PACS: 88.30.M-; 88.30.pn
1. Introduction
Solid Oxide Fuel Cells (SOFCs) have the potential to become a clean profitable power generating technology in the near future, as they display a high energy conversion efficiency and possible cogenerating and internal reforming properties. Materials for SOFCs are constituted of five key stack components, which are the anode, electrolyte, cathode, interconnectors and seals1,2. While good performances are obtained with the classical anode constituted by YSZ-Ni cermet, performance of the cathode still has to be improved1. In order to optimize this, the cathode must have a high mixed ionic-electronic conductivity under an oxidizing atmosphere, a good chemical compatibility, a thermal expansion coefficient similar to that of the electrolyte, enough porosity to allow gaseous oxygen diffusion through the cathode and the cathode/electrolyte interface, a stable structure during manipulation and operation under an oxidizing atmosphere and high catalytic activity3,4.
A perovskite-type oxide follows the formula ABO3, where cations such as La, Sr, Ca and Ba represent the A-site and Cr, Mn, Ni, Fe and Co occupy the B lattice site for most of the materials used as cathodes. While Sr-doping at the A-site in perovskite materials enhances the ionic conductivity, Co doping on the B-site increases the electronic conductivity1.
One of the most commonly used materials for high temperature (800-1000°C) SOFCs is a composite of manganite-based
The perovskite-type LaNiO3, exhibits a high electronic conductivity at room temperature but is unstable above 850°C. Its performance has been improved by doping it with cobalt. Chiba et al. 6, in a set of experiments with LaNiMO3 [M = Al, Fe, Ga, Cr, Mn, Co], found that cobalt promotes the highest conductivity at 812°C, just followed by iron, while the rest of the elements produced conductivity values of up to two orders of magnitude lower. Cobalt doping has been also reported to improve catalytic reactions, since it alters the electronic structure of perovskites 7.
A decrease in the working temperature of the cells would lead to cost reduction and increase of the materials lifetime4,5,6, but it supposes to find new cathode materials with improved kinetics towards oxygen reduction. In this sense, new compositions and structures of perovskite materials are still promising.
Several methods are used to prepare perovskite materials, some using the solid state route, such as mechanical milling5 and calcination of powders in air 6, or by wet chemistry such as spray-freeze-drying 8, surfactants9, Pechini10, sol-gel11, reverse micelle12, nitrate molten salts13, flash microwave14 and polymerization15. The perovskite lattice can tolerate multiple cation substitution with slight changes, which means that many properties can be modified by obtaining the desired material without provoking significant distortion. The modified Pechini method has several important advantages: homogeneity easily achieved, well-controlled morphology, sub-micrometrical particles and specific composition achieved because the mixing of species occurs at the atomic scale.
2. Experimental
In the present work, LaNi x Co 1-x O3 (x = 0.3, 0.5, 0.7) perovskite ceramic, synthesized by sol-gel modified Pechini method, as a cathode material for SOFCs operating at intermediate temperatures were investigated. In addition, the influence of the cobalt amount on electronic conductivity and the densification of the produced material, plus the reactivity of the mixture cathode/electrolyte, were explored. Additionally, suspensions were formulated into an azeotropic mixture of methylethylketone (MEK) and ethanol (ETOH), two additives such as PVB binder and phthalate plasticizer were used, with Zetasperse 2300 as a dispersant. An YSZ substrate was dip-coated on both sides with LaNi x Co 1-x O3 in order to produce a symmetrical cell in order to determine ASR values in a temperature range from 450 to 750°C.
