Since last December, a number of severe pneumonia cases of a novel coronavirus initially called 2019-nCoV have been identified in Wuhan, China a metropolitan city of more than 11 million people in central-east China, a strategic city in the middle of Beijing in the north, Shanghai in the east, and Hong Kong in the south.1,2
The symptom onset date of the first patient identified was Dec 1st, 2019. None of his family members developed fever or any respiratory symptoms. No epidemiological link was found between this first patient and later cases. The first fatal case, who had continuous exposure to the market, was admitted to hospital because of a seven-day history of fever, cough, and dyspnoea. Five days after illness onset, his wife, a 53 years old woman without any known history of exposure to the market, also was hospitalised with pneumonia.
On December 29, 2019, the initial four cases were reported, all related to the Huanan Seafood Wholesale Market, using a surveillance mechanism for «pneumonia of unknown etiology» that was created in the year 2003, during the wave of the severe acute respiratory syndrome (SARS) outbreak with the aim of making an early diagnosis of new pathogens. On February 11th, 2020, the World Health Organization (WHO) announced a new name for the epidemic disease caused by the new virus 2019-novelCoV: coronavirus disease (COVID-19). Finally the International Committee on Taxonomy of Viruses renamed the previously provisionally named 2019-nCoV as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).3
Four coronavirus are endemic in humans: HKU1, NL63, 229E and OC43, that produce illness in the respiratory tract, gut, liver and central nervous system. It’s name comes from the Latin word «corona» (crown) which in turn comes from de Greek «koróne», due to its microscopic appearance surrounded by protein spikes that simulate a solar corona. It is an enveloped +ssRNA virus that measures between 60 and 140 microns belonging to the order Nidovirales, in the family Coronaviridae, and subfamily Orthocoronavirinae. Coronaviruses in general are enveloped with positive-sense single-stranded RNA, and have the largest genome of all RNA viruses, ranging from 26 to 32 kilobases. Two-thirds of the coronavirus genome at the 5’ terminus encodes viral proteins involved in transcribing viral RNA and replication, while one-third at the 3’ terminus encodes viral structural and group-specific accessory proteins.4 The major proteins in coronaviruses are named as follows: S (spike), E (envelope), M (membrane), and N (nucleocapsid) proteins (Figure 1).
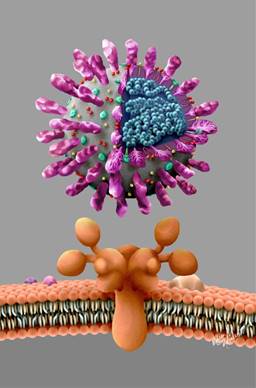
Figure 1: A new coronavirus SARS-CoV-2 model with its respiratory epitelial cell ACEh2 receptor, and its major proteins: S (spike), E (envelope), M (membrane), and N (nucleocapsid) are shown. The surface spike glycoprotein (S-protein) is a key factor in the virulence of coronaviruses, as it is believed to enable it to attach to host epitelial cell shown below. The rights of this Figure belong to the COMMEC, you can reproduce it requesting permission to revistacommec@gmail.com and quoting the source.
For the third time in as many decades, a zoonotic coronavirus has crossed species to infect human populations.5
Given the error-prone characteristic nature of viral RNA-dependent RNA polymerases, viral replication leads to the formation of a quasispecies.6-8 Rather than one virus producing identical progeny during replication, a whole population of viruses is produced, each differing from one another by nucleotide substitutions or deletions as a result of errors incorporated by their RNA polymerase (as a typical RNA virus, the average evolutionary rate for coronaviruses is roughly 10-4 nucleotide substitutions per site per year,9 with mutations arising during every replication cycle). While the majority of these mutations will have neutral or negative effects on viral fitness, a small subset of these mutations and recombinations may probably prove beneficial and enhance the ability for certain variants to replicate despite selective pressures of interest such as the host immune response or an antiviral drug with the consequent adaptation.
Both SARS-CoV and MERS-CoV diseases infect intrapulmonary epithelial cells more than cells of the upper airways.10,11 Consequently, transmission occurs primarily from cases with recognized clinical picture and maybe not that frequent, from people with mild and/or nonspecific signs. It appears that SARS-CoV-2 uses the same cellular receptor as SARS-CoV: the human angiotensin-converting enzyme 2 [hACE2]),12 and not other coronavirus receptors as aminopeptidase N and dipeptidyl peptidase 4,13 despite the presence of amino acid mutations in the SARS-CoV-2 receptor-binding domain.9 The spikes on the surface of the SARS-CoV-2 are glycoproteins composed of two subunits: Subunit S1 binds to ACE-2 on the cell surface; subunit S2 fuses with the cell membrane. Another host enzyme, the serine protease known as TMPRSS2, then promotes celular entry of the aggressor virus. Both ACEh2 and TMPRSS2 are crucial for viral infectivity.
As we know the angiotensin-converting enzyme rapidly removes the two amino acids from the C-terminus of physiologically inactive angiotensin-I to form the physiologically active octapeptide angiotensin-II. ACE is present on the luminal surface of vascular endothelium throughout the body and is predominantly abundant in the rich pulmonary vascular endothelium, which is known to be metabolically very active.14
Some structural work reveals the high-resolution structure of full-length ACE2 in a dimeric assembly. Docking the S protein trimer onto the structure of the ACE2 dimer with the receptor binding domain of the S protein bound suggests simultaneous binding of two S protein trimers to an ACE2 dimer. Structure-based rational design of binders with enhanced affinities to either ACE2 or the S protein of the coronaviruses may facilitate the development of decoy ligands or neutralizing antibodies for suppression of viral infection.15
Since the majority of the COVID-19 transmission is expected only after cough and signs of lower respiratory tract disease have developed and we can expect a fast adaptation of the virus to the human host, and when dealing with this type of receptor, the relationship between these and the supposed association between systolic arterial hypertension and severity of COVID-19 (prognosis) is hypothesized. The biological plausibility of salutary effects (anti-inflammatory and antioxidative) of ACEIs/ARBs in those with COVID-19 is intriguing, but a lot of research in the area is being conducted at the moment.16,17
A recent study showed that serum angiotensin II levels in patients with COVID-19 pneumonia were significantly higher compared with healthy individuals and were linearly associated with viral load and lung injury. Based on this, it can be postulated that SARS-CoV-2 binding to ACE2 may attenuate residual ACE2 activity, skewing the ACE/ACE2 balance to a state of heightened angiotensin II activity leading to pulmonary vasoconstriction and inflammatory and oxidative organ damage, which increases the risk for acute lung injury (ALI).18
As it is likely that SARS-CoV2 will behave more like its close SARS-CoV, it will adapt progressively to the human host, with enhanced binding to hACE2, and with these similarities to SARS-CoV, we can imagine the virus will spread systemically giving rise to the pandemic we all are experiencing,19,20 encompassing the entire planet, with almost one million and a half cases today, 74.4% of which have an active form of the infection and a changing case fatality rate, which has shot up from an initial 2.5% a few months ago to practically double with the deaths of the last dates (the fatality rates for SARS in 2003 was 9.5% and for MERS in 2013: 34.4% but with much fewer cases). Like the rest of the variables, the fatality rate moves dynamically; we know that for young patients (20 years of age) it is very less than 1% (0.03%), but for elderly patients (70 years) it can go up to 8.6%. The same happens with nations, we observe a great variability depending probably for reasons of race, moment of onset of the wave of cases, economic characteristics and status of the health system, population characteristics, geography and climate, among others, as well as the moment in which the Figures are analyzed. The current fatality rate in Italy is 11.9%, while for Germany 1.2% in the same European community. In North America, 2.2% in the USA, half (1.1%) in Canada and 2.4% in Mexico.
