Introduction
The castor bean (Ricinus communis L.), is a plant that belongs to the family Euphorbiaceae (Bauddh et al., 2015). This plant can adapt to a wide variety of climatic conditions. The seeds of castor bean are considered toxic to animals and humans due to the presence of the ricin toxin (Vinayaka et al., 2017).
Recently castor bean crop has been considered as a potential energy crop due to its high non-edible oil content (40 %) (Melo et al., 2008). The oil obtained from the seed of R. communis, is mainly composed of triglyceride and is one of the few naturally pure glycerides due to the fatty acid portion contains almost 900 g kg-1 of ricinoleic acid. The oil is rich in a single hydroxyl fatty acid, ricinoleic acid C18H34O 3 structurally known as cis-12-hydroxyoctadeca-9-enoic acid, hydroxylated fatty acid of 18 carbons having a double bond. The presence of ricinoleic acid gives castor oil its unique properties and unusual versatility. Castor oil differs from other oils because of its high acetyl or hydroxyl value, with an iodine value, viscosity and specific gravity comparable to other oils (Bhuiya et al., 2016).
Recently, ultrasound technology has emerged as a novel tool in food engineering processes (Bhaskaracharya et al., 2009) being this field of research where it has been most studied. High frequency and low frequency ultrasound has been applied successfully to improve the process and/or products (Carcel et al., 2007), maceration (Carcel et al., 2007), microbial and enzymatic inactivation (Vercet et al., 2001), extraction of natural products (Vilkhu et al., 2008), food cutting operations (Arnold et al., 2009), sugar substitution processes (García-Noguera et al., 2010), fermentation (Riener et al., 2010) and among others. The application of ultrasound has been widely studied to extract oil compounds from plant materials (Willems et al., 2008), including almond powder (Riera et al., 2004), and seeds of Copaifera langsdorfii (Stupp et al., 2008), Maclura pomifera and pomegranate seed oil (Goula, 2012). However, this process is complex and its effectiveness depends on many factors, for example, temperature, time, frequency, type of solvent and the solid/liquid ratio. Most studies has focused on the effects of parameters on extraction rate and yield (Vilkhu et al., 2008). The success of ultrasound extraction has been attributed to the propagation of ultrasonic pressure waves and resultant cavitation forces, where the bubbles can explosively collapse and generate localized pressure that causes the breakdown of plant tissue and improves the release of intracellular substances in the solvent (Knorr et al., 2002). According to Vilkhu et al. (2008), the implosion of the cavitation bubbles generates macro-turbulence, collisions between high velocity particles and perturbation in microporous particles of the biomass, which accelerates the diffusion of the eddies and the internal diffusion. Therefore, the objective of this work was to determine the best conditions of ultrasound-assisted extraction of oil from Ricinus communis L. seed.
Material and methods
Ricinus communis L. seed
The R. communis seeds were provided by the Universidad Autónoma Agraria Antonio Narro, where the husk was removed manually of these.
Definition of evaluation parameters
The Soxhlet system by best oil extraction conditions was used, as a conventional reference process in which 3 parameters were evaluated (Pretreatment of the seed, Extraction time, the type of solvent used). In Table 1 is shown the variable used in each parameter.
Table 1 Variables used for each parameter
| Parámetro | |||
| Pretratamiento de la semilla | Sin pretratamiento | Lavado con agua y secado a 50 ºC por 24 h en una estufa Thermo Scientific™ HERAtherm™ | Lavado con agua y secado a 50 ºC por 24 h en una estufa Thermo Scientific™ HERAtherm™ posteriormente las semillas fueron cortadas con un cortador casero dejando tamaños de partícula de aproximadamente 1-2 mm |
| Tiempo de extracción | 2, 4, 6, 8 y 10 h | ||
| Tipo de solvente utilizado | Hexano | Isopropanol | Mezcla hexano:Isoprpanol 1:1 |
Soxhlet extraction process
The extractions were carried out in round bottom flasks of 125 mL coupled to the Soxhlet extraction system in a water bath (IKA® HB10) with temperature control. At each type extraction, 5 g of sample and 50 mL of solvent (ratio 1:10 m/v) were added to each flask and maintained at a range temperature of 63-65 °C for 4 h.
