INTRODUCTION
Sandy beaches are among the most extensive coastal environments in Brazil. In addition to their ecological importance, sandy beaches provide essential ecosystem services (Short and Klein 2016). Approximately 40% of the Brazilian coast is found in the northeastern region of the country. Narrow beaches, particularly those located between sandstone reefs that form strands parallel to the coast, are prevalent in the eastern region of Brazil, which comprises the area to the east of Rio Grande do Norte, Paraiba, Pernambuco, and Alagoas (Zacagnini-Amaral et al. 2016). These reefs protect the coast from highly dynamic coastal energy and increase landscape heterogeneity, which supports high biodiversity. However, sandy beaches are vulnerable due to the impacts of urban, industrial, and port development and tourism, which impose stress on Brazilian beaches while providing essential sources of income for many cities (Schlacher et al. 2007).
Given the crucial roles meiofaunal communities play in marine benthic food chains and their ecological characteristics, the meiofauna present in sandy beaches can provide information that reflects the early signs of environmental change (Moens et al. 2013, Schratzberger and Ingels 2018). Nematodes dominate the meiofauna of most benthic habitats and can reach densities of several million individuals in a square meter (Moens and Vincx 1997). The ecological and biological characteristics of nematodes support their use as bioindicators of environmental change, particularly their tendency to remain present under stressful conditions and their permeable cuticle, which allows these organisms to respond to a wide range of environmental changes that may result in increases or decreases in their abundance (Ferris and Bongers 2006). Despite the high ecological and economic importance of sandy beaches in northeastern Brazil, there is scarce information available on the meiofaunal biodiversity of this region (Fabricio-Maria et al. 2016).
Gaibu Beach, which is located in the state of Pernambuco in northeastern Brazil, was contaminated by a crude oil spill in August 2019. This environmental disaster affected 4,334 km of coastline spanning 11 states in the northeastern and southeastern regions of the country. Indeed, this spill is considered to be one of the worst oil spill disasters in Brazilian history and is among the largest on record in the world (de Araújo et al. 2020, de Santana-Campelo et al. 2021).
The present study aimed to characterize the meiofauna of Gaibu Beach by analyzing the spatial distribution and temporal variation of Nematoda communities and their relationships with granulometry.
MATERIALS AND METHODS
The study area was located on Gaibu Beach (8°19′ S and 34°57′ W), which is found in the state of Pernambuco in northeastern Brazil (Fig. 1). According to the Koppen climate classification, the regional climate can be categorized as Am (humid and tropical with autumn/winter rains; Manso et al. 2006). Gaibu Beach is characterized by large extensions of fringed sandstone reefs and rocky shores (Pereira et al. 2002).
We collected biosedimentological samples in September and December 2014. Above-average rainfall occurred in September 2014 (APAC 2014), although the highest rainfall levels were recorded in May and June 2014 (278.8 and 322.7 mm, respectively) despite being similar to or below those recorded over the last 30 years. Therefore, September was deemed to be “the rainy month” (217.3 mm) in this study, while December was deemed to be “the dry month” (62.0 mm).
We set up 4 fixed and equidistant transects (T1, T2, T3, and T4) that were 100 m apart and perpendicular to the waterline, considering the relevant characteristics of the beach to ensure high homogeneity (Fig. 2). Transect 1 was located in front of beach rocks, and T4 was located where sewage was discharged to the open sea.
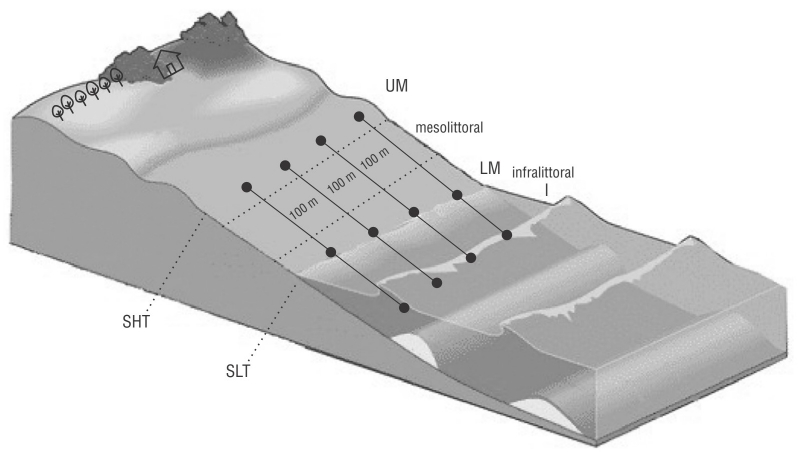
Figure 2 Sampling design: Transects 1-4. Beach zones: upper mesolittoral (UM), lower mesolittoral (LM), and infralittoral (I). SHT: spring high tide; SLT: spring low tide.
