Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Abanico veterinario
On-line version ISSN 2448-6132Print version ISSN 2007-428X
Abanico vet vol.11 Tepic Jan./Dec. 2021 Epub Apr 04, 2022
https://doi.org/10.21929/abavet2021.47
Literature Review
Biochemistry and metabolic pathways of polysaccharides, lipids, and proteins
1Estudiante de Maestría en Ciencias Agropecuarias, Universidad Autónoma Metropolitana. México.
2Departamento de Producción Agrícola y Animal, Universidad Autónoma Metropolitana. México.
3Unidad Académica de Medicina Veterinaria y Zootecnia, Universidad Autónoma de Nayarit. México.
4Facultad de Medicina Veterinaria y Zootecnia, Universidad de Colima. México.
The eukaryotic cells are complex structures, capable of replication and performing a wide range of tasks in multicellular organisms. However, they also obey the laws of chemistry and physics that determine the metabolism of living systems. Consequently, cell biology seeks to understand metabolic processes in terms of reactions of anabolism and molecular catabolism. This review considers the chemical composition and properties of polysaccharides, lipids, and proteins as ultimately responsible for all cellular activities. The atoms and biochemical bonds of these macromolecules determine all cell dynamics, which is why the first part of each chapter reviews the nature of the functional group’s hydroxyl, amino and carboxyl, responsible for the formation of monosaccharides, amino acids and fatty acids. The rest of each chapter analyzes the genesis and lysis of these molecules within each cell organelle, for the formation of acetyl-coenzyme A and the liberation of energy in the Krebs cycle. Thus, the biochemistry of cell metabolism can be understood in terms of the structures and functions of three main organic molecules polysaccharides, lipids and proteins.
Keywords: glycogenogenesis; glycolysis; lipogenesis; lipolysis; proteogenesis; proteolysis
Las células eucariotas son estructuras complejas, capaces de replicarse y realizar una amplia gama de tareas en organismos multicelulares. Sin embargo, también obedecen las leyes de la química y la física que determinan el metabolismo de los sistemas vivos. En consecuencia, la biología celular busca comprender los procesos metabólicos en términos de reacciones de anabolismo y catabolismo molecular. Esta revisión considera la composición química y las propiedades de los polisacáridos, lípidos y proteínas como responsables en última instancia de todas las actividades celulares. Los átomos y enlaces bioquímicos de estas macromoléculas determinan toda la dinámica celular, por lo que en la primera parte de cada capítulo se repasa la naturaleza de los grupos funcionales hidroxilo, amino y carboxilo, responsables de la formación de monosacáridos, aminoácidos y ácidos grasos. El resto de cada capítulo analiza la génesis y lisis de estas moléculas dentro de cada organelo celular, para la formación de acetil- Coenzima A y la liberación de su energía en el ciclo de Krebs. Así, la bioquímica del metabolismo celular, puede entenderse en términos de las estructuras y funciones de tres principales moléculas orgánicas.
Palabras clave: glucogenogénesis; glucólisis; lipogénesis; lipólisis; proteogénesis; proteólisis
ABBREVIATIONS
Aa |
amino acids |
Ac |
acetone |
AcAc |
acetoacetate |
DNA |
deoxyribonucleic acid |
NEFA |
non-esterified fatty acids |
Arg |
arginine |
mRNA |
messenger ribonucleic acid |
tRNA |
transfer ribonucleic RNA |
C |
carbon |
C=O |
carbonyl group |
C16:0 |
palmitic |
C3H3O3 |
pyruvate |
Ca2+ |
calcium ion |
CO2 |
carbon dioxide |
COCH3 |
acetyl group |
COOH |
carboxyl group |
Gln |
glutamine |
GLU |
glucose |
H |
hydrogen |
H2O |
water |
HCO3 |
hydrogencarbonate anion |
N |
nitrogen |
NADPH+H+ |
nicotinamide adenine dinucleotide phosphate |
NH2 |
amino group |
NH4+ |
ammonium ion |
O |
oxygen |
OH |
hydroxyl Group |
PO4 2 |
phosphate group |
TAG |
triacylglycerols |
β-HBA |
β-hydroxybutyrate |
INTRODUCTION
Eukaryotic cells are composed of water, inorganic ions and thousands of organic molecules (Cooper, 2019b). They participate in systems to extract, transform and utilize energy from the environment (Tortora et al., 2019b) which enables organisms to perform mechanical, chemical, osmotic and electrical work (Ameer et al., 2018; Rodwell, 2018a; Melo & Cuamatzi, 2019). Most of these organic molecules belong to one of three classes of polymers: i) polysaccharides, ii) lipids and iii) proteins (Fails & Magee, 2018a). These polymers constitute 80-90% of the weight of most cells (Pavlinov et al., 2019) and they are formed by the bonding (polymerization) of several low molecular weight chemical components: carbohydrates, fatty acids and amino acids, respectively (Guoyao, 2017c). The interaction between these components is dynamic; changes in one component lead to coordination or compensation changes in another (Tortora et al., 2019b). It is biochemistry that describes in molecular terms, this set of interactions (Pol et al., 2014). Considering two metabolic pathways primarily: i) catabolism to obtain acetyl-Coenzyme A (Tortora & Derrickson, 2018b) and ii) anabolism to acquire larger molecules (Pol et al., 2014; Engelking, 2015; Menzies et al., 2016). Thus contributing knowledge and practical applications in medicine (Gundu, 2020), agriculture (Milani et al., 2017), nutrition (Preethi & Sekar 2021) and industry (Wu et al., 2019) but their main concern is the cell as a living organism (Cooper, 2019a).
Therefore, this review provides an overview of the molecular dynamics at the interface of polysaccharide, lipid, and protein metabolism to ground the foundations of cell biology.
PHYSICOCHEMICAL PROPERTIES OF POLYSACCHARIDES
Polysaccharides are organic molecules consisting of more than ten monosaccharides, linked by O-glycosidic bonds (Yang et al., 2015; Guoyao, 2017c). Their general formula contains carbon (C) atoms hydrated with water (H 2 O) molecules (Bender & Mayes, 2018c). Therefore, they exhibit solubility in this fluid and their classification is established based on the position of their carbonyl group (C=O) (Chavarría & Cárabez, 2018). Formed by a C atom bonded to an oxygen atom (O) through a double bond (Cooper, 2019b). If the C=O group is located at the end of the molecule, it is an aldose. If the C=O group is located in the middle of the molecule, it is a ketose (Mckee & Mckee, 2014a; Delbianco et al., 2016).
Polysaccharides are the main biological source of energy storage and consumption (Chavarría & Cárabez, 2018) and are part of the organic structure of all living beings (Cooper, 2019b). Their entry into the organism is from food and their hydrolysis (breaking of O-glycosidic bonds) by amylases produced in the parotid glands ((Kumar & Chakravarty, 2018), and glycogen phosphorylases and glucose-6-phosphatases, produced by the acinar cells of the pancreas (Boticario & Cascales, 2012; Cárabez et al., 2018a)). Subsequent to this hydrolysis, the glucose monomer (GLU), with the chemical formula C6H12O6 (Bender & Mayes, 2018b), is released to be absorbed through the intestinal epithelium (Fails & Magee, 2018a) and distributed through the bloodstream to the different tissues (Dashty, 2013; Oosterveer & Schoonjans, 2014), where it presents five main metabolic pathways: (i) glycogenogenesis, (ii) pentose phosphate pathway (iii) glycogenolysis, (iv) glycolysis and (v) glycogenolysis (Appleton et al., 2013a; Nelson & Cox, 2017b).
