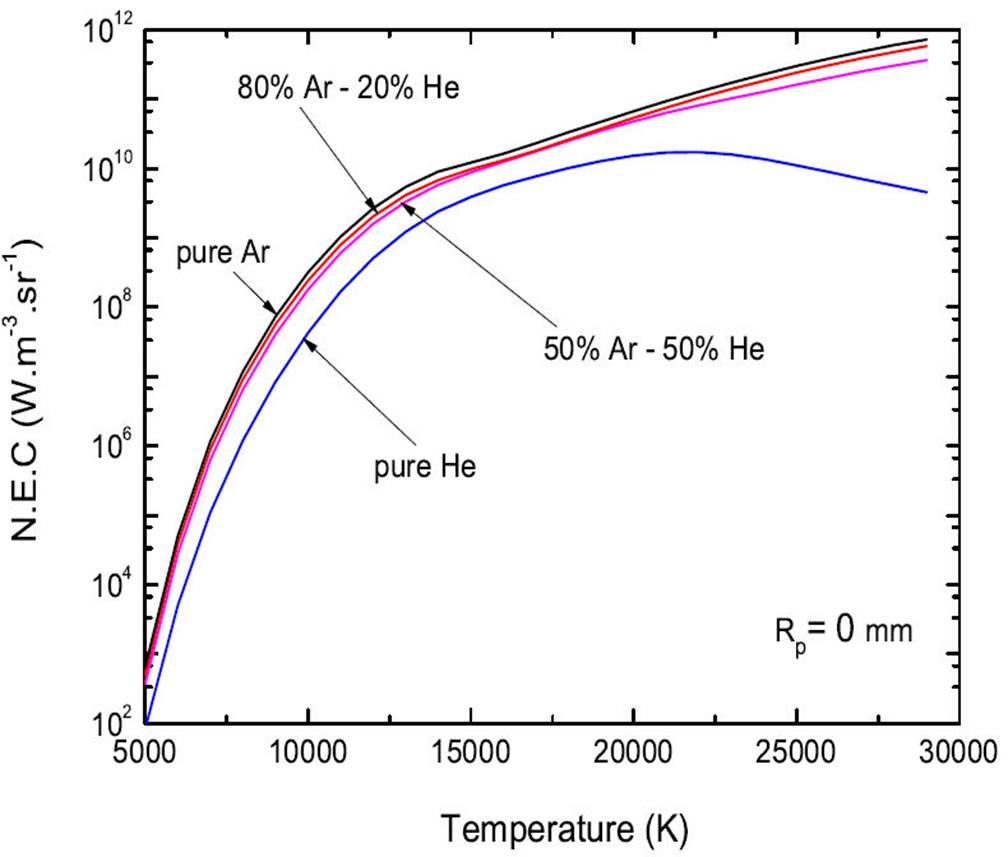
Figure 2 compares the number density obtained in the case of pure plasmas Ar and He and binary mixtures 50%Ar-50%He and 80%Ar−20%He at atmospheric pressure and Rp=0 mm. The ionization energies of Ar and He being close, a slight difference in the number density for these two pure plasmas or mixtures can be noticed in this temperature range. Between 25 000-30 000 K, the number density decreases by some ~ 90% for pure Ar or He plasmas while it decreases by approximately ∼ 17% in the case of a plasma. On the other hand, for temperatures lower than 15 000, the number density for pure He plasmas is largely lower than the number densities for pure Ar. This result is due to the high ionization energy of the neutral He (24.58 eV) in contrast to the ionization energies of Ar (15.76 eV).