Ultrasound-assisted extraction process
The assisted ultrasound oil extraction was carried out using a ultrasonic bath (Zenitron Model TS-200) coupled with a Soxhlet extraction system (as mentioned in section above). The optimum pretreatment and solvent ratio obtained in the previous stage were applied on this stage, varying only the extraction time: 0.5, 1.0, 1.5, 2.0 and 2.5 h. In all cases, at the end of each experiment the mixture was filtered, and oil was recovered in a rotary evaporator (Yamato WATER BATH BM 400 brand) heating above the boiling point of the solvent used. The extraction yields were determined by weight difference.
Analysis of results
All data obtained in the experiment was adjusted to the mathematical model . The results obtained allowed to compared the conventional and ultrasound-assisted extractions for each solvents and times.
Where:
Y= oil yield
Y inf = oil yield at infinite time (maximum yield)
k = oil extraction speed
y = extraction time.
All experiments were carried out by triplicate and the average values were reported. A variance analysis (ANOVA) was performed and Fisher F test (p <0.05) was used a Minitab® software version 17 (Minitab Inc., State College, PA, USA).
Results and Discussion
Figure 1 shows the results obtained during the evaluation stage, the maximum yield (1.54 g of oil/5 g of seed) was obtained in the pretreatment T2, followed by T1 with 1.42 g of oil/5 g of seed and T3 with obtained the lowest yield with 1.27 g of oil/5 g of seed. This behavior is attributed to the fact that in T2 the seeds were free of moisture compared to the T1 control, this may indicate that the presence of moisture in the seed matrix causes the oil extraction yield to decrease. The low efficiency of pretreatment T3 is attributed to the loss of oil during cutting of the seed. Perea-Flores et al. (2011) mentioned that in the microstructure of the seed body of Ricinus communis L. it is highly porous, which favors the extraction of the oil (Perea-Flores et al., 2011) during the pressing or cutting of the seed, which It was evidenced with the low yield of oil extraction.
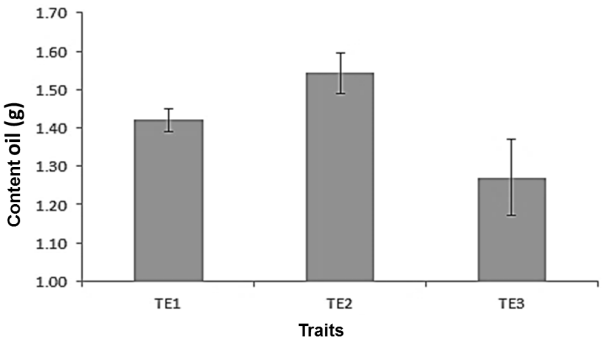
Figure 1 Influence of the pretreatment of the castor bean seed (Ricinus communis L.) for the extraction of its oil, T1: seed without pretreatment; T2: seed washed and dried at 50 ° C for 24 h; T3: seed washed, dried and cut.
An adjustment of the mathematical model was made to analyze the kinetics and make a comparison between conventional and ultrasound-assisted extractions with each of the solvents at different times.
Where:
Y= oil yield
Y inf = oil yield at infinite time (maximum yield)
k = oil extraction speed
y = extraction time.
According to the results with the adjustment to the model (Figure 2) is shown the comparison of the yields obtained during the processes of extraction of oil assisted by ultrasound and by the conventional method, where the best solvent for oil extractions by the conventional method with Soxhlet was the mixture (hexane/isopropanol, ratio 1: 1) obtaining a maximum yield at infinite time of 1.5 g of oil in 5 g of seed, while the extraction with hexane and isopropanol obtained a maximum yield was 1.3 g of oil in 5 g of seed.
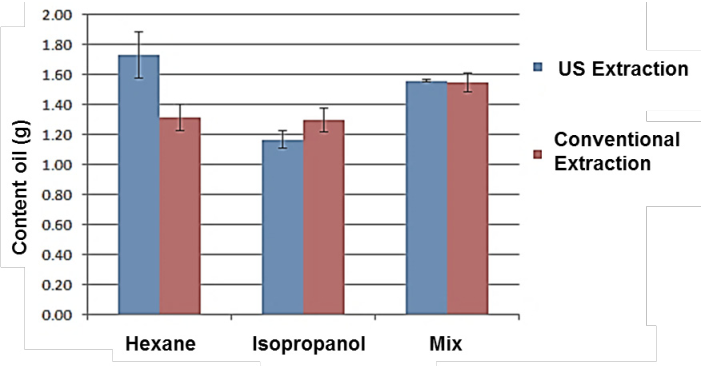
Figure 2 Comparison of maximum yields Y inf infinite time of conventional extraction and ultrasound-assisted (US).