GLYCOGEN ANABOLISM (GLYCOGENOGENESIS)
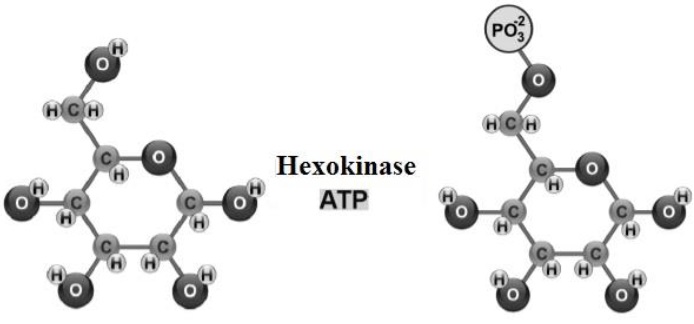
Glycogenogenesis takes place in myocytes (Engelking, 2015) and hepatocytes (Tortora & Derrickson, 2018b), where GLU enters the cytoplasm, to be phosphorylated [addition of a phosphate group (PO 2-)], from adenosine triphosphate (ATP) (Rui, 2014) (Figure 1).
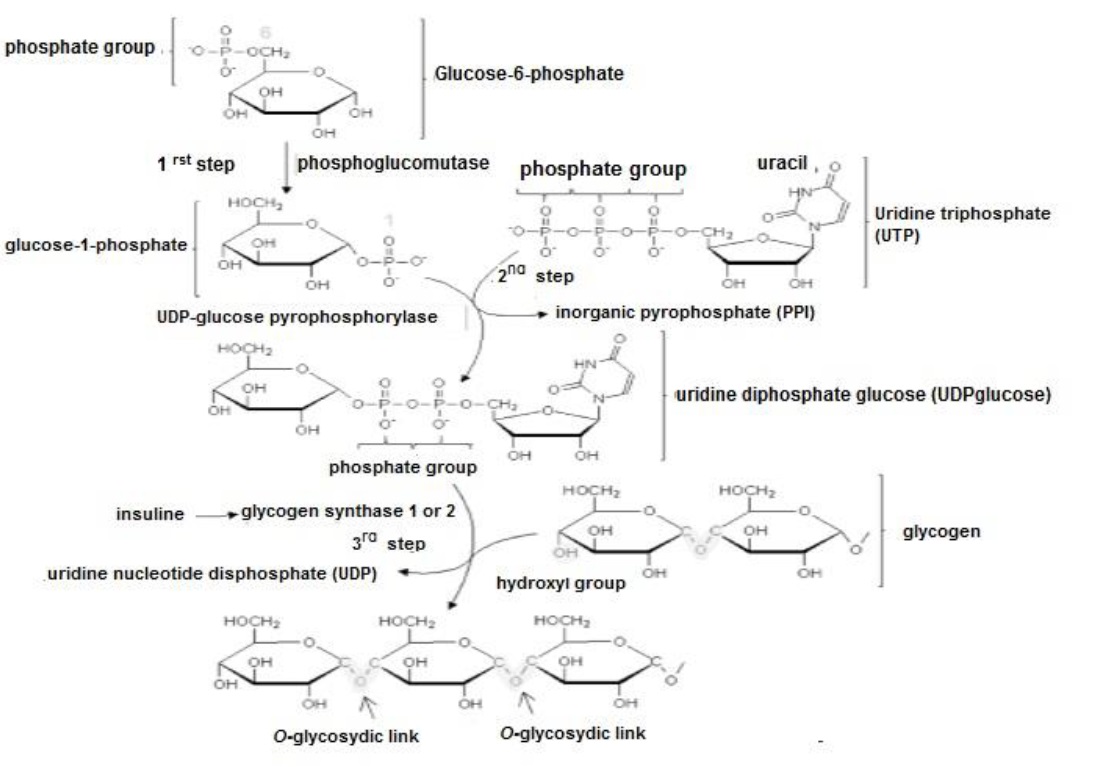
The resulting glucose-6-phosphate is abundant in the cytoplasm of all cells (Litwack, 2018a) and when its levels are elevated, phosphoglucomutase transfers the PO4 group from C6 to C1 synthesizing glucose-1-phosphate (Delbianco et al., 2016). Uridine triphosphate, interacts with glucose-1-phosphate, forming uridine diphosphate glucose (Fox et al., 2017). Insulin activates glycogen synthase 1 expressed in myocytes and/or glycogen synthase 2 expressed in hepatocytes (Gadupudi et al., 2016), so that the hydroxyl (OH) group of uridine diphosphate glucose binds to glycogen (creating an O- glycosidic bond), elongating the polysaccharide (Figure 2).
PENTOSE PHOSPHATE PATHWAY
This process takes place in the cytoplasm and is divided into two, the oxidative phase and the non-oxidative phase (Tortora & Derrickson, 2018b). The oxidative phase, presents of three reactions: i) glucose-6-phosphate is dehydrogenated [loses 2 hydrogens (H)] (Nelson & Cox, 2017b). As product 6-phosphogluconolactone and a nicotinamide adenine dinucleotide phosphate (NADPH+H +) molecule are obtained, and ii) 6- phosphogluconolactone is hydrolyzed and as product 6-phosphoglucanate is obtained (Lee et al., 2019) and iii) 6-phosphoglucanate is decarboxylated [removal of the carboxyl (COOH) group] (Mckee & Mckee, 2014b). As a product ribulose-5-phosphate (ketopentose), an NADPH+H+ molecule and carbon dioxide (CO 2) are obtained (Stincone et al., 2015).
During the non-oxidative phase, ribulose-5-phosphate can undergo isomerization and be transformed into another molecule that has the same atoms, but arranged differently (Madigan et al., 2019a). In other words, it changes its C=O group position to become a ribose 5-phosphate (aldopentose) (Cárabez et al., 2018b). Therefore, the main functions of the pentose phosphate pathway are (i) to synthesize 5-C monosaccharides and (ii) to generate NADPH+H+ (Nelson & Cox, 2017b).
Durante la fase no oxidativa la ribulosa-5-fosfato, puede presentar isomerización y ser transformada en otra molécula que posee los mismos átomos, pero dispuestos de forma distinta (Madigan et al., 2019a). En otra palabras, cambia de posición su grupo C=O para convertirse en a ribosa 5-fosfato (aldopentosa) (Cárabez et al., 2018b). Por lo tanto, las principales funciones de la ruta de las pentosas fosfato son: i) sintetizar monosacáridos de cinco C y ii) generar NADPH+H+ (Nelson & Cox, 2017b).