4.2. N.E.C of continuum
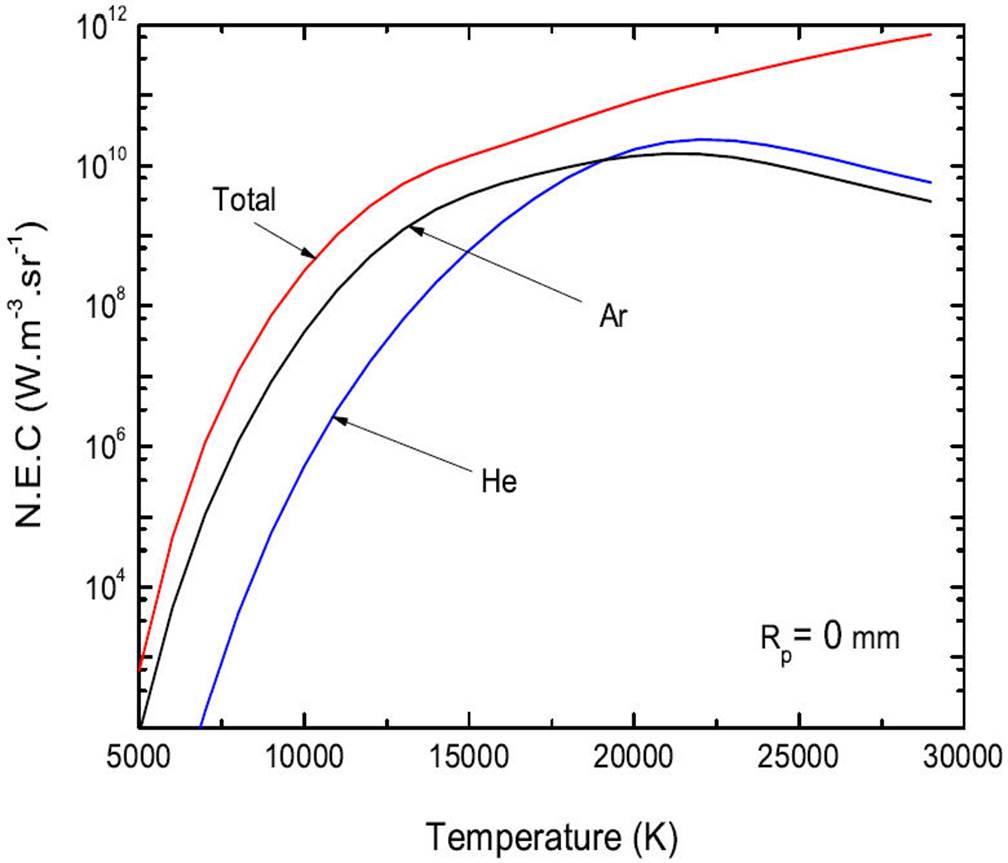
The N.E.C of a plasma of radius Rp at atmospheric pressure and temperature T, is given by expression (3). We calculated the N.E.C of the continuum as a function of the temperature and the thickness of the plasma, the results obtained concerning the 50%Ar-50%He mixture, for plasma thickness Rp = 0 mm are shown in Fig. 3. The contribution to the influence of each of the 2 species is represented there. Rp = 0 mm corresponds to the fictitious case of an optically thin plasma, for which absorption is considered zero. We note that He does not take a large part in the total radiation of the continuum.
It is, therefore, Argon species that mainly contributes to the radiation of the mixture 50%Ar-50%He.
For temperatures T ( 19000 K, Ar is the species that radiates the most. Indeed, the densities of the ions Ar+ and Ar2+ are majority at these temperatures.
4.3 Contribution of lines to the N.E.C
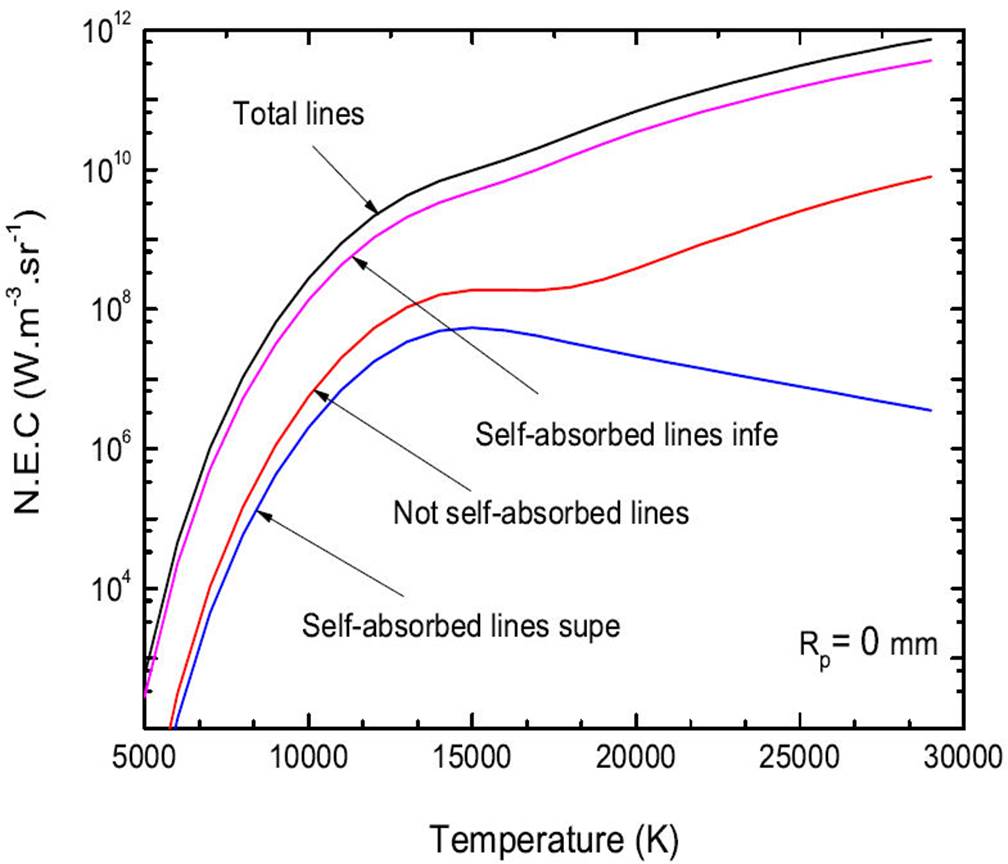
In 4, we show the N.E.C of lines of optically thin plasma of a mixture 50%Ar-50%He at atmospheric pressure and R p = 0 mm. It can be seen from Fig. 4, the total radiation emitted by the lines results from the overlay of the following contributions:
The self-absorbed lines with a wavelength less than 200 nm.
The self-absorbed lines with a wavelength superior than 200 nm.
The not self-absorbed lines.
It can be seen that the self-absorbed lines whose wavelength is lower than 200 nm are the majority given the total radiation emitted by the lines.
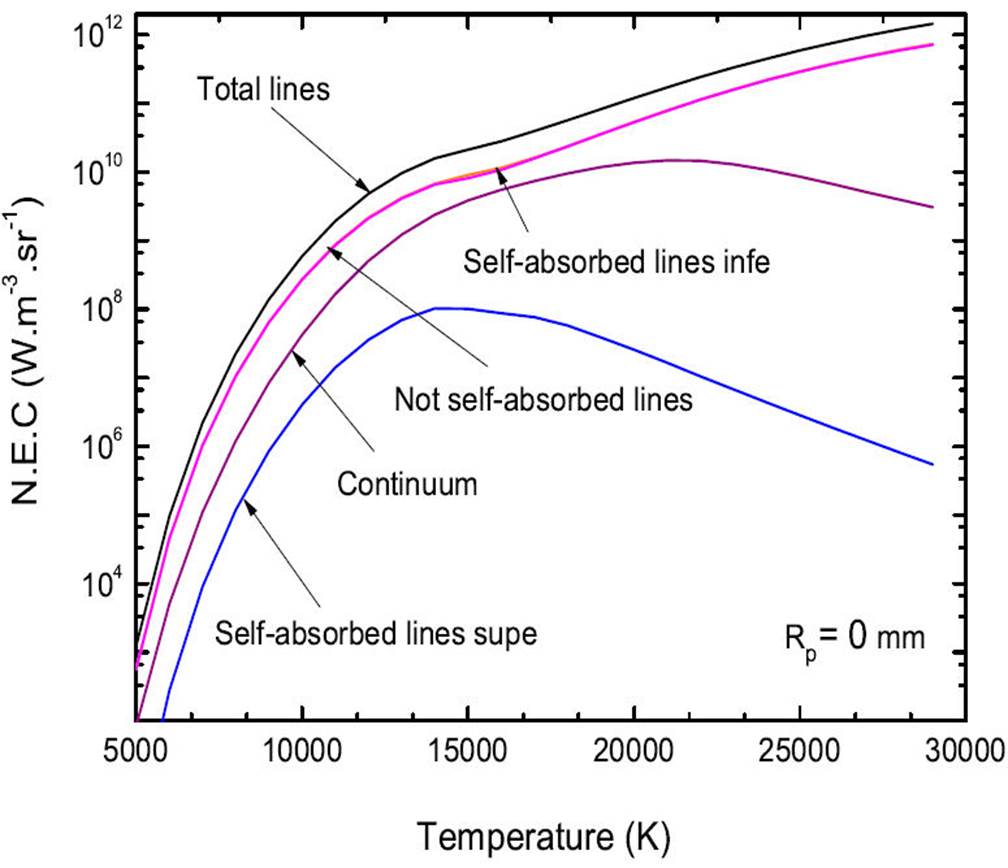
Figure 5 shows the components of the total N.E.C of a optically thin plasma of pure Ar at atmospheric pressure and Rp = 0 mm. The results show that total net radiation from lines and in particular resonance lines (self-absorbed) including the wavelength is less than 200 nm and lines not self-absorbed. This figure allows to better estimate this part of radiation represented by these lines that can reach nearly ( 98% of total radiation at low temperatures (5000 K ≤ T ≤ 14 000 K) and high temperatures (T ≥ 20 000 K). In these temperature intervals, the contribution of the continuum, is therefore negligible. In that region of temperatures the contribution of self-absorbed lines as well as the total lines in the total N.E.C decrease slightly but however, the majority still remains at almost ∼ 87% to 14 000-20 000 K.