But the worst may come in the near future as a wave of cases occurs in the most unprotected regions of the world such as Africa, Latin America and the Caribbean. Currently the country with the highest number of cases is the USA with more than 400,000 and unfortunately 12,867 deaths (but with projections that could reach more than 100,000) and soon several European nations besides Italy and Spain and with much smaller populations, will exceed the original number of cases in China (82,000). The challenge for the health systems of countries with developing economies, great territorial extension and population such as Mexico, at the start of the accelerated phase of the epidemic is enormous. Just over 5,523 mechanical ventilators are registered in this country, with 2,500 ICU beds and 50,000 public hospital beds for a total population of 130 million Mexicans. It is known the mathematical modeling of Dr. Marc Lipsitch, researcher and professor at the Harvard University School of Public Health in Boston; he predicted that something between 40% and 70% of the world’s adult population could become infected with the new coronavirus this year, a later adjustment of that calculation placed the infected ones between 20% and 60% of people over 18 years of age, which is still a huge number: between 940 million and 2,820 million human beings.
In the beginning of the epidemic, the fast movement of the Chinese central public health authorities, clinical, and scientific communities facilitated recognition of the clinical disease and initial understanding of the epidemiology of the pandemic, and after the viral genome sequencing, the development of sensitive quantitative reverse-transcriptase-polymerase-chain-reaction assays to rapidly detect the virus was assured. Genome sequences of the SARS-CoV-2 sampled from nine patients who were among the early cases of this severe infection in Wuhan, are almost genetically identical, which suggests very recent emergence of this virus in humans and that the outbreak was detected relatively rapidly.21
Taking into consideration the typical surveillance pyramid and Its relation to outbreak containment, we can consider a huge base constituted by mild or asymptomatic cases; these are patients that typically do not seek health care, so do not get a medical diagnosis and may easily spread the virus to their contacts and community. The early detection of cases is then critical (anosmia, hyposmia, lower respiratory symptoms followed by fever) and initially a history of travel to China or close contact with a person known to have COVID-19 diagnosis in the 14 days prior to symptoms onset. We still have key questions regarding any emerging virus as what is the role of overall pathogenicity in our ability to contain emerging viruses, prevent large-scale spread, and prevent them from causing a pandemic or becoming endemic in the human population?, what is the shape of the mentioned disease pyramid?, what proportion of infected people develop disease?, what proportion of those seek health care?, how widespread is the virus in its reservoir?, among others.22 All of these are good research questions.
Public health measures, that included quarantining in the community as well as timely diagnosis and strict adherence to universal precautions in nosocomial settings, were very important in controlling SARS and MERS in the past. The institution of similar measures will be important and hopefully, successful in reducing the transmission of the new pandemic (it is now clear that human-to-human transmission has been occurring and that the epidemic has been gradually growing in recent weeks), so we can undertake many important actions to try to shovel it as shown in Table 1.
Table 1: The most important actions to undertake during the pandemic are shown.
| Actions to undertake |
|---|
| Society & primary caregivers education & communication |
| Public health measures (including quarantining in the community) |
| Timely diagnosis |
| Strict adherence to universal precautions (health care settings) |
| International cooperation |
| Fighting economic & social consequences |
| Research |
The substantial involvement of nosocomial transmission in both SARS-CoV and MERS-CoV outbreaks suggests that such transmission is a serious risk with other newly emerging respiratory coronaviruses as the one we are facing now, so that personal protective equipment (PPE) is a very important resource in health institutions. Age and coexisting conditions (such as diabetes mellitus 2 or heart disease) were independent predictors of adverse outcome in SARS-CoV and MERS-CoV in the past.
Now, what is the source of the pandemic?, to investigate and understand the issue of genetic diversity, time origin and evolutionary history of COVID-19 outbreak in China and Thailand, a total of 12 genome sequences of the virus with known sampling date (December 24th 2019 and January 13th 2020) and geographic location (primarly Wuhan city, China and Bangkok, Thailand) were analyzed and it was estimated that the COVID-19 originated in Wuhan on November 9th 2019 (95% credible interval: September 25th and December 19th 2019), and that Wuhan was at that time the larger hub for the spread of the outbreak in China and the rest of the world, moving now to Italy and central Europe as well as the United States of America and Latin America.23-25
Wuhan is a strategic metropolitan city in central-east part fo China, as already mentioned, where the «pneumonia of unknown etiology» surveillance mechanism was used,26 with the definition showed in the Table 2.27,28 Although the first cases were reported on late December 2019, some authors have estimated that 86% of all infections were undocumented (95% CI: [82%-90%]) prior to January 23rd, 2020 travel restrictions.29
Table 2: Operational definition of a COVID-19 case originally used in the outbreak in China. This definition has required different adaptations over time as the pandemic has progressed in different regions of the world.