GLYCOGEN CATABOLISM (GLYCOGENOLYSIS
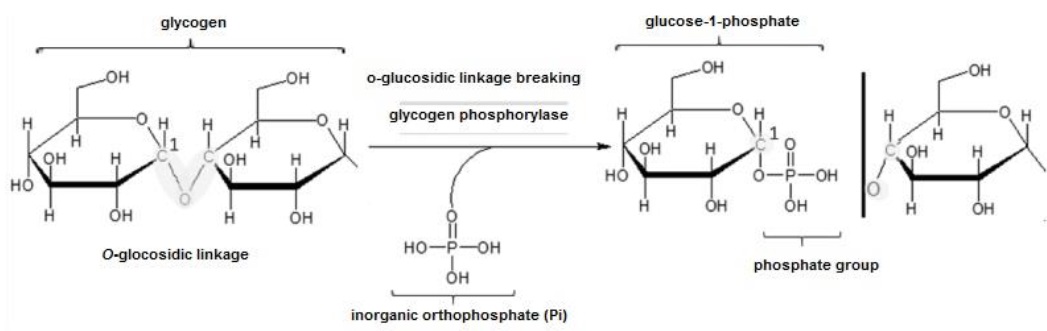
This process takes place in the cytoplasm of almost all cells, although particularly in muscle myocytes and liver hepatocytes (Mckee & Mckee, 2014c). When blood GLU levels are low, adrenaline or epinephrine in muscle and glucagon in liver activate protein kinases (Ahern, 2019d), and these perform phosphorylation to glycogen phosphorylase, thereby activating this enzyme (Mckee & Mckee, 2014c). Glycogen phosphorylase catalyzes the transfer of an inorganic orthophosphate at C1 of glycogen ((Fox et al., 2017), and this change breaks the O-glycosidic bond and releases glucose-1-phosphate (Figure 3). Glucose-1-phosphate is transformed into glucose-6-phosphate by transferring the PO 2- group from C1 to C6 (Ahern, 2019d).
GLUCOSE CATABOLISM (GLYCOLYSIS)
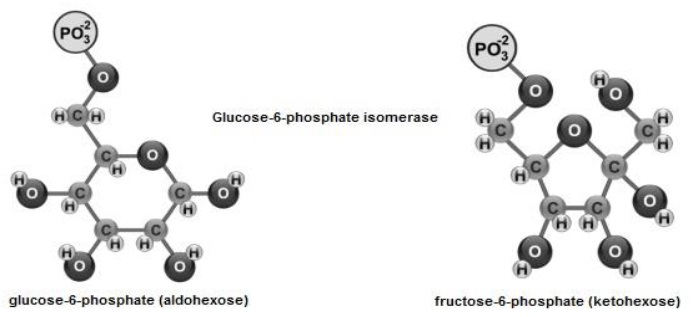
This process consists of the degradation of glucose-6-phosphate to obtain acetyl- coenzyme A from pyruvate (C 3 H 3 O 3) (Ferrier, 2017b). It takes place in the cytoplasm where glucose-6-phosphate (aldohexose), shows isomerization (Mckee & Mckee, 2014c) and it is transformed into fructose-6-phosphate (ketohexose) by shifting its C=O group (Figure 4).
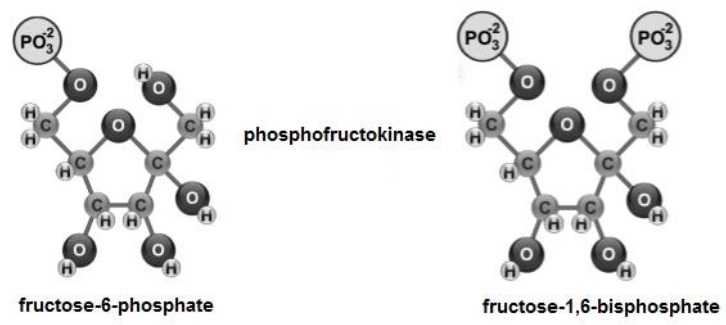
Fructose-6-phosphate, is phosphorylated (Figure 5), from ATP at C1 and C6 (Tortora et al., 2019a), to give rise to fructose-1,6-bisphosphate (Delbianco et al., 2016; Ferrier, 2017a).
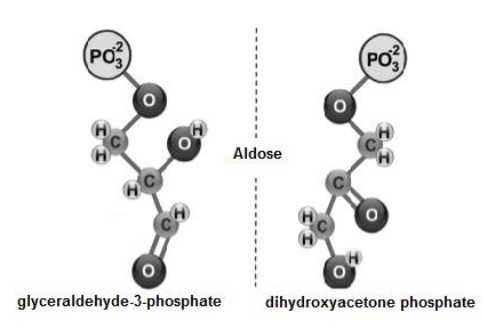
Subsequently, fructose-1,6-bisphosphate (Figure 6) is cleaved into two: i) glyceraldehyde-3-phosphate and ii) dihydroxyacetone phosphate (Melo & Cuamatzi, 2019).
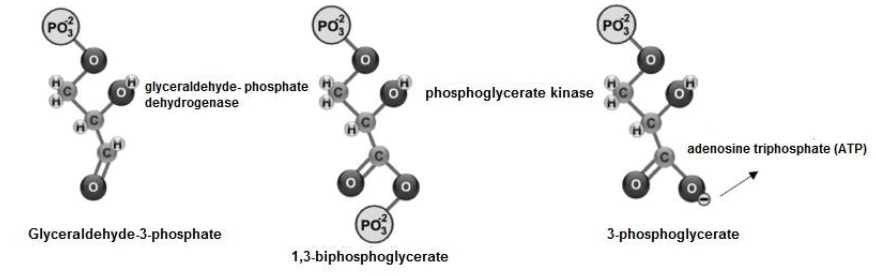
El gliceraldehido-3-fosfato es oxidado y fosforilado, en los C1 y C3 formando 1,3- bifosfoglicerato () (Figura 7). Posteriormente, transfiere su grupo PO 2−, para sintetizar ATP ) y se transforma en 3-fosfoglicerato.
Glyceraldehyde-3-phosphate is oxidized and phosphorylated, at C1 and C3 forming 1,3- bisphosphoglycerate (Mckee & Mckee, 2014c) (Figure 7). Subsequently, it transfers its PO42- group, to synthesize ATP (Ahern, 2019b) and is transformed into 3- phosphoglycerate (Voet et al., 2016; Tortora & Derrickson, 2018b).
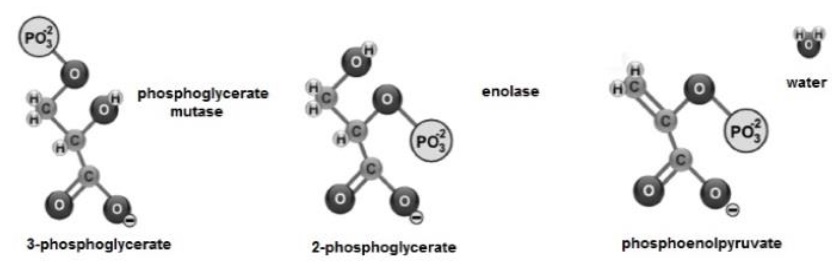
The 3-phosphoglycerate shows isomerization and its PO 2- group changes from C3 to C2, transforming the molecule into 2-phosphoglycerate (Nelson & Cox, 2017b). Next, enolase promotes the formation of a double bond (Voet et al., 2016), eliminating a molecule of H2O and forming phosphoenolpyruvate (Guoyao, 2017f; Bender & Mayes, 2018a) (Figure 8).