4.4. Influence of the proportion in N.E.C
In industrial plasma processes such as plasma spraying or gas−tungsten arc welding, He is widely used with Ar. Ar is used for its high mass density whereas He increases the enthalpy of the plasma. The presence of He also increases the thermal conductivity of the mixture compared with the pure gases and limits the penetration of the surrounding gas. Consequently, the mixture exhibits a higher viscosity due to its high ionization energy. However, He can create a departure from equilibrium because of the high excitation potential of its first excited levels and of its light mass which facilitates the diffusion phenomena.
Figure 6 shows the evolution with the temperature of the net emission coefficient total of 4 ternary plasmas Ar−He composed of: pure Ar, 80%Ar-20%He, 50%Ar-50%He and pure He. We note that as the proportion of He increases (and the proportion of Ar decreases), the values of the N.E.C decrease, ternary plasmas Ar−He. We can deduce that it is because the proportion of Ar decreases as plasma radiation intensity decreases. It is clear that the He only plays a role at very high temperatures (centre of plasma) and for important proportions. This phenomenon can be explained by a higher ionization potential for He than Ar.
4.5 Influence of pressure
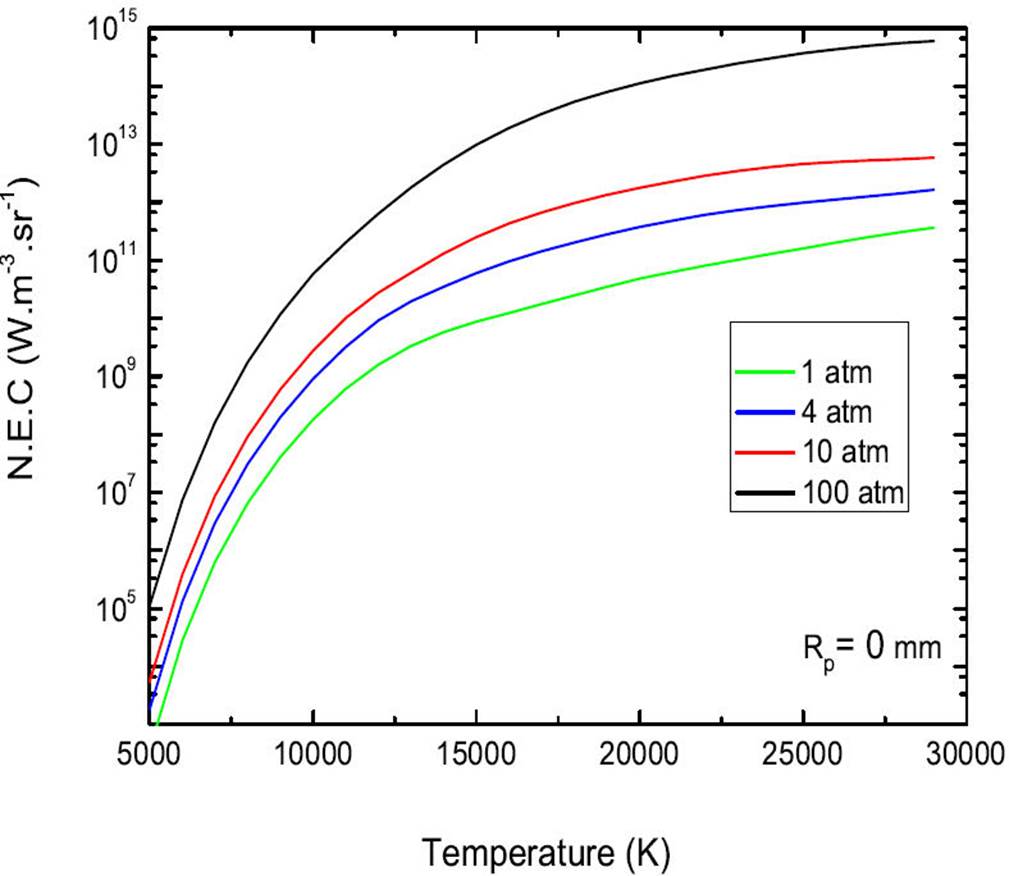
We now proceed to examine the sensitivity of N.E.C to the pressure p. In Fig. 7, we show the N.E.C of a mixture 50%Ar-50%He for a radius and four values of the selected pressures p = 1, 4, 10 and 100 atm. This result has a double interest, show the influence of the pressure on the total N.E.C and show the linearity of this coefficient with it. We note first of all that the increase in pressure increases the net emission factor. The N.E.C depends only on