| Illness without a causative pathogen identified that fulfills the following criteria |
|---|
| • Fever (≥ 38o°C) |
| • Rx evidence of pneumonia |
| • Low or normal white-cell count or low lymphocyte count |
| • No symptomatic improvement after antimicrobial treatment for 3 to 5 days following standard clinical guidelines |
| • Or fulfilled the abovementioned first 3 criteria and had an epidemiologic link to the Huanan Seafood Wholesale Market or contact with other patients with similar symptoms |
It was seen that from the initial 41 persons hospitalised in China with SARS-CoV-2 pneumonia, 2/3 had been exposed to the Huanan Seafood Wholesale Market since they were tenants of it, and 33 of the 585 animal specimens taken from that market by the sanitary authority, had evidence of the virus. When this market was closed by the Chinese government, there were about 150 live animals, of 75 different species, living in unhygienic conditions with people and in close proximity to products of animals slaughtered and exposed for sale to the public. Another factor of probable importance in the dispersion of the disease of this specific Wuhan site is its proximity to the Hankou railway station, just two blocks away. There is evidence that human-to-human transmission30 has already occurred among close contacts since the middle of December 2019.
The results of the viral genome sequence made by the Chinese researchers31 in conjunction with other reports, showed a similarity of 75 to 80% to the SARS-CoV and even more closely related to several bat coronaviruses (86.9 to 95%). Figure 2 The close phylogenetic relationship to RaTG13 provides evidence for a bat origin of SARS-CoV-2. These authors then successfully isolated the virus (named nCoV-2019 BetaCoV/Wuhan/WIV04/2019), in Vero and Huh7 cells using a bronchoalveolar lavage fluid (BALF) sample from an ICU patient that underwent a bronchoscopic procedure and based on that findings, they conclude that this new disease should be transmitted through the airway, although they couldn’t rule out other clinical possibilities.32
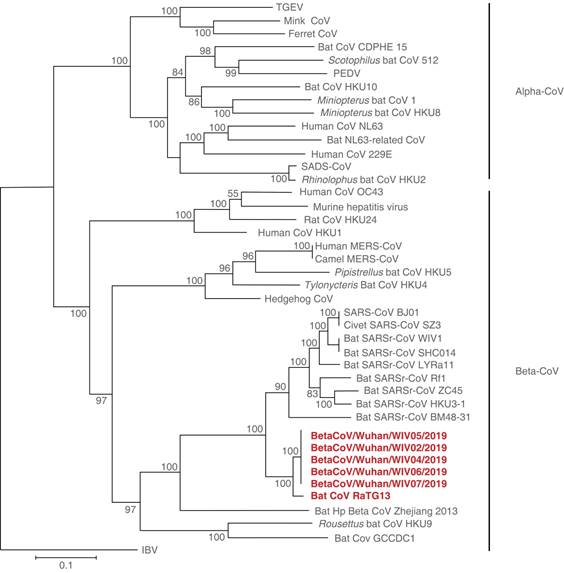
Figure 2: Phylogenetic tree based on nucleotide sequences of complete ORF1b of coronaviruses, showing a close similarity between SARS-CoV-2 and bat coronaviruses.13
So as far as we know, both SARS-CoV and MERS-CoV are believed to originated in bats (2003 and 2013), and these infections were transmitted directly to humans from market civets and dromedary camels, respectively.33 SARS-CoV-2 virus possibly followed the same way to the human from the pangolin, a mammal from Asia and Africa in danger of extinction, that is trafficked for its meat as food and its scales used in traditional Chinese medicine, although it is possible that other species are also part of this chain. More generally, the COVID-19 outbreak linked to SARS-CoV-2 again highlights the hidden virus reservoir in wild animals and their potential to occasionally spill over into human populations.
About the early transmission in China, we know some of the epidemiologic characteristics and transmission dynamics of COVID-19, although caution must be taken, given that the Figures of the pandemic in general have been in continuous change and evolution, as this is a new disease.
The median age of the first Chinese patients was 59 years (range 15 to 89), and 240 of the 425 patients (56%) were male and more likely to have exposure to the Huanan Seafood Wholesale Market, characteristic that soon desapeared as the epidemic progressed. There were no cases in children below 15 years of age, while the proportion of cases in health care workers worryingly increased.
The age group most frequently affected, was the age group between 45 and 64 years (about 50% of total cases).
The mean incubation period was estimated to be 5.2 days (95% confidence interval [CI], 4.1 to 7.0); the 95th percentile of the distribution was 12.5 days (95% CI, 9.2 to 18);34 which provides the basis to support a 14-day quarantine for exposed persons.
At the very beginning the epidemic growth rate was 0.10 per day (95% CI, 0.050 to 0.16) and the doubling time was 7.4 days (95% CI, 4.2 to 14). Using the serial interval distribution above, it was estimated the reproductive number or R0 to be 2.2 (95% CI, 1.4 to 3.9); it is considered in general, that an epidemic will increase as long as this number is > 1, and control measures aim to reduce it to < 1 (as an example the R0 of SARS at the beginning of this century was around 3).35
The duration from illness onset to first medical visit for first cases was estimated to have a mean of 5.8 days (95% CI, 4.3 to 7.5), while the mean duration from onset to hospital admission was 12.5 days (95% CI, 10.3 to 14.8), and shorter later on, with a mean of 4.6 days (95% CI, 4.1 to 5.1) and 9.1 days; 95% CI, 8.6 to 9.7) respectively.
So taking into account the possible bias, first published information in peer review journals stablish COVID-19 to be a disease of young-middle age economically active working people, with very few cases occurring in children and a not negligible proportion of cases in health care workers, not as high as during the SARS and MERS outbreaks.36 It is estimated that the health personnel attack rate, although variable between countries is higher than 10% (3.8% in China, of them 14.8% with severe or critical illness), so we should always consider in first place how better protect our staff, remembering that aerosol-generating procedures in the ICU include: endotracheal intubation, bronchoscopy, open suctioning, administration of nebulized treatments, manual ventilation with a bag before intubation, physical proning of the patient, disconnecting the patient from the mechanical ventilator, non-invasive positive pressure ventilation, performing a tracheostomy, and of course cardiopulmonary resuscitation; all these procedures must be performed by the healthcare worker who is most experienced.