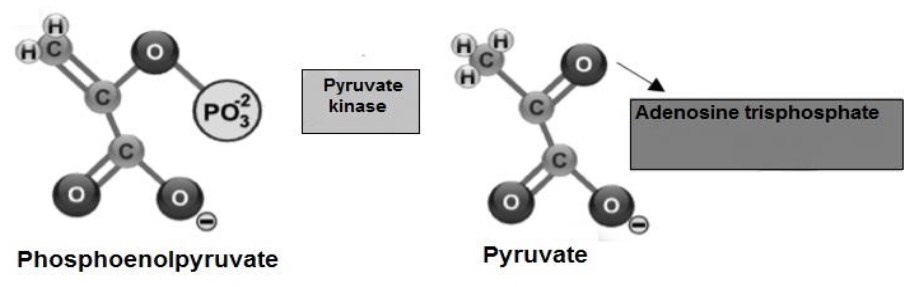
Phosphoenolpyruvate transfers its PO4 2- group (Cárabez et al., 2018a), to synthesize ATP (Ahern, 2019b) and is transformed into C3H3O3 (Botham & Mayes, 2018d), a molecule that is drawn into the mitochondrial matrix, using the proton-motive force generated by the respiratory chain (Fails & Magee, 2018b; Madigan et al., 2019c) (Figure 9).
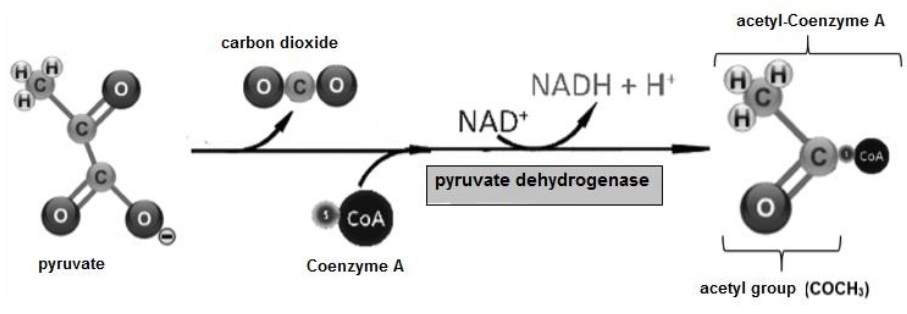
The fate of C3H3O3 produced in glycolysis depends on the availability of O. Under anaerobic conditions C3H3O3 undergoes reduction by adding H atoms to form lactate (Tortora & Derrickson, 2018b). Under aerobic conditions C3H3O3 presents decarboxylation and its COOH group is released as CO2 (Stincone et al., 2015), the rest of the molecule presents oxidation, to form the acetyl group (COCH 3). Finally, Coenzyme A is transferred to the COCH3 group forming acetyl-Coenzyme A (Guoyao, 2017f) (Figure 10).
PHYSICOCHEMICAL PROPERTIES OF LIPIDS
Lipids constitute an energy storage depot in adipocytes (Guoyao, 2017a). They participate in the formation of phospholipid membranes of eukaryotic cells and their organelles (Schoeler & Caesar, 2019). In the bloodstream, they transport fat-soluble vitamins e.g. A for soft tissue and mucosal formation (Botham & Mayes, 2018c), D for calcium ion (Ca 2+) absorption (Jameson, 2017), E as an antioxidant and erythrocyte formation (Madigan et al., 2019c) and K that contributes in coagulation (Guoyao, 2017a). They also act as a thermal insulator in subcutaneous tissues to retain body heat (Mas, 2018b).
Their entry into the body is from food and their hydrolysis (breaking of ester bonds) by lipases and esterases produced by the acinar cells of the pancreas (Ahern, 2019c).
Following this hydrolysis, non-esterified fatty acids (NEFA) and triacylglycerols (TAG) are released (Tortora et al., 2019a), to be absorbed via the intestinal epithelium (Pol et al., 2014; Guoyao, 2017d), and transported to the hepatocytes of the liver (Botham & Mayes, 2018c). Where they are packaged into very low density lipoproteins (Wadhera et al., 2016), for subsequent export to peripheral tissues (Wang et al., 2016). The NEFA obtained during this process are necessary to synthesize acetyl-Coenzyme A (Appleton et al., 2013d).
TRIACYLGLYCEROL ANABOLISM (LIPOGENESIS)
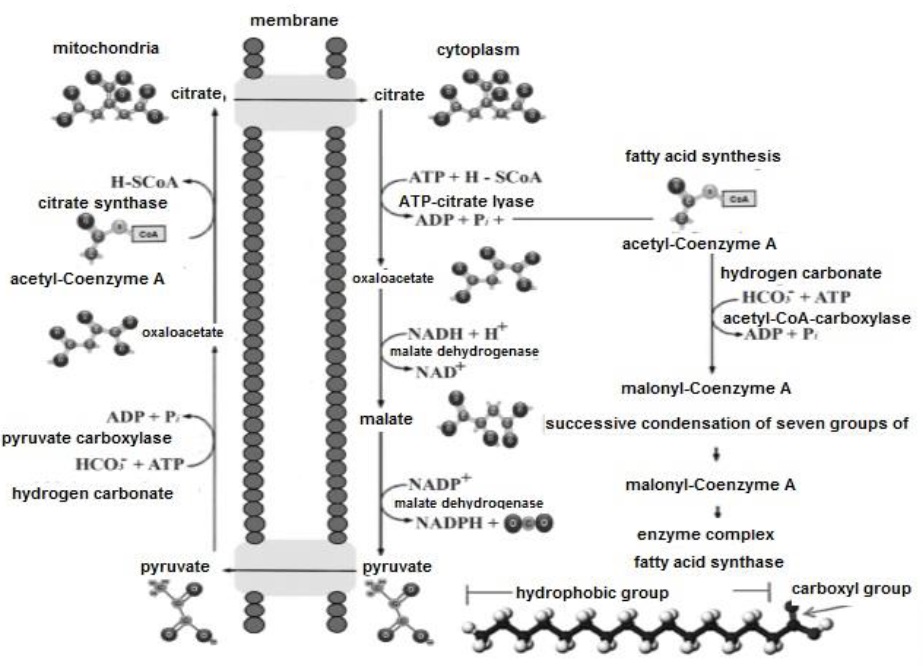
Lipogenesis initiates in the mitochondria, with the production of acetyl-Coenzyme A (Cooper, 2019a). Because the mitochondrial membrane is impermeable to the passage of acetyl-Coenzyme A (Friedman & Nunnari, 2014), the tricarboxylate system (Figure 11) and citrate synthase are required to convert it to citrate (Nunes-Nesi et al., 2013), via C- binding (Ameer et al., 2018), thereby ensuring its entry into the cell cytoplasm (Botham & Mayes, 2018c). Citrate is then transformed back into acetyl-coenzyme A by ATP-citrate lyase (Nunes-Nesi et al., 2013; Botham & Mayes, 2018c), yielding oxaloacetate and adenosine diphosphate (Mas, 2018a; Tortora & Derrickson, 2018a).