temperature and pressure, and therefore the total density of the species. It therefore seems correct to consider the radiation proportional to the pressure. This result has a large importance to the extent that numerical models not only use also proportional to the pressure. Indeed, the profile pressure could alter the profile of a line and by these results allow us to conclude that the dependence of the N.E.C on the pressure is related not only to increase the total density of the species but also to phenomena of the widening of the lines. It can be seen from Fig. 7, these results highlight the increase in the N.E.C with pressure. Indeed, the main contribution to the N.E.C comes from line emission and the population number densities of the corresponding emitting levels are increased at high pressure. For a given temperature, the population densities of all chemical species are linked to the pressure through the perfect gas law. Thus, it is often assumed that the N.E.C is directly proportional to the pressure.
4.6 Influence of the plasma sizes
In all the results shown previously in Figs 3-7, the plasma radius Rp was taken to be constant at the value of 0 mm. We have investigated the influence of the Rp on the N.E.C.
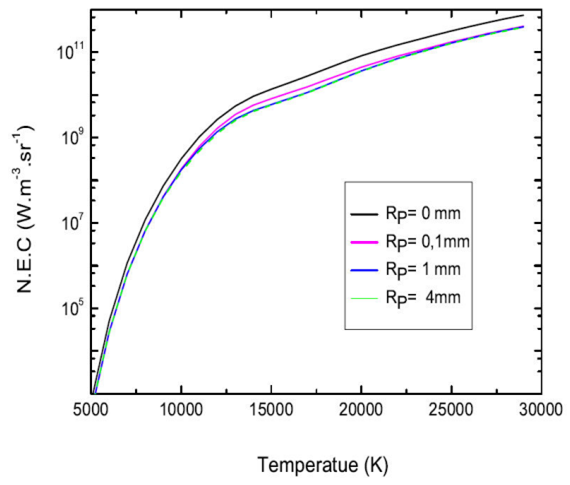
The N.E.C depends not only on the temperature and the plasma radius but also on the pressure. Figure 8 shows the different components of the N.E.C a mixture of 50%Ar-50%He at atmospheric pressure, in an interval of temperature ranging from 5000-30 000 K and for three selected values of plasma radius (i.e. Rp = 0.1 and 4 mm). It is broken down into two phases.
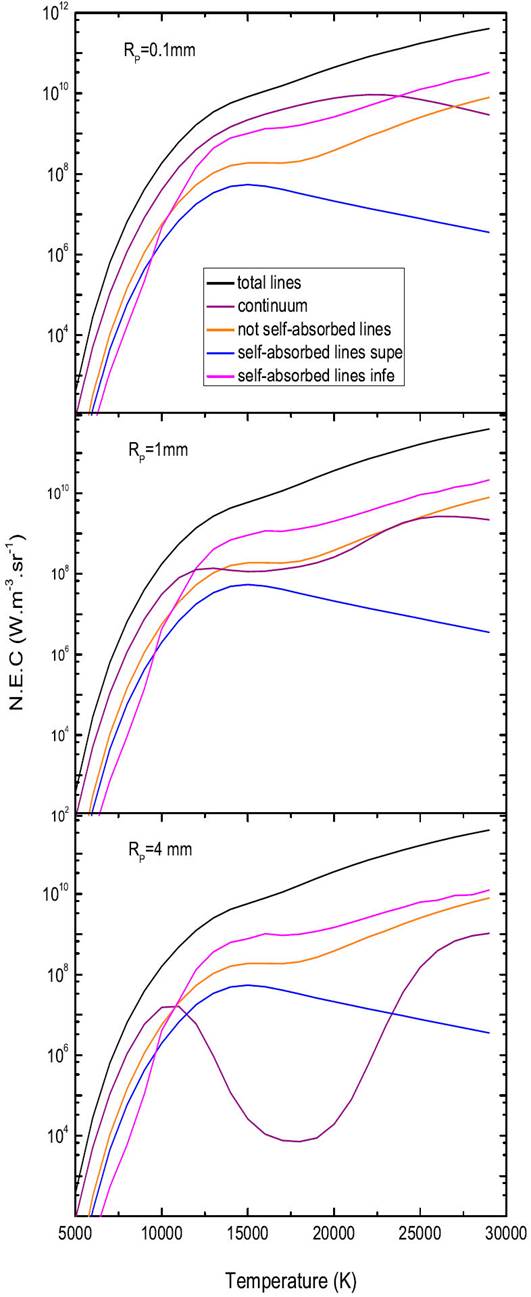
Figure 8 The influence of the plasma radius (Rp =0.1,1 and 4 mm) upon the N.E.C from a mixture 50%Ar-50%He, at atmospheric pressure.