As mentioned, these initial reports have some important limitations, as the initial focus of case detection was only directed to patients with proven pneumonia, loosing the proportion of cases who could present with just gastrointestinal symptoms, as well as asymptomatic infections specially in childs and young people, early infections with atypical presentations, and it is likely that infections of mild clinical severity have been under-ascertained among the confirmed cases.32,37
In the retrospective cohort study of adult patients in two hospitals in Wuhan, China, mean age was 56 years old (46-67) and it was found a borderline increasing odds of in-hospital death associated with older age (odds ratio 1.10, 95% CI, 1.03-1.17; p = 0.004).38
In subsequent larger series with more tan 1,000 cases, the mean age of COVID-19 was 47 years (interquartile range, 35 to 58) and the mean incubation time was four days (interquartile range, 2 to 7).39 Since Mexico is basically a country of young people, the median age of COVID-19 is currently 41 years with a little less than three thousand cases.
Other authors have calculated a COVID-19 basic reproduction number (R0) of 2.24-3.58. Kids have milder symptoms or are asyntomatic but with the potential of being infectious contagious. Chinese series report only 0.9% cases in pediatric ages, we can hypothesize that probably due to some mechanism of relative immaturity/ or dysfunction-like of their ACEh2 receptors.
One of the lessons we learned from an early German communication is that this disease can be transmissible from the incubation period and that there may be totally unspecific signs in the first week of the disease, in addition to its high contagiousness.40
In general, a respiratory infection has a clinical behavior that depends on the balance of different variables, namely the particular virulence of the infecting agent (contagiusness), the level of exposure or massive inoculum and the personal inmune system status.
It is known to all that COVID-19 clinical course is highly variable between individuals: it varies in severity from asymptomatic infection 1% (maybe more, it is unclear),15,29,41 to mild and moderate respiratory illness (80%), to severe clinical forms with acute respiratory failure (ARF) (15%) and critical illness with ARDS/fatal outcome 4% (in Italy the proportion of ICU admissions represented 12% of the total positive cases, and 16% of all hospitalized patients);42 mortality rate in this setting can be as high as 49%. It has non-specific signs and symptoms and fever may be intermitent or prolonged, and generally not present since the beginning of the disease. There is potential for clinical deterioration during the second week of illness.
Clinical presentation includes anosmia and disgustia at the beginning in mild cases, dry cough, fever and malaise with mialgia, arthralgia or fatigue, shortness of breath, nausea, vomiting and diarrhea as well as other uncommon signs and symptoms (sore throat, productive cough, headache, hemoptisis, diarrhea). Severe cases usually develope ARF at arrival (not necessarily ARDS) and systemic hypotension (60%) (Table 3).43
Table 3: The main clinical, laboratory and radiological manifestations of the disease are shown.
| COVID-19 Characteristics |
|---|
| Clinical |
| Fever (83-98%) |
| Cough (46-82%) |
| Myalgia or fatigue (11-44%) |
| Shortness of breath (31%) |
| Nausea, vomiting and diarrhea (10%) |
| Hypotension (60%) |
| Uncommon signs and symptoms |
| Sore throat, productive cough, headache, hemoptysis, diarrhea |
| Subtle symptoms |
| Anosmia, hyposmia |
| Disgusia |
| Laboratory |
| Leukopenia (9-25%) |
| Lymphopenia (63%) |
| Leukocytosis (24-30%) |
| Elevated transaminases (37%) |
| High CRP (> 80%) |
| Elevated D-Dimer (> 60%) |
| Normal procalcitonin (> 80%) |
| High ferritin (> 50%) |
| Positive rRT-PCR for SARS-CoV-2 |
| Radiological |
| Chest X-ray |
| Abnormal in 96% severe cases, and 100% ICU cases |
| Bilateral consolidation (98%) |
| CT scan |
| Bilateral multilobar and subsegmental areas of peripheral consolidation |
| For non-ICU patients: bilateral ground-glass opacity and subsegmental areas of consolidation |
In an effort to find variables with a poor prognosis, Huang et al. analyzed the data on Chineses ICU admitted critically ill patients vs non-severe patients.44 Most of the infected patients were men (73%); only a third had underlying diseases (32%), including diabetes (20%), hypertension (15%), and cardiovascular disease (15%). It was found to be a disease of young people in middle age, with a median age of 49·0 years (IQR 41.0-58.0), in this cohort, no children or adolescents were infected. 32% of patients were admitted to the ICU to treat progressive hypoxaemia. Besides, any of the following variables was useful to predict a bad outcome: age (p = 0.60), sex (p = 0.24), smoking history (p = 0.31), diabetes (0.16), hypertension (0.93), cardiovascular disease (0.32), COPD (0.14), malignancy (0.49) and chronic liver disease (0.68), similarly any of the following signs and symptoms showed significant differences: fever (0.68), cough (0.35), myalgia or fatigue (0.38), sputum production (0.32), headache (0.10), haemoptysis (0.46) and diarrhoea (0.66). In this small early report, the only variables that showed clinical significance to predict a bad outcome were ARF signs: dyspnoea (p = 0.001), tachypnea > 24 bpm (p = 0.002) and systolic hypertension (0.01), as well as the delay to get medical care (p = 0.002). Compared with non-ICU patients, ICU patients had higher plasma levels of IL2, IL7, IL10, GSCF, IP10, MCP1, MIP1A, and TNFα only during the advanced stage of disease, suggesting that the cytokine storm was associated with disease severity. Another subsequent retrospective, multicentre cohort study yielded some different data.
Interestingly a pre-epidemic Chinese multicenter prospective registry of viral community acquired pneumonia (CAP) did not showed any significant difference between influenza and non-influenza viral infection groups (where usual coronavirus were included) in terms of CURB-65 > 3, PaO2/FiO2 ratio < 200 mmHg, clinical stability, or 90-day mortality, but coronavirus resulted to be with metapneumovirus the most frequent producer of ARF during hospitalization, and was frequently complicated with sepsis related with longer length of hospital stay. We do not know if we can extrapolate these results to SARS-CoV-2 (probably not), but curiously the authors conclude that more attention should be paid to CAP associated with the non-influenza virus, even in immunocompetent population.45
Zhou et al looking for risk factors to predict poor prognosis, found in a retrospective cohort study of adult in-patients in two hospitals in Wuhan, China, that 91 (48%) of them had a comorbidity, with hypertension being the most common (58 [30%] patients), followed by diabetes (36 [19%] patients) and coronary heart disease (15 [8%] patients), but a relatively small percentage of patients with pulmonary disease both in China and Italy. Multivariable regression showed increasing odds of in-hospital death associated with older age (odds ratio 1.10, 95% CI 1.03-1.17, per year increase; p = 0.0043), higher sequential organ failure assessment (SOFA) score (5.65, 2.61-12.23; p < 0.0001), and D-dimer greater than 1 μg/mL (18.42, 2.64-128.55; p = 0.0033) on admission. Additionally, elevated levels of blood IL-6, high-sensitivity cardiac troponin I, lactate dehydrogenase and lymphopenia were more commonly seen in severe COVID-19 illness.