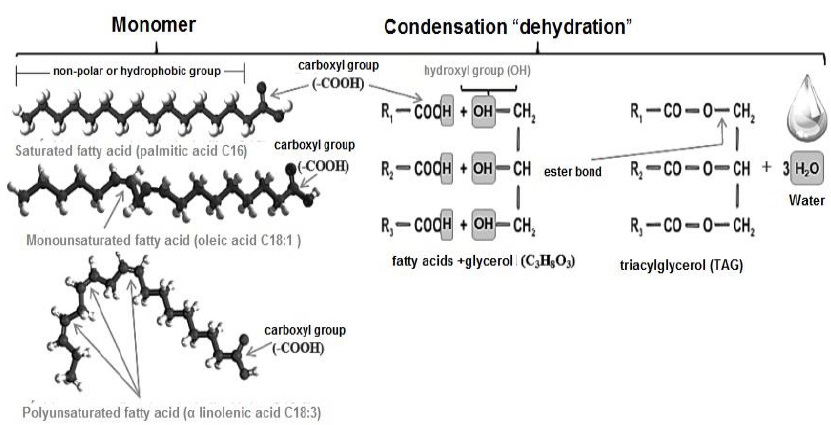
Lipogenesis is an endergonic process, therefore, acetyl-Coenzyme A must be activated by carboxylation through its binding to the hydrogencarbonate anion (HCO -) in an ATP- consuming reaction (Botham & Mayes, 2018a), catalyzed by acetyl-CoA carboxylase (Cooper, 2019b). As a result, acetyl-Coenzyme A is converted to malonyl-Coenzyme A (Nelson & Cox, 2017c). In turn, oxaloacetate is reduced by malate dehydrogenase to malate, and this in turn converted to C3H3O3 by malic enzyme, producing NADPH+H+ (Appleton et al., 2013e; Dashty, 2013). Subsequently, the fatty acid requires elongation, via the protein complex fatty acid synthase (Pol et al., 2014). This complex performs condensation, reduction, dehydration and again reduction, coupling malonyl-Coenzyme A groups with acetyl-Coenzyme A (Nelson & Cox, 2017c). The two reductions mentioned, require NADPH+H+ (Dashty, 2013), and during elongation two C groups are added to the fatty acid, always synthesizing hexadecanoic or palmitic (C16:0), as the final product (Guoyao, 2017d). Subsequently, C16:0 is released from the protein complex and can be elongated by introducing C into its chain, to produce other larger fatty acid molecules (Botham & Mayes, 2018c), and/or unsaturated by introducing double bonds into its chain (Cooper, 2019a). TAG synthesis, takes place in the smooth endoplasmic reticulum (Quintero, 2014).
Once different NEFAs are obtained, the lipid ester bond, is established by joining the three OH groups of glycerol (Nelson & Cox, 2017c) (Figure 12), and the COOH group (the polar part) of three fatty acids (Botham & Mayes, 2018c). This bond is a condensation or dehydration where 3 molecules of H2O are lost (Smith, 2020b). Due to this bonding, the polar groups attached to the carbohydrate are not accessible (Pratt et al., 2016). Consequently, non-polar or hydrophobic molecules, highly insoluble in water, are formed (Dowhan & Bogdanov, 2016).
TRIACYLGLYCEROL CATABOLISM (LIPOLYSIS) AND KETOGENESIS
When glycogen stores in the cytoplasm of myocytes and hepatocytes decrease, carnitine palmitoyltransferase is activated (Longo et al., 2016), stimulating transport of NEFA into the liver mitochondria (Merritt et al., 2020; Wang et al., 2020). Wang et al., 2020). Where β-oxidation, leads to a decarboxylation of the NEFA (Wanders et al., 2020), the COOH group is released as CO2 and the rest of the molecule exhibits dehydrogenation, establishing the COCH3 group (Botham & Mayes, 2018b). Coenzyme A, is transferred to the COCH3 group and forms acetyl-Coenzyme A (Guoyao, 2017f). This molecule combines with oxaloacetate for entry into the Krebs cycle (Appleton et al., 2013c). If its oxidation is complete, CO2 and pairs of H atoms will be released (Friedman & Nunnari, 2014), which will donate their electrons to perform oxidation-reduction reactions (Madigan et al., 2019c), H2O formation and energy storage in the form of ATP (Jump, 2011).
However, if oxaloacetate is not sufficient, acetyl-Coenzyme A accumulates within the mitochondrion (Longo et al., 2016). Subsequently two molecules of acetyl-Coenzyme A react to form acetoacetyl-CoA, in a reaction catalyzed by thiolase (Merritt et al., 2018). Acetoacetyl-CoA condenses with another acetyl-Coenzyme A molecule to form β- hydroxy-β-methylglutaryl-CoA (Mas, 2018a). From this molecule acetoacetate (AcAc) is metabolized, a ketone body that leaves the mitochondria and in the cytoplasm of the hepatocyte can be reduced to β-hydroxybutyrate (β-HBA) (Selvaraj et al., 2020) or slowly and spontaneously decarboxylated to acetone (Ac) (Deemer et al., 2020).
PHYSICOCHEMICAL PROPERTIES OF PROTEINS
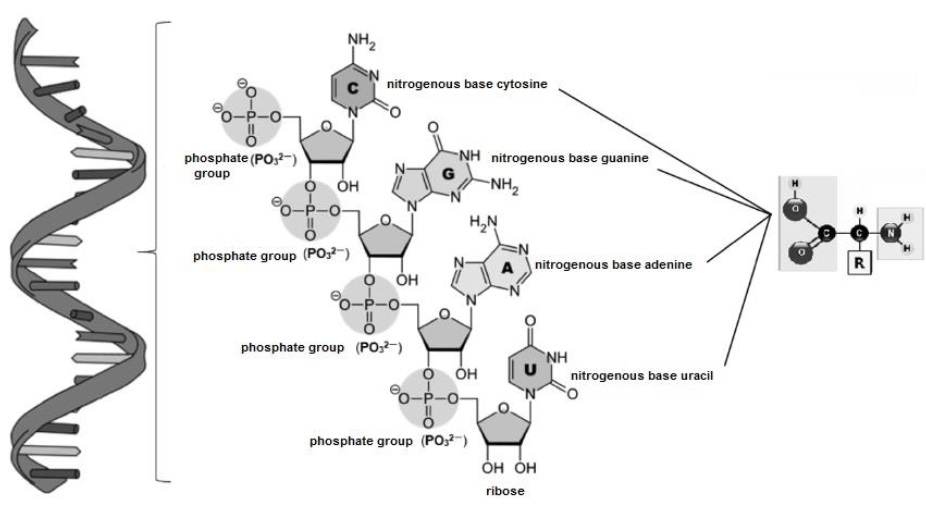
Of the three biomolecules discussed, proteins are the only ones that contain nitrogen (N) atoms (Ferrier, 2017c). They are constituted by the combination of 20 amino acids (aa) (Ahern, 2019a), linked by a peptide bond (Guoyao, 2017b). This covalent type bond, unites the amino group (NH 2) of one aa and the COOH group of another, with the formation of a H2O molecule (Madigan et al., 2019b). Proteins actively participate in cellular homeostasis ((Cooper, 2019b), e.g., transporting O (Guoyao, 2017b), structuring immunoglobulins (Kenneth & Casey, 2017) and constituting enzymes (Ahern, 2019c).