Three replicas of each sedimentological sample were collected with a PVC sediment corer (diameter: 2.5 cm2, length: 10 cm; made in the laboratory) without cutting the sediment into layers. The upper mesolittoral, lower mesolittoral, and infralittoral zones were sampled in each transect with the sediment corer during low tide. All samples were fixed in 4% formalin. In total, we collected 48 biosedimentological samples per sampling period (rainy and dry). The same procedure was used to collect granulometry samples, although no replicas were collected nor were these samples fixed.
Sediment grain size was determined from dry sediment samples using sieves with different mesh sizes (2, 1, 500, 250, 125, and 0.063 mm; Suguio 1973). We classified sediments according to the Wentworth scale (Buchanan et al. 1984). The textural parameters were calculated using the equations of Folk and Ward (1957). The data obtained were analyzed with the software SYSGRAN 3.0 (Camargo 2006).
In the laboratory, we extracted the meiofauna from the sediment samples by wet sieving and manual elutriation (Elmgren 1973). The supernatant was passed through 0.044 and 0.5-mm mesh sieves. The meiofauna was identified to the highest taxonomic level possible (order or class) and counted by group using a Dollfus plate and an Olympus stereomicroscope (×10 to ×400). The first 50 nematodes were removed, placed on embryo dishes for diaphanization following the methods of De Grisse (1969), and mounted on permanent glass slides. We identified specimens to the genus level using the pictorial key of Warwick et al. (1998) and the Nemys digital database (Nemys 2022). The feeding type classification was based on those of Wieser (1953, 1960).
For each biosedimentological sample, we calculated faunal density (individuals per 10 cm-2), richness (number of taxa present), and relative abundance (%). After the data were square-root transformed, we used a 2-factor permutational multivariate analysis of variance (PERMANOVA; sampling moment = month; beach zone = zone) to compare communities. We used the Bray-Curtis similarity coefficient of the meiofaunal communities to conduct a nonmetric multidimensional scaling (nMDS) analysis and used the same procedure for the nematode community (mean of the replicas). We used a similarity percentage (SIMPER) routine to evaluate the contribution of each taxon to community dissimilarity (>50%). We performed a BEST-BioEnv analysis using the Spearman correlation coefficient to analyze the influence of granulometry on the meiofaunal distribution. All analyses were performed in Primer v.6.1. (Clarke and Gorley 2006).
RESULTS
In September and December 2014, the sediment varied from fine to medium sand in both the lower mesolittoral and infralittoral zones. The average grain size ranged from 0.18 to 0.83 mm, and there were no significant differences between months (PERMANOVA: t = 1.44; P = 0.08) or beach zones (PERMANOVA: t = 1.03; P = 0.38). The BEST-BioEnv analysis indicated no significant correlations between granulometric characteristics and the meiofaunal (P = 0.55) or nematofaunal (P = 0.07) communities. However, the BEST-BVSTEP analysis revealed significant correlations between the meiofaunal communities and granulometric parameters (Rho value = 0.958; P = 0.01), although the same was not observed for nematofaunal communities (Rho value = 0.455; P = 0.94).
No meiofauna was present in the samples collected during December (dry month) in the upper mesolittoral zone nor in half of the samples collected in September (rainy month). The PERMANOVA indicated that a significant difference (Global R = 0.31, P = 0.007) was present between the upper mesolittoral zone and the other 2 beach zones (lower mesolittoral and infralittoral), which formed a group. The lower mesolittoral and infralittoral zones were later reanalyzed excluding the upper mesolittoral zone.