The median time from onset of symptoms to first hospital admission was 7.0 days (4.0-8.0), to shortness of breath was 8.0 days (5.0-13.0), to ARDS was 9.0 days (8.0-14.0), to mechanical ventilation was 10.5 days (7.0-14.0), and to ICU admission was 10.5 days (8.0-17.0). In a series it was found that half of the patients treated for mild COVID-19 infection, still had coronavirus for up to eight days after symptoms disappeared, with a continuous shedding of the virus;46 more severe infections may have even longer shedding times (up to 20 days, IQR 17.0-24.0), The longest observed duration of viral shedding in survivors was 37 days, and more in the ICU patients that eventually died; so taking into account that the 95th percentile of the distribution of the incubation period is 12.5 days (95% CI, 9.2 to 18) we have to reconsider if the «quarantine» period of only 14 days applies to all cases.
Signs of ARF were equally significantly different between survivors and deceased, but arterial hypertension (p = 0.53) or any other sign or symptom were not. A low lymphocyte count was present more often in non-survivors (76%), p < 0.0001. In this series, the following laboratory variables were observed more frequently in non-survivors: anaemia, low platelet count and albumin level, high ALT, creatinine, lactate dehydrogenase and creatine kinase level; high-sensitivity cardiac troponin I and a prolonged prothrombin time. A high D-dimer, serum ferritin and IL-6 level, usually with a normal or low procalcitonin level at arrival. Among adult patients, cardiovascular disease and hypertension were the most common underlying diseases, followed by diabetes mellitus. Fever was the most common symptom but not necessarily present from the start (92.8%; n = 258), followed by cough (69.8%; n = 194), dyspnoea (34.5%; n = 96), myalgia (27.7%; n = 77), headache (7.2%; n = 20) and diarrhoea (6.1%; n = 17). Rhinorrhoea was noted in only 4.0%, a sore throat in 5.1% and pharyngalgia in 17.4% of patients with relevant clinical information.3 As in many other conditions, the elderly and those with comorbidities (diabetes, heart disease, lung disease), have a higher risk of developing serious forms of the disease and require hospitalization and probably ICU and mechanical ventilation, with which their expected mortality could be higher, a well described phenomenon in pneumonia, ARDS and Critical Care Medicine. Previous interesting studies in macaques inoculated with SARS-CoV found that the oldest had stronger host innate responses to virus infection than younger adults, with an increase in the differential expression of genes associated with inflammation, whereas expression of type I interferon beta was reduced.47 The age-dependent defects in T-cell and B-cell function and the excess production of type 2 cytokines could lead to a deficiency in control of viral replication and much more prolonged proinflammatory responses, potentially leading to worst outcomes.48 COVID-19 death patients are more frequently seen in the elderly and those with chronic underlying diseases as already mentioned,49 its case-fatality rate with comorbidities is 73.3%, higher than SARS (46%) and MERS (60%). Nevertheless the median age of patients admitted to Italian ICU’s was only 63 (IQR, 56-70) years old, which resulted to be the same as the median age of all the positive Italian cases with COVID-19, suggesting that, to date, older age alone is not a risk factor for admission to the ICU at least in a society like that.50
In general different series describe the most common laboratory abnormalities among hospitalized patients: leukopenia, leucocytosis, lymphopenia, elevated transaminases, with normal serum levels of procalcitonin on admission; high ferritine and D-dimer levels already mentioned, but the definitive diagnosis is made through the real time-reverse-transcriptase polimerase-chain-reaction testing (RT-PCR)51 of SARS-CoV-2 virus determination, that extract the viral RNA, lower cycle threshold (Ct) values in the first four days of the infection indicate higher viral loads. This test is usually done through a nasopharingeal swab, but others have used oropharingeal swab, serum test and stool test. Also this determination can be done in BALF with a very high performance, pharingeal washes, tracheal aspirates and sputum. No urine or pleural fluid RT-PCR for this disease has been found. Given the lack of a reference standard for diagnosing COVID-19, the sensitivity and specificity of diagnostic testing are unknown, but it has been reported to be 93% (95% CI, 28.9-33.2) in BAL fluid, 46% (95% CI, 29.8-37.9) in fibrobronchoscopic brushing, 72% (95% CI, 29.3-33.0) in sputum, 63% (95% CI, 13.7-35.0) in nasal swabs, 32% (CI 95%, 31.2-33.1) in pharyngeal swabs, 29% (CI 95%, 29.4-33.5) in feces, 1%(CI 95%, 0.0-36.4) in blood, and 0% in urine; then its specificity is high (low false positives), but its sensitivity not (possible high false negatives).52,53 There are different trademarks in the market, the first to be available were from China (genetic primers and probes only), Thailand, Hong Kong, Japan, Germany, USA (CDC) as well as different local home tests, all of them detect virus, with no detection of humoral response for convalescent phase. There are no reliable rapid tests yet in the market.
There have been monitoring of viral antibody levels in isolated patients at different times of disease evolution (seven, eight, nine, and eighteen days after disease onset). A clear trend of IgG and IgM titre increase (as a marker of acute infection decreased at the last day 18th) has been observed.13 Serum antibodies ideally should be tested among health-care workers before and after their exposure to SARS-CoV-2 infected patients for identification of asymptomatic infections.44
Regarding radiological abnormalities the plain chest film is unspecific, showing bilateral air-space opacities with no pleural effusion, but in chest CT images were detected among all patients. Of the 41 original patients, 40 (98%) had bilateral involvement (Table 3). The typical findings of chest CT images of ICU patients on admission were bilateral multiple lobular and subsegmental areas of consolidation. The representative chest CT findings of non-ICU patients showed bilateral ground-glass opacity and subsegmental areas of consolidation. Later chest CT images showed bilateral ground-glass opacity, whereas the consolidation had been resolved (Figure 3).44,54 So, the ground-glass opacities, the multilobar involvement and the subpleural or peripheral distribution (with central sparing) are almost universal in COVID-19 patients. Consolidation and septal thickening are less frequent, as well as bronchiectasis and wall thickening. There is not pleural effusion, lymphadenopathy or lung nodules.