They enter the body from food and are hydrolyzed (peptide bond breaking) by peptidases or proteases and aminotransferases, produced by the acinar cells of the pancreas (Ahern, 2019c). Following this hydrolysis, aa are released (Rodwell, 2018a), to be absorbed through the intestinal epithelium (Guoyao, 2017e; Piña & Flores, 2018), and transported to the hepatocytes of the liver (Appleton et al., 2013b)Appleton et al., 2013b), for subsequent export to peripheral tissues (Fernández & Peimbert, 2018).
Within the cell cytoplasm, aa can lose their NH2 group and as carbon skeletons function as: i) substrate to synthesize C3H3O3 and subsequently acetyl-Coenzyme A (Appleton et al., 2013d), ii) structure purines and neurotransmitters (Rodwell, 2018b) and iii) participate in proteogenesis (Rodwell, 2018a; Madigan et al., 2019b) or ureogenesis (Nelson & Cox, 2017a) mainly.
PROTEIN ANABOLISM (PROTEOGENESIS)
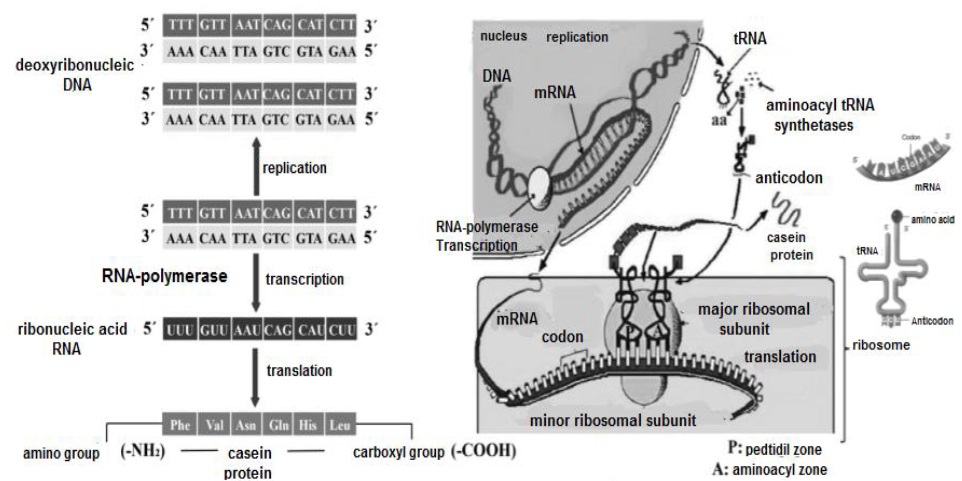
Proteogenesis (Figure 13), begins in the cell nucleus (Noller, 2017), with the transcription of transfer ribonucleic acid (tRNA) (Nelson & Cox, 2017d; Madigan et al., 2019d). Subsequently, the enzyme RNA polymerase performs the transcription of messenger ribonucleic (mRNA) from a deoxyribonucleic (DNA) sequence (Liu et al., 2013), which serves as a template or mold for the genetic information (Litwack, 2018b). The mRNA is transported to the rough endoplasmic reticulum and its ribosomes (Weil, 2018b). During initiation, a bridge is formed between the minor and major ribosomal subunit (Weil, 2018a).
For their part, tRNA (Figure 14), have to bind with different aminoacyl-tRNA synthetases (Rodnina & Wintermeyer, 2016), to expose the NH2 group of their nitrogenous bases (cytosine, guanine, adenine and uracil) and attach the COOH group of the different aa (Smith, 2020a).
The aa transported on the tRNA enter the ribosomal complex, which has two binding sites: i) the P or peptidyl site and ii) the A or aminoacyl site (Berk et al., 2006). Translation is carried out in ribosomes, by reading triplets (three by three nucleotides) called: codon for mRNA and anticodon for tRNA (Ingolia, 2014). The first stage of translation, begins when the 5' end of the mRNA is inserted into the minor ribosomal subunit (Nelson & Cox, 2017d), exposing the initiator codon adenine-uracil-guanine or AUG for binding to the first anticodon uracil-adenine-cytosine or UAC, at the peptidyl site (Angov, 2011), originating methionine as the first aa (Madigan et al., 2019d).
Subsequently, when the peptidyl site and the aminoacyl site are occupied simultaneously, the peptidyl transferase enzyme establishes a peptide bond between the aa by inserting the former into the latter (Weil, 2018a). Then, in elongation codon and anticodon are precisely associated according to the complementarity of their bases (Dutta & Nandi, 2012), and this sequence of steps is repeated according to the number of aa contained in the polypeptide (Madigan et al., 2019b). As a completion of this process, different proteins and enzymes mainly hydrolases are translated (Swiderek et al., 2015).
PROTEIN CATABOLISM (PROTEOLYSIS) AND UREOGENESIS
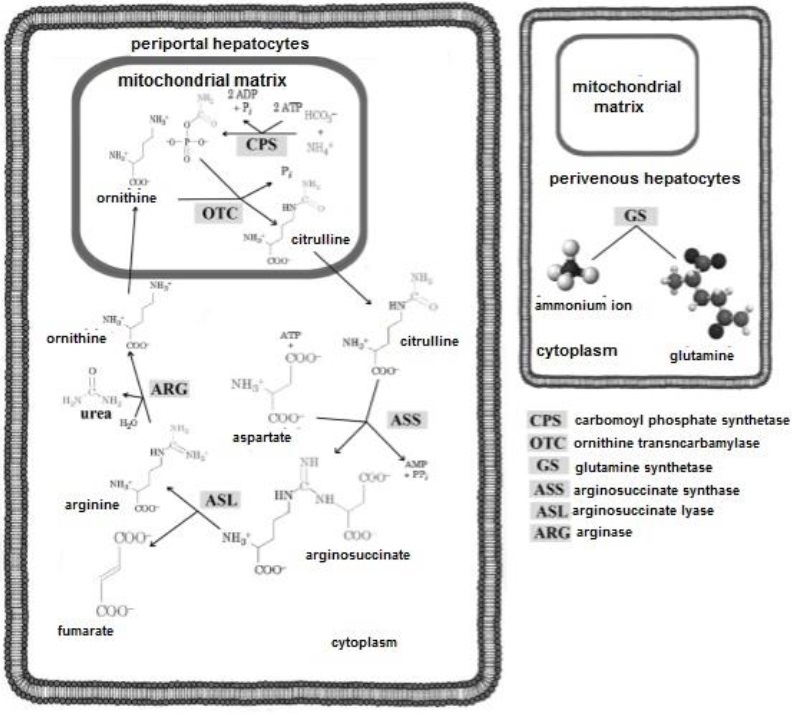
After the gastric and enzymatic digestion of proteins, the breaking of their peptide bonds, and the release and absorption of aa (Piña & Flores, 2018), ammonium ion (NH +) is also obtained (Rodwell, 2018a). This molecule travels to the liver, where its first contact is with periportal hepatocytes (Guoyao, 2017e), which possess in their structure ureagenic enzymes in charge of urea synthesis (Figure 15). In the mitochondria of periportal hepatocytes, HCO3-, NH4+ and ATP (Appleton et al., 2013b) are condensed to form carbamyl phosphate (Friedman & Nunnari, 2014). Ornithine enters the mitochondrion and carbamyl phosphate gives up its carbamyl group to form citrulline (Weiner et al., 2015).