On the other hand, the obtained results from the adjustment by ultrasound-assisted extractions are showed in Figure 2, where best yields value are obtained using hexane (1.7 g 73.5%) and the solvent mixture (1.5 g) as solvent, 73.5%), these results are higher than those reported by Perdomo et al. (2013) with an maximum efficiency of 56% using the conventional extraction method with Soxhlet for oil extraction from R. communis. seed. In Figure 3 the initial rates of both processes are compared, and the solvent that showed a higher initial extraction rate was hexane reaching 0.58 g.h-1, followed by isopropanol with an initial rate of 0.5 g.h-1 and the mixture showed a lower rate with a 0.49 g.h-1, which indicates that the initial extraction rates are not affected by the type of solvent when a conventional extraction is performed, on the other hand higher extraction rates were obtained when the process was assisted by ultrasound, with a rate of 3.7 g.h-1 with isopropanol, a rate of 1.8 g.h-1 when using the mixture and a rate of 0.79 g.h-1 with hexane.
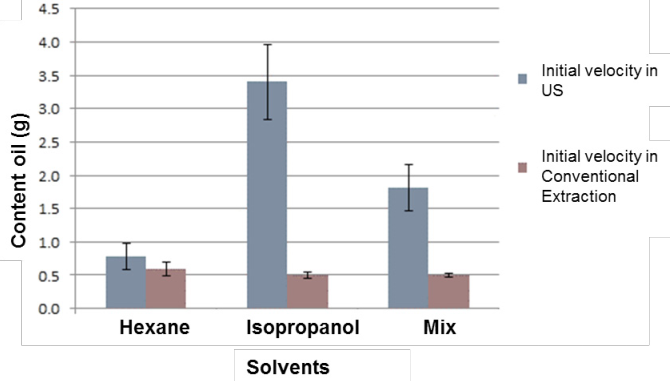
Figure 3 Comparison of initial speeds k in conventional extraction and ultrasound-assisted extraction (US).
The comparison and analysis of the results obtained from the extraction with hexane and the mixture (Figure 3) for the extraction rates with ultrasound inferred that the best treatment is the mixture of solvents in both cases since the oil yield between these two is not significant, but when comparing the rate bars in Figure 3 a positive effect is observed when the mixture of solvents is used, since it exceeds twice the rate obtained with hexane. The results obtained indicate that when extraction with ultrasound is carried out, the solvent directly influences the initial extraction rate, with a 3 fold as in the case of the mixture compared with the assistance of the ultrasound in contrast when only Soxhlet system was used, where the type of solvent did not influence the initial rate. The difference in speed shown during the treatment with ultrasound can be due to the molecular structure and chemical properties of the solvents (Akowuah et al., 2005), since hexane is a molecule of larger size than isopropanol, which takes longer time to penetrate the matrix of the seed and the speed of extraction is slower while isopropanol penetrates more quickly in the matrix by its structure and a faster extraction is made. In the extractions with the conventional method cannot appreciate the difference in speeds because the cavitation that occurs when using ultrasound is not generated, which favors the solvent of smaller structural size and the yields respond to the polarities of the solvents since while hexane is a non-polar compound and isopropanol is a polar compound, extractions are favored when the mixture is used since the triglycerides present in the oil have a polar fraction for the carbonyl group and a fraction non-polar represented by the hydrocarbon chain that makes it up; and both parts dissolve when the mixture of solvents is used, favoring the extraction and obtaining a higher yield.
Conclusions
The results obtained showed that the ultrasound had a positive effect in the oil extraction process of Ricinus communis L. and that the treatment with the solvent mixture (isopropanol/hexane 1: 1, v/v) resulted to be the best option in both methods, obtaining an extraction yield of 73.5% and a rate of 1.8 g.h-1 with ultrasound, which the rate is almost 4 times higher compared to the conventional method that obtained a rate of 0.49 g.h-1 with a yield of 70%, so that the process assisted by ultrasound showed a better results in the extraction process in a lesser time.











 text in
text in 