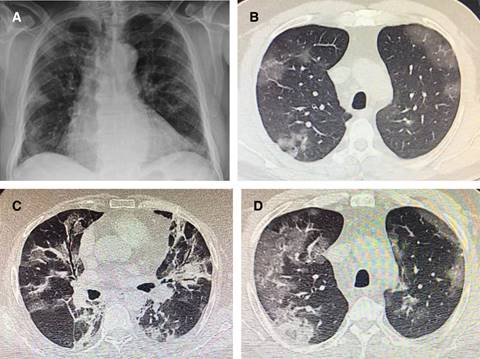
Figure 3: Plain chest film and CT Scanner from COVID-19 different cases. A) AP projection showing some peripheral bilateral multilobular shadows. B) CT section showing upper lobes peripheral ground glass opacities. C) Another case with alveolar filling with air bronchogram in the middle lobe and lingula plus ground glass pattern. D) Peripheral ground glass opacities with some alveolar filling and septal thickening.
At the beginning of the actual health crisis in Wuham, Chinese physicians found that around 10% of patients had criteria to be admitted to the ICU because of progressive hipoxemia and that 5% entered to ECMO, but later on these data were adjusted downwards, when observing that only 5% of the sick population required the ICU services. Covid-19 associated ARDS reached a terrible 49% mortality in China, usually accompanied by a hyperinflammation and cytokine storm syndrome (severe ARDS as defined by the Berlin definition has an expected associated mortality of 45% (CI 95%, 42-48%) but in the other hand some preliminary data form Italy show a much lesser mortality of only 26%, but still with 58% of their patients admitted in the ICU, so this is not conclusive.50,55 Described direct lung injury ARDS mortality such as that caused by viral pneumonia, is currently well below the first Figure.56 But we can ask ourselves what explains this high mortality?, is it basically the overwhelming magnitude of the pandemic and the surprise and uncertainty that this produces?57 Is it fear of contagions that modifies attitudes and proven work systems to start improvising? Is it a matter of lack of mechanical ventilators or human fatigue and insufficient experience of the human factor with hard cases?, is the intrinsic virulence of the SARS-CoV-2 virus?, is the frequent complicating appearance of ventilator associated pneumonia, sepsis, cardiac dysfunction and thrombosis?, or simply a different physiopathology that we haven’t fully understood? (endotelial dysfunction with vascular desregulation and loss of hypoxic vasoconstriction vs a hiperangiotensin II state induced vasoconstriction in the presence of shunt, resulting in death space, worsening of V/Q mismatch and silent non-responsive units to PEEP our best friend?). Probably each society has its own reality, finally we are living something never seen before in modern human history, but I personally see with concern the number of early deaths in Mexico in the phase in which we are in the epidemic, having the contingency plan ready and even with full ICU capacity for many more cases in the different states of the republic.
The deep analysis of mechanical ventilation in ARDS is beyond the scope and primary purpose of this review, but we can ensure the correct application of the alveolar protection strategy, with optimal PEEP setting, and neutral or mild negative liquid balances. I want to emphasize that the time of a pandemic is not the optimal time to try to launch respiratory therapies that have not been proven or with which our working group has no experience at all, are excessively expensive or take up many man-hours to perform, we are experiencing a health crisis of terrible dimensions; in such a way that a simple and early volume controlled ventilation, with low tidal volume, good monitoring and a correct selection of PEEP level, initially tailored to fit the intriguing and unique COVID-19 lung elastance and recruitability, timely prone position, good hemodynamic and parenteral fluid management, will surely be sufficient, as no specific treatment exist.
My advice if your team has experience and the proper resources, is to provide a supervised, cautious, short and careful trial of non-invasive mechanical ventilation, strictly in mild cases (Berlin definition),55 but with inspiratory power not just CPAP (like pressure support ventilation) to decrease enough the patient inspiratory effort, in Lombardy only 11% of patients could be managed with noninvasive ventilation.50 If this management is successful, the question that you will have to answer in that case is what that patient does in your ICU occupying a bed in pandemic times. I consider that high-flow O2 does not have a clear role in these patients with shunt mediated progressive hypoxemia and may only delay the start of mechanical ventilation; its home use in some mild cases may be reasonable.
In one series high-flow O2 and non-invasive ventilation were used in 61% and 44% of non-survivors patients respectively.
In Figure 4 you will find the author’s personal view of these aspects of COVID-19 associated ARDS ventilatory management, more for the experience gained over time than for knowledge of this particular new virus that has surprised us all.
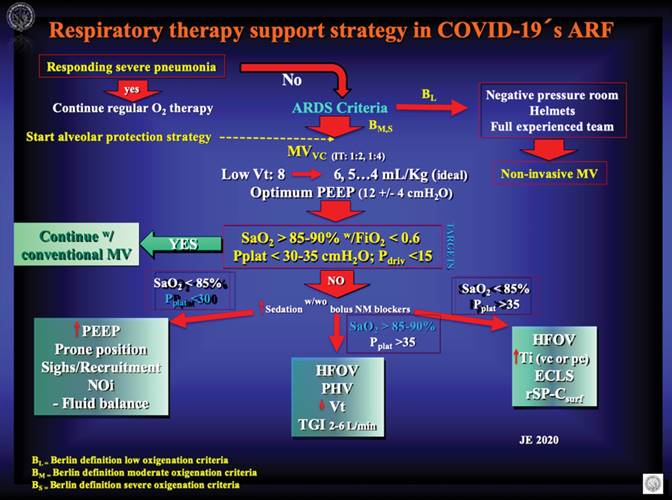
Figure 4: The picture shows the autor’s personal view of how to provide advanced respiratory therapy support to patients affected with COVID-19 related Acute Respiratory Failure (ARF ). If you have a hospitalized patient with a severe pneumonia that is responding to regular management, you must continue with regular respiratory therapy maneuvers (O2 by face mask, etc.). If the patient reach ARDS criteria advance your management to a short course of non-invasive mechanical ventilation in Berlin low oxigenation criteria ARDS if you have the appropiate resources, if not or in case of Berlin moderate or severe oxigenation criteria go to protected endotracheal intubation and invasive mechanical ventilation with alveolar protective strategies. Use low Vt’s and optimum PEEP with special attention to lung elastance and alveolar recruitment possibility. Always have a clear idea of what your targets are in terms of oxigenation and pressure in the airway, if you reach them, you are ok with conventional ventilation!, but if not you must try any of the three scenarios measures depending on the case. ARF = acute respiratory failure; ARDS = acute respiratory distress syndrome; MVvc = volume control mechanical ventilation; Vt = tidal volumen; PEEP = positive expiratory ending pressure; SaO2 = O2 arterial saturation; FiO2 = inspired O2 fraction; Pplat = plateau pressure; Pdriv = driving pressure; NOi = Inhaled nitric oxide; HFOV = high frequency oscilatory ventilation; PHV = permissive hypercanea ventilation; TGI = tracheal gas insufflation; Ti = inspiratory time (volumen controled or pressure controled); ECLS = extracorporeal lung support; rSP-Csurf = recombinant surfactant.