After removing the upper mesolittoral samples, the PERMANOVA revealed a significant difference in meiofaunal communities between sampling periods (rainy and dry months; t = 2.16; P = 0.002). This result can be seen in the nMDS analysis (Fig. 3), although no significant difference between the communities of the lower mesolittoral and infralittoral zones was present (t = 1.18; P = 0.178). The PERMANOVA also indicated a significant difference in nematofaunal communities between the dry and rainy months (t = 1.89; P = 0.001; Fig. 4), although no significant difference between the communities of the lower mesolittoral and infralittoral beach zones was present (t =1.19; P = 0.204).
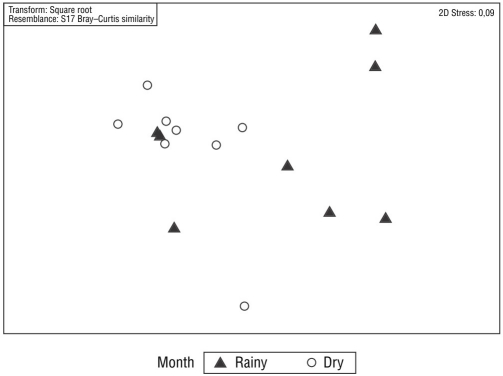
Figure 3 Multidimensional scaling (MDS) analysis of meiofaunal communities of Gaibu Beach per sampling period.
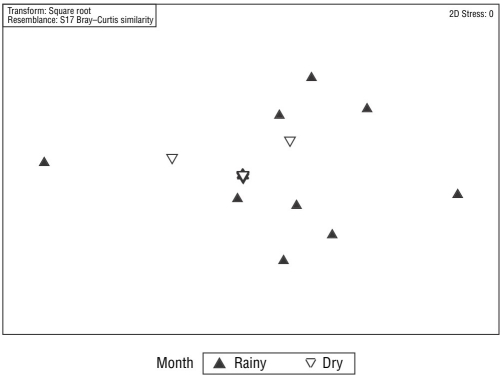
Figure 4 Multidimensional scaling (MDS) analysis of nematode communities of Gaibu Beach per sampling period.
The mean meiofaunal density ranged from 19.27 to 527.86 ind.·10 cm-2 in the rainy month of September and 13.8 to 1,556.77 ind.·10 cm-2 in the dry month of December. The meiofauna comprised 8 taxa and 6 phyla: Nematoda, Arthropoda (Copepoda and Ostracoda), Tardigrada, Annelida (Oligochaeta and Polychaeta), Platyhelminthes (Turbellaria), and Mollusca (Bivalvia). Community richness based on these taxa varied from 3 to 5 groups in September and 3 to 7 groups in December (Fig. 5). Nematoda was the dominant taxa in all samples (Fig. 6). The SIMPER analysis showed that Nematoda and Copepoda were responsible for over 60% of the dissimilarity between communities (Table 1).
Table 1 Similarity percentage analysis of the meiofaunal community of Gaibu Beach. Av. Diss. = average dissimilarity; Av. Abund. = average abundance; Diss. = dissimilarity; SD = standard deviation; Contrib. = contribution; Cum. = cumulative.
| Rainy and dry groups | ||||||
| Av. Diss. = 68.63 | ||||||
| Rainy group | Dry group | |||||
| Species | Av. Abund | Av. Abund | Av. Diss. | Diss./SD | Contrib.% | Cum. % |
| Nematoda | 7.21 | 17.50 | 29.28 | 1.44 | 42.66 | 42.66 |
| Copepoda | 3.84 | 8.35 | 12.04 | 1.04 | 17.54 | 60.21 |
| Tardigrada | 3.14 | 0.68 | 7.29 | 0.63 | 10.62 | 70.83 |
| Turbellaria | 0.44 | 2.46 | 5.30 | 1.14 | 7.73 | 78.55 |
| Kinorhyncha | 2.08 | 0.00 | 4.79 | 0.51 | 6.98 | 85.53 |
| Oligochaeta | 1.96 | 1.01 | 4.15 | 1.03 | 6.04 | 91.57 |
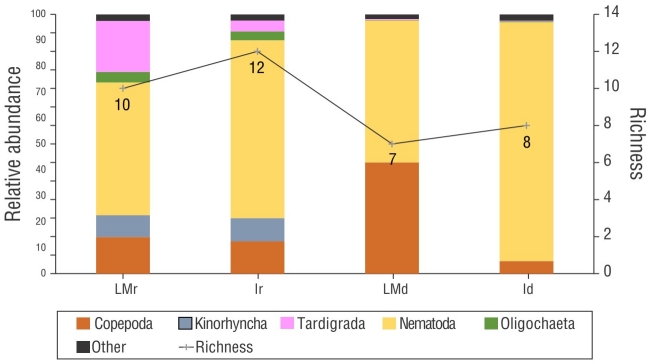
Figure 5 Richness and relative abundance of meiofaunal communities of Gaibu Beach in 2 sampling periods (September and December 2014). LMr = lower mesolittoral in the rainy month. Ir = infralittoral in the rainy month. LMd = lower mesolittoral in the dry month. Id = infralittoral in the rainy month.