Citrulline exits the mitochondria into the cytoplasm, where it binds to aspartate, forming arginosuccinate (Menzies et al., 2016). Arginosuccinate is cleaved into two: i) arginine (Arg) and ii) fumarate. Arg is hydrolyzed by arginase releasing urea and ornithine (Nelson & Cox, 2017a). The latter enters the mitochondria to initiate another turn in the cycle (Rodwell, 2018a). Urea in turn, can travel to the kidney (Guoyao, 2017b) and be excreted in urine (Marini & van Amburgh, 2003). The NH4+ ion that is not metabolized into urea, has contact with perivascular hepatocytes, which possess in their structure glutamine synthetase (Piña & Flores, 2018), which converts NH + ion into glutamine (Gln). This polar or hydrophilic aa, presents affinity for H2O (Appleton et al., 2013a). Therefore, it favors the transport and excretion of NH + ion in urine (Rodwell, 2018a).
ADENOSINE TRIPHOSPHATE ANABOLISM (KREBS CYCLE)
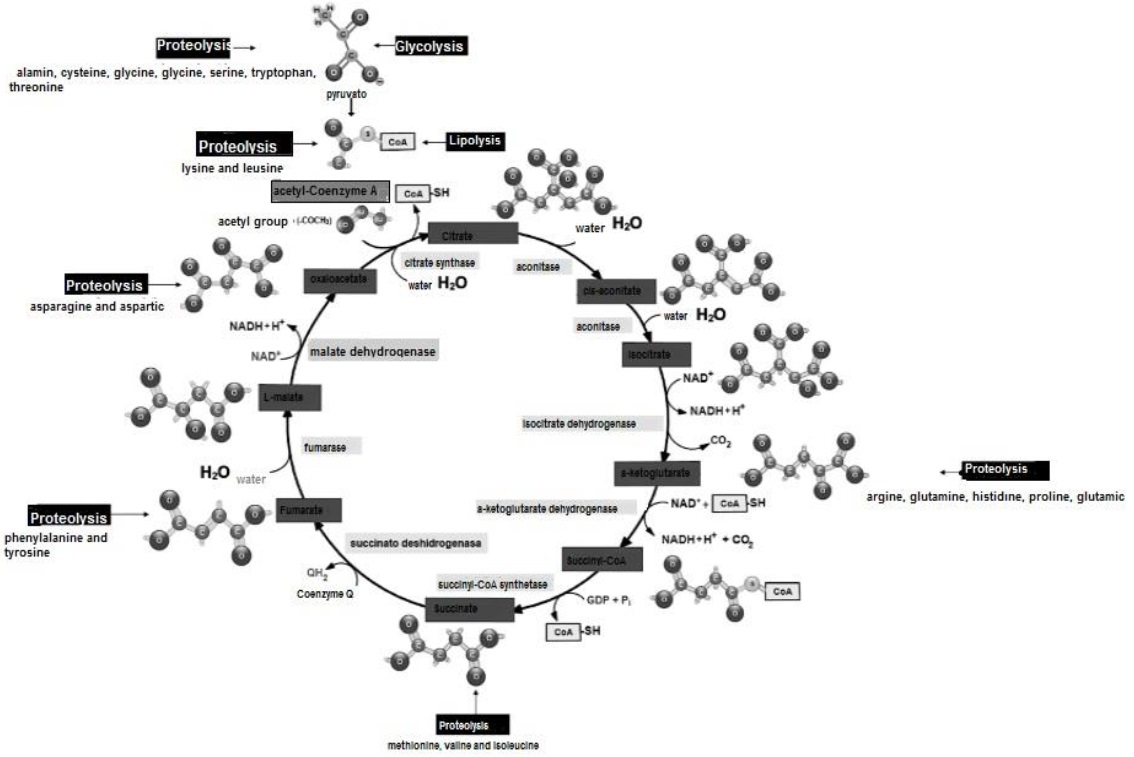
The Krebs cycle was discovered by Hans Adolf Krebs (Appleton et al., 2013c). It is part of mitochondrial gas exchange (Madigan et al., 2019c) and allows the release of stored energy from acetyl-Coenzyme A in the form of the nucleotide ATP (Botham & Mayes, 2018d). Acetyl-Coenzyme A binds its COCH3 group to bind with oxaloacetate to form citrate via a condensation reaction (Menzies et al., 2016; Verschueren et al., 2019). During a complete turn of the cycle and through hydrolysis, oxidative decarboxylation and h ydration (Figure 16), citrate is converted back to oxaloacetate (Appleton et al., 2013d).
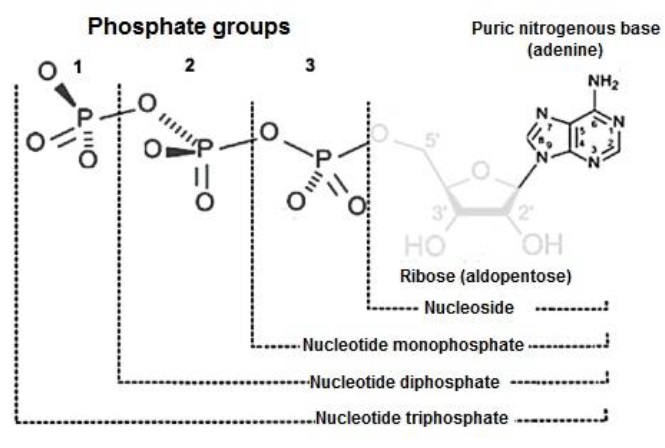
The C atoms released in the process form CO2 (Madigan et al., 2019c). The Krebs cycle consumes per turn one acetyl-Coenzyme A and three NAD+ (Nelson & Cox, 2017e). It produces for each turn two CO2 and three NADPH+H+ (Friedman & Nunnari, 2014). For each acetyl-Coenzyme A entering the Krebs cycle, 12 ATP are produced (Appleton et al., 2013c), each consisting of a purine or purine nitrogenous base (adenine), linked to a ribose (aldopentose) and three PO4 2- (Botham & Mayes, 2018a) (Figure 17).