Three compositions of LaNi
x
Co
1-x
O3 (x: 0.3; 0.5 and 0.7) were synthesized via a polymeric route. This method follows a procedure similar to that reported by Pechini9. Reagent grade metal nitrates of La(NO3)3 6H2O, Ni(NO3)3 6H2O and Co(NO3)26H2O were mixed and dissolved in deionized water. Chelating and polymeric agents such as hexamethylenetetramine (HMTA), acetylacetone (Acac) and acetic acid were mixed in the appropriate molar ratio with the cations to obtain the solution. The ratio of HMTA to metal ions was 3 while the HMTA/Acac ratio was 1:1. The resulting solution was maintained at 65°C for 10 min under constant magnetic stirring. A mix was formed and then heat-treated at 300°C for 1 h, resulting in precursory amorphous powders. These materials were calcined at 1000°C for 5 h at a heating rate of 5°C/min. LNC37, LNC55 and LNC73 were used as labels for LaNi0.3Co2.7O3, LaNi0.5Co0.5O3 and LaNi0.7Co0.3O3 respectively. Amorphous precursors were characterized by high temperature X-ray diffraction (HT-XRD) on a Bruker D8 advanced diffractometer, equipped with an Anton Paar HTK 1200 N high temperature chamber and a Vantec rapid detector (Cu K
3. Results and discussion
3.1 Phase analysis
According to the HT-XRD results (Fig. 1), an amorphous material was observed from room temperature to 350°C with crystallization starting above 350°C. Whereas peaks corresponding to the perovskite were evidenced from the beginning of crystallization for LNC37 and LNC55, an intermediate domain was observed for