It has been described some histopathological information by Chinese colleagues; these findings are similar to those seen in SARS and MERS and consistent with a diffuse alveolar damage with cellular fibromyxoid exudates (desquamation of pneumocytes and hyaline membrane formation), pulmonary oedema with hyaline membrane formation, suggestive of ARDS. Interstitial mononuclear inflammatory infiltrates, dominated by lymphocytes, were seen in both lungs. Multinucleated syncytial cells with atypical enlarged pneumocytes characterised by large nuclei, amphophilic granular cytoplasm, and prominent nucleoli were identified in the intra-alveolar spaces, showing viral cytopathic-like changes. Some liver injury was also documented.58
Others have described an extensive thrombosis phenomena as something new, but we have information for many years that this is the case in ARDS, in which we can distinguish from the histological point of view three phases, one exudative (from day 1 to approximately the 7th), one proliferative (from 7th to 21st) and another fibrotic (> day 21st); the first with fibrin thrombi and platelets, the second with macrothrombi and the third with arterial tortuosity, mural fibrosis and hypertrophy of the media at the vascular level. This may be a new virus, but the body’s response should not vary much; pulmonary vascular injury and pulmonary hypertension are important complications of ARDS.59,60
Pulmonary vascular lesions follow a temporal sequence that correlates with the duration of alveolar damage, and maybe this is the main difference in COVID-19. Multiple postmortem studies have shown markedly reduced vascular filling of lung specimens, and in fact thromboembolic are the most consistently observed vascular lesion, present in as many as 95% of ARDS patients,61 specially if they die,61,62 that is not new.
Thromboemboli detected only by light microscopy (microthrombi) are as prevalent as macrothrombi, but tend to be distributed throughout all phases of ARDS.61 The clinical prevention of this condition and the search of better clinicopathological correlations are guaranteed.
In these moments we can ask ourselves what to do as we are only physicians. The answer is a lot!, just to mention some of the most important actions: to actively participate in our society through education and communication, who better than the medical community in direct contact with cases?, we must lead the public health measures
(including quarantining in our community), to participate in the process of timely diagnosis, to follow strict adherence to universal precautions in our health care settings, to prepare our ICU’s63 and to design good research between others, ideally clinical trials should be structured to promote maximum learning from around the world, such as through the use of master protocols or adaptive platform designs (Table 1).64
The complications will surely be similar to those observed in atypical, viral pneumonias, etc.65 In addition to severe pneumonia, respiratory failure and ARDS that we have already discussed (29%), we know that viral infection can also cause sepsis syndrome and even shock. It has been determined that sepsis occurred in nearly 40% of adults with community-acquired pneumonia due to viral infection. In the actual pandemia it has been found that more than half of patients develope sepsis. More than 70% of patients had leukopenia or normal procalcitonin, with no bacterial pathogens detection on admission. Recently a multinational panel of experts proposed relevant questions, reviewed literature for direct and indirect evidence on the management of COVID-19 in critically ill patients in the ICU, and identified relevant and recent systematic reviews related to supportive care. Based on this analysis and their assessment, the Surviving Sepsis Campaign COVID-19 panel has issued some statements, which include four best practice statements, nine strong recommendations, and 35 weak recommendations.66
Other COVID-19 complications include acute kidney injury, acute cardiac injury (12%; in a series using high-sensitivity cardiac troponin I during hospitalization it was found high in more than half of those who died). Heart failure in COVID-19 USA patients is > 40%,67 secondary infection (10%), encephalitis, rhabdomyolysis, hepatic failure, disseminated intravascular coagulopathy, intrabdominal hypertension and abdominal compartment syndrome, deep venous thrombosis and pulmonary embolism.58
The mortality rate appears to be relatively consistent with current trends, between 2.5% and 3.5%; although with increasing numbers in the face of fatigue of health systems worldwide. This would make the COVID-19 the least deadly of the three most pathogenic human coronaviruses. Nonetheless, this relatively lower mortality rate may be outweighed by the virulence of COVID-19. With more than one and a half million cases and almost 85,000 deaths, the total death toll from COVID-19 has exceeded that of both MERS-CoV and SARS-CoV combined.68 Despite the whole world’s efforts to understand COVID-19, many issues remain unclear. First, one report has demonstrated the presence of SARS-CoV-2 in patient stools. However, whether SARS-CoV-2 can be transmitted through the faecal-oral route remains unclear. Second, previous studies showed that SARS-CoV and other coronaviruses could survive on environmental surfaces and inanimate objects; however, the presence of SARS-CoV-2 in the environment has not been reported. The survival data for similar viruses suggest that enveloped ones can remain infectious on surfaces long enough for people to come in contact with them, posing a risk for exposure that leads to infection and possible disease transmission. Previous SARS-CoV has been reported to survive for 36 hrs on stainless steel.69
In a situation of low temperature in the air (4 oC) and low relative humidity in the air (20%), viruses become less inactive (they live longer). This inactivation is faster at medium temperature (20 oC) than with cold air at all relative humidity levels; they were inactivated more rapidly at 40 °C than at 20 °C, and there was greater survival at low than at high relative humidity. The results of the statistical analysis suggest that relative humidity has a greater effect on viral inactivation than atmospheric temperature, but there are interactions between both factors. All this relationships between atmospheric temperature, relative humidity in the air, and virus inactivation is complex and still not entirely clear and may vary depending on the virus type, there is no specific information of SARS-CoV-2.70 There is growing evidence that establishes a relationship between greater air pollution (PM2.5) and a greater probability of death from COVID-19.