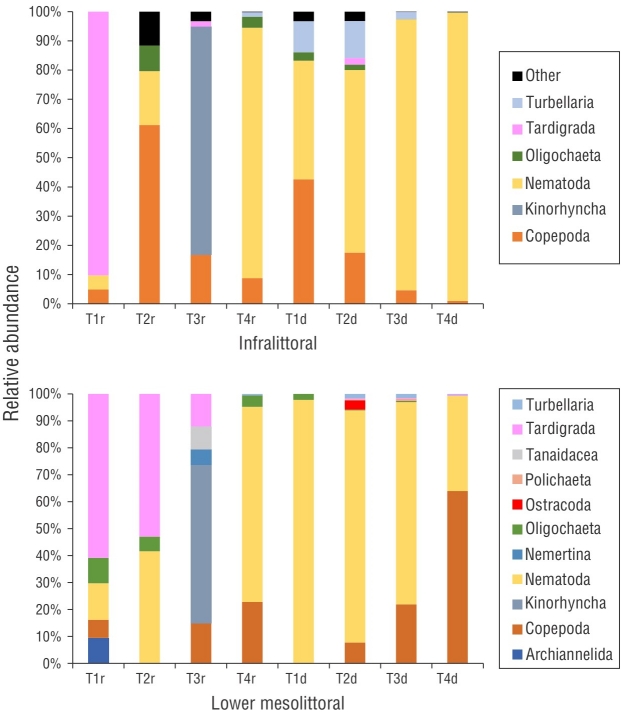
Figure 6 (a) Relative abundance of meiofauna taxa of the infralittoral zone; (b) relative abundance of the meiofauna taxa of the lower mesolittoral zone. T = transect; r = rainy month (September); d = dry month (December).
The nematofaunal density of Gaibu Beach ranged between 1.04 to 443.49 ind.·10 cm-2 in September and 13.22 to 1,296.09 ind.·10 cm-2 in December. The nematofauna was composed of 5 orders, 16 families, and 45 genera (Table 2). Within the 16 families identified, Xyalidae showed the highest number of genera (8), followed by Comesomatidae and Thoracostomopsidae (5 each).
Table 2 Nematoda genera recorded on Gaibu Beach (Pernambuco, Brazil).