LNC73 with crystallization of the perovskite only above 525°C. The HT-XRD shows small peaks at 350°C corresponding to the residue, after the combustion of organics and nitrates, plus the formation of an intermediate phase of the perovskite. Thermogravimetric analysis was reported by Ivanova et al. 17, in the case of LaNi0.5Co0.5O3, where an intermediate product formed at 350-400°C, a spinel and a carbonate (La2O2CO3) were identified, which is in agreement with our HT-XRD results.
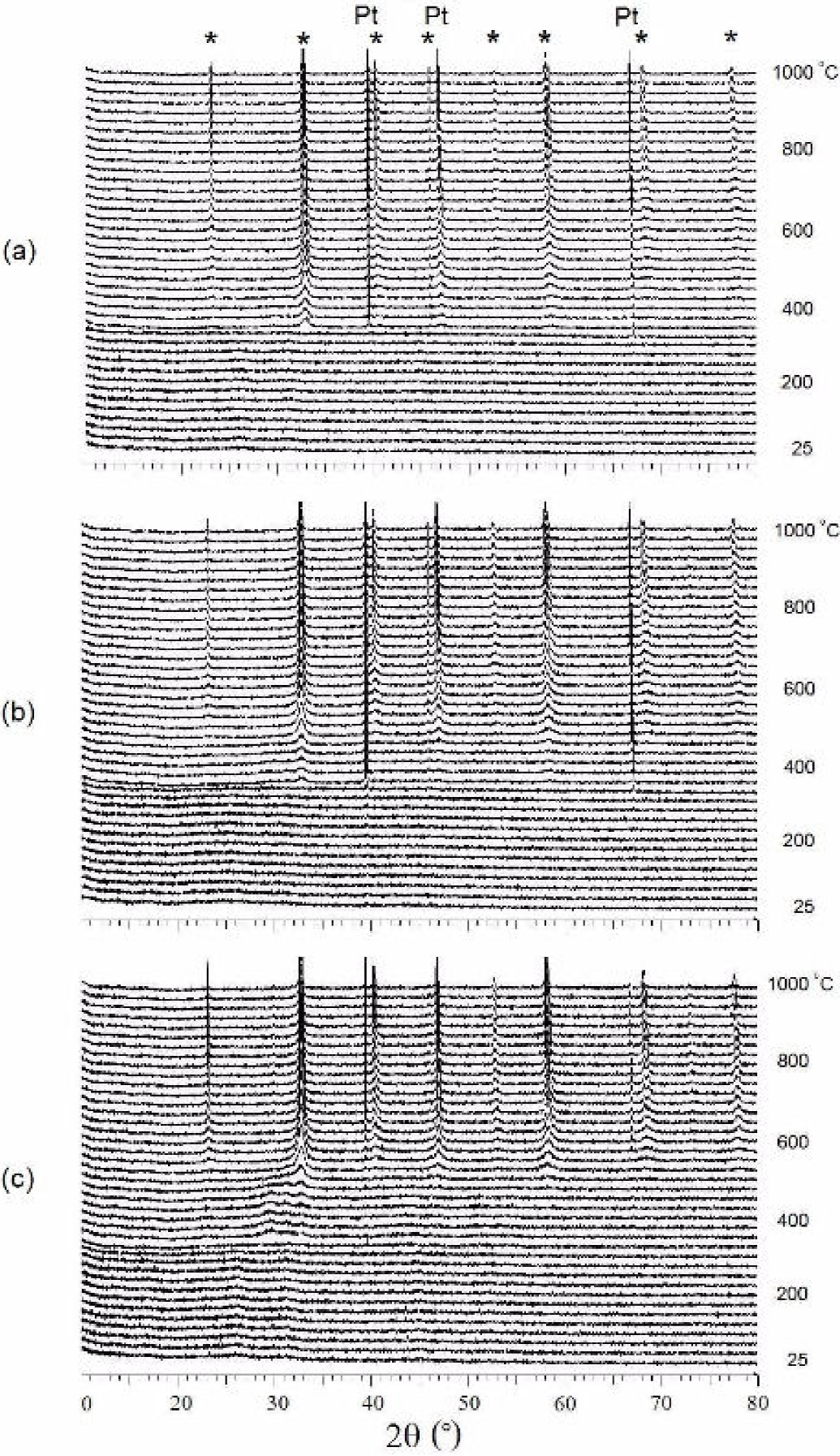
Figure 1. High-temperature XRD showing the crystallization profiles of the perovskite (*) in (a) LNC37, (b) LNC55 & (c) LNC73.
Unit cell parameters were refined in an R
In all of the cases, the perovskite structure is obtained after 600°C, where a well-defined XRD peak can be identified around that temperature, after which the structure becomes crystalline, gradually forming the final structure at 1000°C. One advantage of this method is the time taken to reach crystallization, which is only 5 h or less to convert the amorphous powder into a full crystalline material. In comparison, solid state procedures require a longer amount of time at the same temperature to obtain the phase in a pure state or at least with enough order to be fully detected.
It can be inferred that the method may have influenced the structure and properties, as reported before for other materials15. In brief, it is well known that the cathode performance depends on its microstructural and morphological properties, for which reason these features must be well controlled and understood.