Previous studies have shown that coronaviruses could be efficiently inactivated using surface disinfectants with 62-71% ethanol, 0.5% hydrogen peroxide or 0.1% sodium hypochlorite within 1 min, but other biocidal agents such as 0.05-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were less effective. However, current investigation of the efficacy of commonly used disinfection agents against SARS-CoV-2 is lacking.71
Finally with regard to COVID-19 treatment as all we know there is none, specific anticoronaviral therapies are still in development, although a lot of research has been organized in the last few months.
Three general methods are being explored,72 the first is to try existing broad-spectrum antiviral drugs using standard assays, the second screening of a chemical library containing many existing compounds or databases, and third the development of new specific drugs based on the genome and biophysical understanding of individual coronaviruses.
Drugs like Lopinavir /Ritonavir, Ribavirin, Nucleoside analogues, Neuraminidase inhibitors, Remdesivir, peptide (EK1), abidol, RNA synthesis inhibitors (such as TDF, 3TC), anti-inflammatory drugs (such as hormones & other molecules), inhaled α-ketoamide inhibitors73 and others alone or in combination, are not licensed by the FDA in the USA, EMA in Europe or COFEPRIS in Mexico and we need more time to collect information on its usefulness and safety in critically ill human beings.
A few words deserve the frequent combination of azithromicin and chloroquine in COVID-19 patients since the beginning of the epidemic in Wuhan. The first, an interesting bacteriostatic macrolide that have received considerable attention for their anti-inflammatory and immunomodulatory actions beyond the antibacterial effect; both in vitro and in vivo studies have provided evidence of its efficacy in respiratory viral infections including rhinovirus (RV), respiratory syncytial virus (RSV), and influenza virus, but not in SARS-CoV-2. As example, this molecule significantly increase rhinovirus 1B- and rhinovirus 16-induced interferons and interferon-stimulated gene mRNA expression and protein production, and significantly reduce rhinovirus replication and release as well as viral load.74-76
The second, chloroquine an old drug that has been used to treat malaria and other conditions for many years and is a repurposed drug with great potential to treat COVID-19, a viral disease through a mechanism that is not well understood (inhibition of pH-dependent steps of the replication of several viruses,77 immunomodulatory effects, suppression of the production/release of TNF-α and IL-6, a novel class of autophagy inhibitor, which may interfere with viral infection and replication, interference with the glycosylation of cellular receptors).78 Both are experimental in the actual COVID-19 pandemic.
SCCM and ESICM concluded in their recent «Surviving Sepsis Campaign» guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) with a weak recommendation against the routine use of lopinavir/ritonavir, standard intravenous immunoglobulins or convalescent plasma, as well as that there is insufficient evidence to issue a recommendation on the use of other antiviral agents, recombinant rIFNs, alone or in combination with antivirals, chloroquine or hydroxychloroquine or tocilizumab in critically ill adults with COVID-19.
A weak suggestion on usage of empiric antimicrobials/antibacterial agents, over no antimicrobials was done with a remark of assessing for de-escalation on a daily basis, and re-evaluate the duration of therapy and spectrum of coverage based on the microbiology results and the patient’s clinical status.
Regarding the old corticosteroids discussion in ARDS they suggest in mechanically ventilated adults with COVID-19 and ARF (but without ARDS), against the routine use of systemic corticosteroids, but in its presence, just a part of the experts panel and because of the very low-quality evidence available, made only a weak suggestion of using systemic corticosteroids, over not using them.66
Many of us thought that the discussion of steroids use in ARDS was over since Roger C Bone in a classic study described a trend toward increased incidence of ARDS with the use of methilprednisolone (MPSS) (32% vs 25% with placebo; p = 0.10), fewer reversion of ARDS (31% vs 61% with placebo; p = 0.005), higher mortality (52% vs 22% with placebo; p = 0.004). Concluding that early treatment of septic syndrome (the predecessor of the systemic inflammatory response syndrome, SIRS), with MPSS does not prevent the development of ARDS. Additionally, MPSS treatment impeded the reversal of ARDS and increased the mortality rate in patients with ARDS (specially with renal or hepatic failure).79
In addition to other authors, Chinese physicians resuscited them in 2003 when SARS hit their nation, pointing out in retrospective publications that timely use of corticosteroids was an effective strategy for increased lung shadows and increased dyspnea, improving significantly the clinical symptoms and reducing the degree of disease progression, and accelerating the absorption of lung lesions.80,81
With pandemic COVID-19 steroids were used in 18.6% of patients in China, with a higher percentage among those with severe disease than nonsevere disease (44.5% vs. 13.7%), with no conclusive results,39 although others suggest a mild benefit with its use, something difficult to evaluate with so few cases.82
Recently many guidelines are being published for diagnosis and treatment of COVID-19.83,84
Last, it has been considered, based on the progress of research in different countries of the world, that the first vaccine for this disease could be ready in little less than 18 months.
Conclusions
The pandemic of COVID-19 has become a real clinical threat to all, specially healthcare workers worldwide. However our knowledge about this new virus is still limited and evolving.
This is not a disease of old people, but they are at risk for worst clinical outcomes including death. Most patients will experience mild to moderate illness.
The effective option of specific antiviral therapy and vaccination are currently under active evaluation and development in different countries. What we must do now is aggressively implement infection control measures to prevent the spread of SARS-CoV-2 via human-to-human transmission. Public health authorities should keep monitoring the situation, as the more we learn about this novel virus and its associated outbreaks, the better we can respond.3
Emerging infectious diseases and epidemics are inevitable, and we must learn from recent lessons to prepare in the best way for the near future.
All of us need to evaluate and organize our local resources and prepare our ICU’s always keeping in mind to first protect our staff.
Strict adherence to nosocomial universal precautions are necessary. For the most critically ill, the logic points to apply the same lung protection measures in mechanical ventilation while building scientific evidence.
Update frequently your COVID-19 information and knowledge, because the more we know, the less we fear.











 nueva página del texto (beta)
nueva página del texto (beta)