| Order | Family | Genus |
| Chromadorida Chitwood, 1933 | Chromadoridae Filipjev, 1917 | Neochromadora Micoletzky, 1924 |
| Actinonema, Cobb, 1920 | ||
| Endeolophos Boucher, 1976 | ||
| Cyatholaimidae Filipjev, 1918 | Marylynnia (Hopper, 1972) Hopper, 1977 | |
| Paracanthonchus Micoletzky, 1924 | ||
| Paracyatholaimus Micoletzky, 1922 | ||
| Pomponema Cobb, 1917 | ||
| Selachinematidae Cobb, 1915 | Gammanema Cobb, 1920 | |
| Latronema Wieser, 1954 | ||
| Synonchiella Cobb, 1933 | ||
| Araeolaimida Coninck& Schuurmans Stekhoven, 1933 | Axonolaimidae Filipjev, 1918 | Axonolaimus De Man, 1889 |
| Odontophora Bütschli, 1874 | ||
| Synodontium Cobb, 1920 | ||
| Comesomatidae Filipjev, 1918 | Comesoma Bastian, 1865 | |
| Paracomesoma SchuurmansStekhoven, 1950 | ||
| Hopperia Vitiello, 1969 | ||
| Paramesonchium Hopper, 1967 | ||
| Sabatieria Rouville, 1903 | ||
| Desmodorida De Coninck, 1965 | Desmodoridae Filipjev, 1922 | Molgolaimus Ditlevsen, 1921 |
| Metachromadora Filipjev, 1918 | ||
| Polysigma Cobb, 1920 | ||
| Epsilonematidae Steiner, 1927 | Epsilonema Steiner, 1927 | |
| Microlaimidae Micoletzky, 1922 | Bolbolaimus Cobb, 1920 | |
| Microlaimus De Man, 1880 | ||
| Monoposthiidae Filipjev, 1934 | Monoposthia De Man, 1889 | |
| Monhysterida Filipjev, 1929 | Xyalidae Chitwood, 111951 | Cobbia De Man, 1907 |
| Daptonema Cobb, 1920 | ||
| Paramonhystera Steiner, 1916 | ||
| Promonhystera Wieser, 1956 | ||
| Pseudosteineria Wieser, 1956 | ||
| Rhynchonema Cobb, 1920 | ||
| Theristus Bastian, 1865 | ||
| Xyala Cobb, 1920 | ||
| Linhomoeidae Filipjev, 1922 | Eleutherolaimus Filipjev, 1922 | |
| Terschellingia De Man, 1888 | ||
| Enoplida Filipjev, 1929 | Thoracostomopsidae Filipjev, 1927 | Enoploides Ssaweljev, 1912 |
| Enoplolaimus De Man, 1893 | ||
| Epacanthion Wieser, 1953 | ||
| Mesacanthion Filipjev, 1927 | ||
| Paramesacanthion Wieser, 1953 | ||
| Ironidae de Man, 1876 | Thalassironus de Man, 1889 | |
| Oxystominidae Chitwood, 1935 | Halalimus de Man, 1888 | |
| Enchelidiidae Filipjev, 1918 | Eurystomina Filipjev, 1921 | |
| Oncholaimidae Filipjev, 1916 | Metoncholaimus Filipjev, 1918 | |
| Oncholaimus Dujardin, 1845 |
In September, the most abundant nematofaunal genera in the lower mesolittoral zone were Daptonema Cobb, 1920 (27%) and Theristus Bastian, 1865 (20%). The most abundant genera in the infralittoral zone were Bolbolaimus (21%) and Theristus (19%). In December, Mesacanthion dominated the lower mesolittoral zone (30%) along with the notable presence of Theristus and Neochromadora Micoletzky, 1924. In the lower mesolittoral zone, Theristus and Neochromadora comprised 17% of the community, whereas in the infralittoral zone, Theristus and Neochromadora comprised 23% and 35% of the community, respectively (Fig. 7). The SIMPER analysis indicated that Theristus; Neochromadora; Metachromadora Filipjev, 1918; Daptonema; and Eleutherolaimus Filipjev, 1922 were responsible for over 50% of the dissimilarities between communities (Table 3).
Table 3 Similarity percentage analysis of the nematofaunal community of Gaibu Beach between the rainy (September) and dry (December) months. Av. Diss. = average dissimilarity; Av. Abund. = average abundance; Av. Sim. = average similarity; Diss. = dissimilarity; SD = standard deviation; Contrib. = Contribution; Cum. = cumulative.