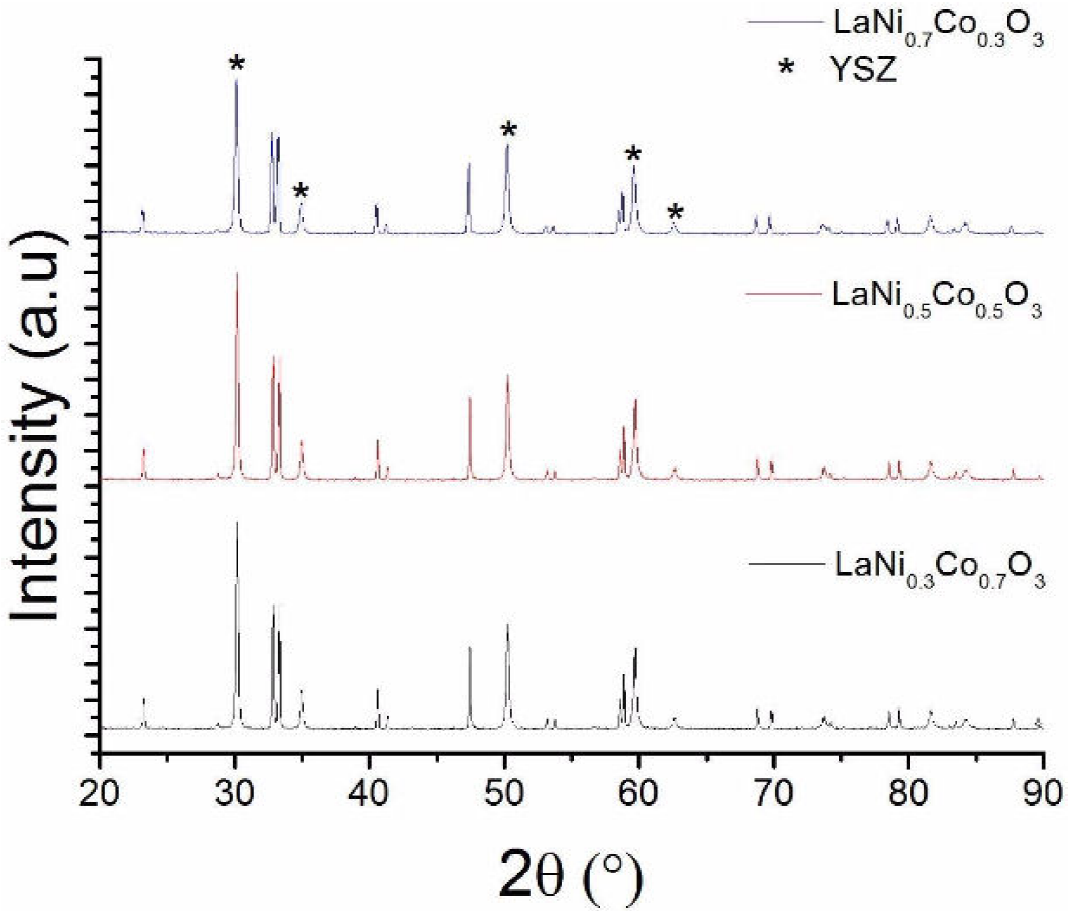
Preliminary stability tests were made by mixing the materials (LCN) with YSZ, then firing the mixture at 1000°C for 5 h, well above the typical SOFC working temperatures, which are around 700°C. For the three cathode materials, well-defined peaks corresponding to YSZ and the perovskite material can be observed in Fig. 2, where no impurity peaks are discerned in the spectrum, which suggests that no reaction appears at the interface and proves that these materials produced by the polymeric route have a good potential for SOFC cathodes. Of course, further studies are needed to judge on the feasibility of these new cathodes interacting with different electrolytes such as gadolinium doped ceria (GDC).
3.2 Morphology
Figure 3 show SEM micrographs of the calcined samples at 1000°C. In Fig. 3 (a-c), a mixture of YSZ and the cathode materials (50%/50% weight) are shown, while the pure perovskites are shown in Figs. 3 (d-f). Figure 3 (a-c) show fine particles
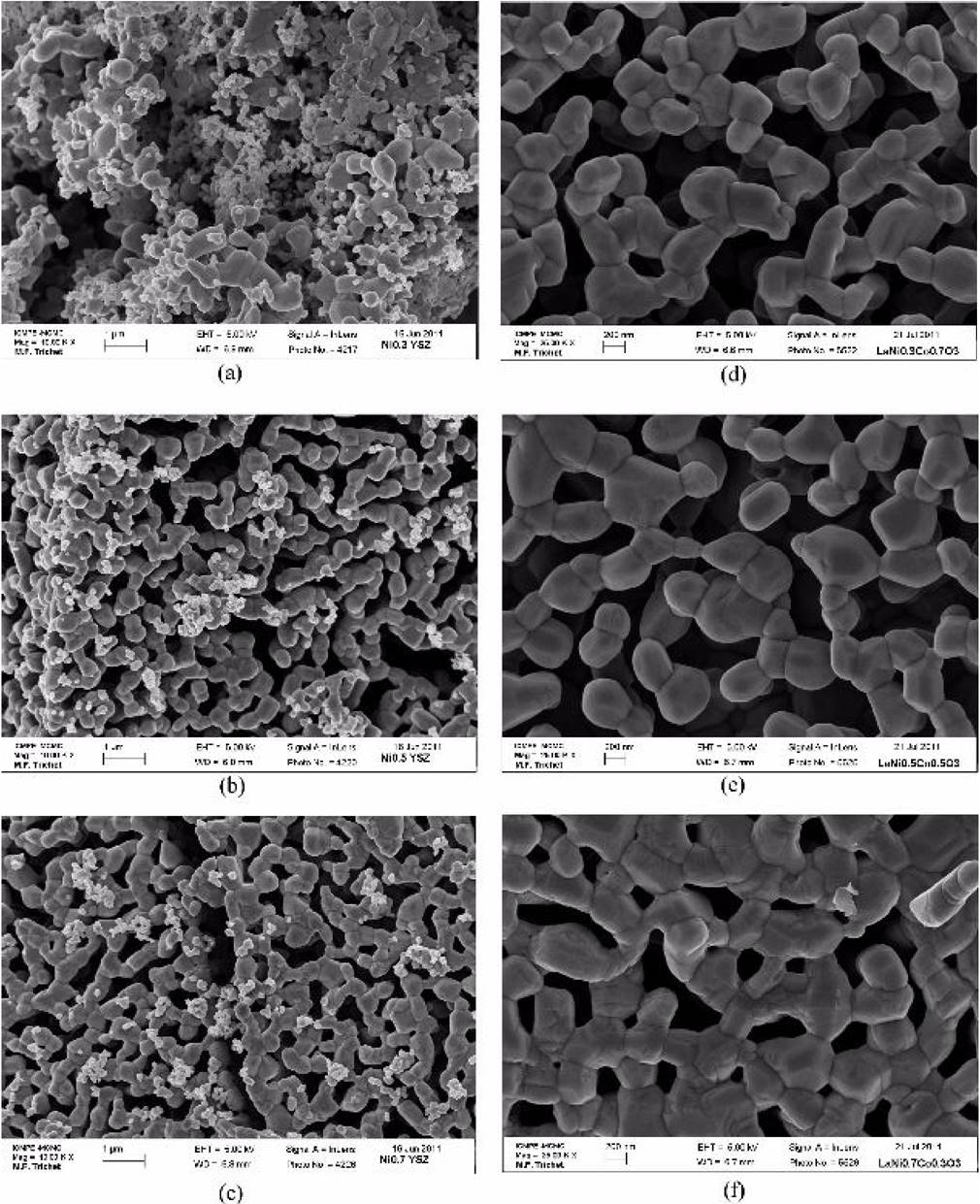
Figure 3. SEM micrographs showing the mixture of YSZ/LaNi x Co 1-x O3, where x = 3, 5, 7 in (a), (b) and (c) respectively and the crystallized LaNi x Co 1-x O3 material, where x = 3, 5, 7 in (d), (e) and (f) respectively