The first go from 5000 K to 10 000 K, the radiation of self-absorbed lines is still almost all the radiation with almost ( 90% of the coefficient total N.E.C for the radius is equal to Rp = 0 mm, the emission of the not self-absorbed lines, the atomic continuum is then negligible on the other hand when the radius increases in radius is equal to Rp = 0.1 mm, Rp = 1mm and Rp = 4 mm the radiation from the continuum is becoming the dominant. The second phase, including the temperatures between 10 000 K and 30 000 K, the contribution of the self-absorbed lines with a wavetongue inside 200 nm remains greater than the continuum. As a result, the self-absorbed lines are no longer the only cause of total radiation. In effect, thus the contribution of the emission of not self-absorbed lines and the atomic continuum make up nearly half of the net emission factor which will be all the more important as the thickness of the plasma will be large. These results provide a better understanding of the mechanisms involved in total radiation, their influence as a function of temperature and the thickness of the plasma, and in particular the importance of the resonance lines and their absorption.
Figure 9 represents the total N.E.C of a composite plasma 50%Ar-50%He at atmospheric pressure, for plasma thicknesses of (Rp = 0 mm, 0.1 mm, 1 mm and 4 mm). We find that at a fixed temperature, the total radiation of the plasma decreases as the ray of the plasma increases, thus reflecting the effect and the importance of the absorption phenomenon that increases with the optical thickness. Most of the radiation is absorbed from the very first millimetres. LHowever, for temperatures below ∼ 12000 K, this total N.E.C varies little with the plasma radius.
5. Conclusions
The objective of this work is to calculate the radiation emitted by a thermal plasma formed by an Ar-He mixture. This radiation results from the superposition of several contributions (continuum and atomic lines). By crossing the plasma, only one fraction of the radiation manages to escape from the environment.
The first part of this paper is devoted to calculating the composition of the plasma at equilibrium which is based on the laws of thermodynamic equilibrium and from the knowledge of the functions of partition. The parameters involved in these calculations are temperature, pressure and the proportions of the mixture.
In the second part of this study, we calculated the radiative properties of established plasmas in a mixture (Ar-He) in a range of temperatures from 5000-30 000 K and for different pressure values. This study was carried out by the N.E.C method which assumes that the plasma is supposed to be homogeneous, spherical and isothermal. The results obtained show that:
For an optically thin Rp, the N.E.C of the lines of a plasma results from the superposition of self-absorbed lines whose wavelength is less than 200 nm, self-absorbed lines with wavelength is greater than 200 nm and the not self−absorbed lines, that note self-absorbed lines with a wavelength of less than 200 nm are majority of the total radiation emitted from the lines.
The emission coefficient of the continuum for low temperatures Ar is more emissive than He. When the temperature increases the species He is the species that radiates the most.
The increase in pressure leads to an increase in the N.E.C, it therefore seems that the radiation is proportional to the pressure.
However, for temperatures below ∼ 12 000 K, this coefficient total N.E.C varies little with the plasma radius.
This result is of great importance as the numerical models not only use the net emission factor to estimate radiative losses which lead to any modelling in plasma (calculation of thermal energy temperature profile). Analysis of the radiation emitted by a plasma also provides some information on this medium (temperature electronics, density of charged particles and degree of ionization).











 text new page (beta)
text new page (beta)