| Rainy (September) and Dry (December) Groups | ||||||
| Av. Diss. = 84.28 | ||||||
| Rainy group | Dry group | |||||
| Species | Av. Abund | Av. Abund. | Av. Sim. | Diss./SD | Contrib. % | Cum.% |
| Theristus | 1.10 | 2.83 | 13.35 | 1.15 | 15.85 | 15.85 |
| Neochromadora | 0.83 | 1.83 | 10.06 | 0.76 | 11.93 | 27.78 |
| Metachromadora | 0.04 | 1.93 | 7.97 | 0.98 | 9.46 | 37.24 |
| Daptonema | 1.11 | 1.08 | 6.76 | 1.00 | 8.03 | 45.26 |
| Eleutherolaimus | 0.44 | 1.04 | 4.68 | 1.23 | 5.55 | 50.81 |
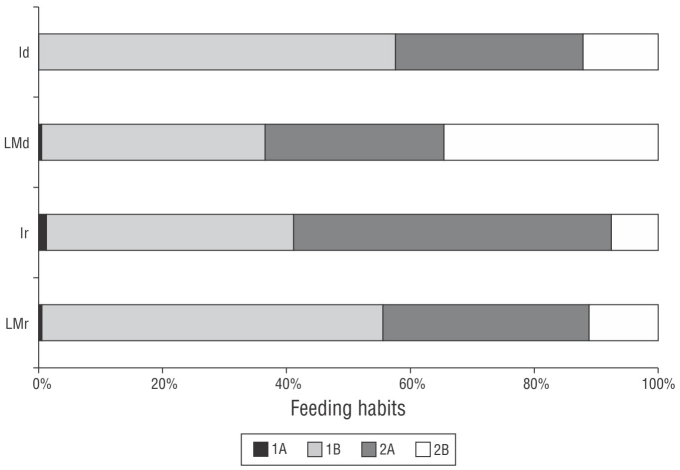
Figure 7 Trophic structure of the nematofaunal community of Gaibu Beach in 2 sampling periods (September and December 2014). LMr = lower mesolittoral in the rainy month. Ir = infralittoral in the rainy month. LMd = lower mesolittoral in the dry month. Id = infralittoral in the dry month. 1A: selective deposit feeders; 1B: nonselective deposit feeders; 2A: epistrate feeders; 2B: predators/omnivorous.
The trophic structure of the nematofaunal community included 4 feeding groups. A co-dominance (>50%) of nonselective deposit feeders (1B) was observed in the infralittoral zone in December and in the lower mesolittoral zone in September along with epistrate feeders (2A, >50%) in the infralittoral zone in September. The predators/omnivores (2B) feeding group were most abundant in the lower mesolittoral zone in December. Selective deposit feeders (1A) were present in the infralittoral zone in September but absent from this zone in December (Fig. 8).
DISCUSSION
Despite the environmental heterogeneity of the study area, meiofauna were absent in most mesolittoral samples. The disordered utilization of Gaibu Beach by visitors, sellers, and constructions observed during sampling trips suggests that anthropogenic factors may have contributed to the absence of a meiofaunal community in the upper mesolittoral zone of the study area. As has been reported in previous studies, vehicle movements and intense trampling, especially in the upper littoral and supralittoral zones, interferes with species life cycles and may even result in species being lost from the community (dos Santos-Reis and Rizzo 2019, Santos et al. 2021).
The meiofauna of Gaibu Beach followed the quantitative patterns that have been described in other tropical sandy beaches on the northeastern coast of Pernambuco (Castro et al. 1999, Souza-Santos et al. 2003, Pinto and Santos 2006, Guilherme et al. 2016). The meiofaunal densities in this study were similar to those found in Tamandaré Beach (Souza-Santos et al. 2003) and Guadalupe Beach (Guilherme et al. 2016), which are also located on the southern coast of Pernambuco. However, the meiofaunal densities in this study were lower than those found in other sites on the southern (Castro et al. 1999) and northern coasts of Pernambuco (Pinto and Santos 2006), which are relatively isolated, moderately conserved, and associated with estuarine environments, which may influence their sedimentary characteristics and consequently the densities of their meiofaunal communities.
The meiofaunal density of Gaibu Beach reported in this study agrees with what was described by Kotwicki et al. (2005), who expected densities of 538 ± 137 ind·10 cm-2 for tropical sandy beaches. Sewage discharge that flows directly into the sea near T4 may be causing eutrophication in the area (Bongers and Ferris 1999), which intensifies algae growth and biota proliferation and consequently may increase nematode density (Moreno et al. 2011).
The relationships between meiofauna and granulometric characteristics are well known, so much so that these topics are often studied together. However, these relationships are not the only things that determine the structure of the nematofaunal community, and other variables beyond granulometric characteristics can be strong drivers of community structure. In this study, the absence of significant correlations between the nematofaunal community and granulometric characteristics suggests that other factors affect the structure of these benthic communities. Indeed, Fonseca et al. (2014) did not find any significant correlations between nematofaunal richness and sedimentary characteristics and concluded that other factors interact with granulometric characteristics to determine community structure.