Given that the electrochemical reaction can only occur at the triple-phase boundaries (TPBs), area is an important factor for reaction rate, therefore, it is more probable that LNC37 and LNC55 will be able to extend the TPB length, due to its open, less dense structure, thus enabling the contact between the perovskite, electrolyte and oxygen anions (O2-), improving the material properties and its feasibility as a new cathode for SOFC.
The particles shown in Fig. 3(d), with the highest proportion of Co, are more likely to be spherical in shape, having the smallest particle size (307 nm), while the smaller amount of Co produces significant faceting of the surface (grains) with a polygonal shape, creating a denser, more sintered structure with coarser grains (432 nm). Therefore, finer powders give rise to larger specific areas at the cathode/electrolyte interface and, consequently, a larger amount of triple phase boundary points.
LNC37 has the smallest grain size of 307 nm, in comparison with LNC55 showing a particle size of 416 nm, has a higher number of planar faces and appears to be in an intermediate stage of sintering. Even when the material is totally crystallized, the volume of the structure can be reduced by increasing the heating time at 1000°C, allowing the grains to grow through grain boundary diffusion. Further studies will be made using a full dense single phase material, which may have poor oxygen flow due to the lack of porosity, but with the probability of higher mechanical resistance. When conductive paths become clearer (LNC55 and LNC73), as the conductive particles are closer to each other, as shown in Fig. 3, then conductivity improves.
3.3 Area-specific resistance analysis
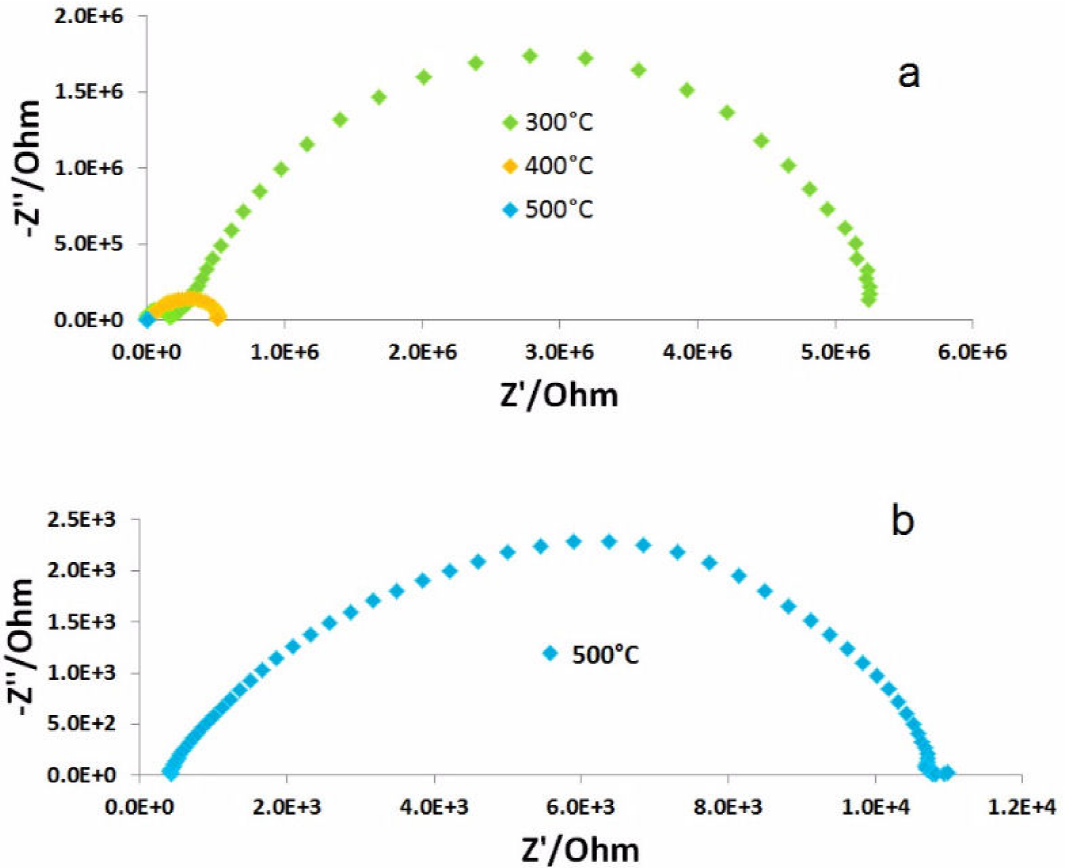
Area-specific resistance (ASR) as a function of temperature was obtained for each composition to determine the materials performance as electrode. All samples exhibit the same behavior, with resistivity decreasing with temperature, as shown in Fig. 4.
Intermediate work temperatures in the range of 800 to 500°C are considered, the ASR results at 762°C for the LNC73, LCN55 and LNC37 were 2.35
4. Conclusions
A cathode material with different compositions of LaNixCo
1-x
O3 (x =0.3, 0.5, 0.7) was synthesized without phase contamination. The material crystallization began at 350°C according to high-temperature XRD which also confirmed the presence of pure compounds after sintering at 1000°C. The influence of composition on the structure was investigated; finding out that LNC55 has an open structure with rounded grains and is likely to have a better performance when used as a SOFC cathode, as shown by the ASR results. LNC55 has an intermediate structure showing the possibility of controlling the microstructure through the composition of the material, opening the possibility to design a material with the desired properties. According to our results, the importance and flexibility of the modified Pechini method is evidenced, and it can be used to design and produce cathode materials. The ASR performance of the materials in the range from 500 to 800°C for each compound showed that LNC55 got the lowest resistance (102.07











 text new page (beta)
text new page (beta)