The time period considered in this study included a change from rainy to dry seasons, and significant differences in meiofaunal communities were found between seasons. In the rainy month of September, meiofaunal richness and density were lower than those in the dry month of December. These findings agree with what has been reported in other studies. For example, Alongi (1990) and Souza-Santos et al. (2003) indicated that during the rainy season, when salinity decreases, superficial sediment erosion can reduce meiofaunal density.
Nematoda and Copepoda were the main taxa responsible for the dissimilarities between meiofaunal communities. According to Coull (2009), Nematoda is the most abundant group in sediments and constitutes 60-90% of meiofaunal communities, followed by Copepoda, which constitutes 10-40% of these communities, and thus the dominance of these taxa in this study was expected. The high abundance of Tardigrada in our study during the rainy month may have resulted from the presence of beach rocks on T1, which likely modulated the hydrodynamic characteristics of the transect. Tardigrades constitute a permanent component of the meiofaunal community and have also been found to be abundant in sediments in other studies (Albuquerque et al. 2007, Verçosa et al. 2009).
Among the 16 nematode families identified in this study, Xyalidae was the most abundant and diverse, which agrees with what has been reported for the northeastern Brazilian coast (Venekey et al. 2010). Theristus was dominant in the rainy and dry months and contributed the most in the beach zones to the observed temporal variation in the structure of the meiofaunal community according to the SIMPER analysis. Fonseca et al. (2014) stated that Theristus showed a broad distribution that was correlated with average grain size. The high abundance of Daptonema in this study agrees with what has been previously reported indicating that this genus is commonly an abundant nematofaunal taxa present in various environments, especially in sandy beaches (Pereira-Gomes de Melo et al. 2013) with well-selected sands (Moreno et al. 2008) or fine sand (Corbisier et al. 2008).
Four trophic types were identified in both sampling periods, although selective deposit feeders constituted less than 2% of the meiofaunal community. In contrast, nonselective deposit feeders dominated the community followed by epistrate feeders, which agrees with what was reported by Pereira-Gomes de Melo et al. (2013). The high representativeness of nonselective deposit feeders on Gaibu Beach agrees with what was reported by Alongi (1990), who stated that this group is dominant during warm months due to the elevated number of bacteria, protozoa, and microphytobenthos present. In this study, the highest abundance of nonselective deposit feeders on Gaibu Beach occurred in the dry month of December. Although we did not measure organic matter content in the sediment, the dominance of nonselective deposit feeders suggests that the sediments in the study area have high organic loads (Danovaro and Gambi 2002).
The low representativeness of predatory animals may be related to the types of sediment found on Gaibu Beach. According to Gallucci et al. (2005), these predators increase in dominance in environments with relatively large grain sizes and consequently relatively high permeability. Furthermore, anthropogenic impacts on the beach can also play important roles in determining the structure of meiofaunal communities, as nematode predators are quite sensitive to environmental disturbance (Bongers and Ferris 1999).
Abiotic data, such as salinity, organic matter, and chlorophyll a measurements, were not collected in this study, as we aimed to characterize the meiofaunal community of Gaibu Beach and its relationship to the sediment present. Thus, additional studies and environmental impact assessments are needed to evaluate the interactions among the main environmental stressors present on Gaibu Beach and their influence on the meiofaunal community in both the short- and long-term. Braga et al. (2003) consider beach degradation due to mining, sewage pollution, coastal plain flooding, and damage to protected areas to be the main environmental problems present in the area. These problems were observed during our study and should be evaluated based on the environmental quality indicators for recreational beaches of Barbosa de Araújo and Costa (2008), specifically considering the indicators of domestic effluents, bathing areas protected by reefs, urban expansion, and the use of tides for leisure activities.
In conclusion, seasonal variation was present in the meiofaunal community of Gaibu Beach. Nematoda were dominant in the dry month of December, while a more diverse meiofaunal community was present in the rainy month of September with a dominance of other taxa. Differences were also present between communities among transects (in the same beach zone and season), which suggests that various abiotic drivers affect community structure beyond granulometric characteristics. Further studies of the biodiversity of the meiofauna present on the sandy beaches in northeastern Brazil are urgently needed due to the insufficient data available for these environments and the extreme anthropogenic and natural stress these beaches are currently under.











 texto en
texto en 