For each GLU (C6H12O6) entering the cycle, two C3H3O3 are produced, which in turn produce two acetyl-Coenzyme A (Nelson & Cox, 2017e). Therefore, for each GLU (C6H12O6) entering the Krebs cycle, four CO2, six NADPH+H+ and 24 ATP molecules are produced (Friedman & Nunnari, 2014).
The information presented in previous paragraphs, shows how the biomolecules that constitute living organisms, interact to maintain and perpetuate life, governed by the same physical and chemical laws that govern the inert universe. The frontier of knowled ge was organized around central principles or questions of biochemistry and how cells use a relatively small set of carbon-based metabolites to create polymeric molecules, supramolecular structures and information reservoirs. The chemical structure of thes e components defines their cellular function, the end result of which is the transformation and self-perpetuation of that compilation of biomolecules, in short, life.
CONCLUSIONS
Eukaryotic cells are composed of water, inorganic ions and organic molecules. They contain carbon chains with hydroxyl, amino and carboxyl functional groups, responsible for the formation of cell tissue. These structures obey the laws of chemistry and physics that determine the metabolism of living systems. Animals, possessing a high chemical complexity and a robust microscopic organization, constitute in their molecular anabolism and catabolism, systems of extraction, transformation and utilization of monosaccharides, amino acids and fatty acids. For the formation of acetyl-Coenzyme A and the release of its energy in the Krebs cycle. Thus, the biochemistry of cellular metabolism can be understood in terms of the structures and functions of three main classes of organic molecules polysaccharides, lipids and proteins.
ACKNOWLEDGMENTS
This work was supported by the National Council of Science and Technology (CONACyT- Mexico) (CONACyT-México) and the project: Metabolic profiles and their implications in veterinary medicine (Universidad de Colima).
REFERENCES
Ahern K. 2019a. Amino acids: 20 building blocks of life. In: Ahern K, Biochemistry and Molecular Biology: How Life Works. Virginia, United States: The Teaching Company. Pp. 29-40. ISBN: 978-1-25-983793-7. [ Links ]
Ahern K. 2019b. Breaking down sugars and fatty acids. In: Ahern K, Biochemistry and Molecular Biology: How Life Works. Virginia, United States: The Teaching Company. Pp.120-126. ISBN: 978-1-25-983793-7. [ Links ]
Ahern K. 2019c. Enzyme regulation in cells. In: Ahern K, Biochemistry and Molecular Biology: How Life Works. Virginia, United States: The Teaching Company. Pp. 84-92. ISBN: 978-1-25-983793-7. [ Links ]
Ahern K. 2019d. How animals make carbs and fats. In: Ahern K, Biochemistry and Molecular Biology: How Life Works. Virginia, United States: The Teaching Company. Pp.158. ISBN: 978-1-25-983793-7. [ Links ]
Ameer F, Munir R, Zaidi N. 2018. Lipid metabolism. Reference Module in Biomedical Sciences. 1(1):1-4. ISSN: 9780128012383. http://dx.doi.org/10.1016/B978-0-12-801238-3.64998-X [ Links ]
Angov E. 2011. Codon usage: nature's roadmap to expression and folding of proteins. Biotechnology Journal. 6(6):650-659. ISSN: 1860-7314. https://onlinelibrary.wiley.com/doi/10.1002/biot.201000332 [ Links ]
Appleton A, Vanbergen O, Dominiczak MH. 2013a. Metabolismo de los hidratos de carbono. En: Horton-Szar D, Lo Esencial en Metabolismo y Nutrición. Barcelona, España: Elsevier Health Sciences. Pp. 23-40. ISBN: 978-84-9022-416-8. [ Links ]
Appleton A, Vanbergen O, Dominiczak MH. 2013b. Metabolismo de las proteínas. En: Horton-Szar D, Lo Esencial en Metabolismo y Nutrición. Barcelona, España: Elsevier Health Sciences. Pp. 71-82. ISBN: 978-84-9022-416-8. [ Links ]
Appleton A, Vanbergen O, Dominiczak MH. 2013c. Metabolismo energético I: ciclo ATC. En: Horton-Szar D, Lo Esencial en Metabolismo y Nutrición. Barcelona, España: Elsevier Health Sciences. Pp. 13-17. ISBN: 978-84-9022-416-8. [ Links ]
Appleton A, Vanbergen O, Dominiczak MH. 2013d. Metabolismo energético II: generación de ATP. En: Horton-Szar D, Lo Esencial en Metabolismo y Nutrición. Barcelona, España: Elsevier Health Sciences. Pp. 17-23. ISBN: 978-84-9022-416-8. [ Links ]
Appleton A, Vanbergen O, Dominiczak MH. 2013e. Transporte y metabolismo de los lípidos. En: Horton-Szar D, Lo Esencial en Metabolismo y Nutrición. Barcelona, España: Elsevier Health Sciences. Pp. 45-70. ISBN: 978-84-9022-416-8. [ Links ]
Bender AD, Mayes AP. 2018a. Glycolysis & the oxidation of pyruvate. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. New York, United States: McGraw-Hill Education / Medical. Pp.400-404. ISBN: 978-1-25-983794-4. [ Links ]
Bender AD, Mayes AP. 2018b. Overview of metabolism & the provision of metabolic fuels. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. New York, United States: McGraw-Hill Education / Medical. Pp. 336-342. ISBN: 978-1-25-983794-4. [ Links ]
Bender AD, Mayes AP. 2018c. Physiologically important carbohydrates. n: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. United States: McGraw-Hill Education / Medical. Pp. 363-381. ISBN: 978-1-25-983794-4. [ Links ]
Berk V, Zhang W, Pai RD, Cate JH. 2006. Structural basis for mRNA and tRNA positioning on the ribosome. Proceedings of the National Academy of Sciences of the United States of America. 103(43):15830-15834. ISSN: 0027-8424. https://doi.org/10.1073/pnas.0607541103 [ Links ]
Botham MK, Mayes AP. 2018a. Bioenergetics: the role of ATP. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. United States: McGraw-Hill Education / Medical. Pp. 277-291. ISBN: 978-1-25-983793-7. [ Links ]
Botham MK, Mayes AP. 2018b. Fatty acid oxidation: ketogenesis. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. United States: McGraw-Hill Education / Medical. Pp. 512-534. ISBN: 978-1-25-983793-7. [ Links ]
Botham MK, Mayes AP. 2018c. Lipids of physiological significance. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. United States: McGraw-Hill Education / Medical. Pp. 483-511. ISBN: 978-1-25-983793-7. [ Links ]
Botham MK, Mayes AP. 2018d. The respiratory chain and oxidative phosphorylation. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. United States: McGraw-Hill Education / Medical. Pp. 308-335. ISBN: 978-1-25-983793-7. [ Links ]
Boticario BC, Cascales AM. 2012. Metabolismo de los carbohidratos. En: Boticario BC , Cascales AM, Digestión y Metabolismo Energético de los Nutrientes Plasencia, España: UNED. Pp. 86. ISBN: 978-84-615-8137-5. [ Links ]
Cárabez TA, Sánchez AJ, Riveros RH. 2018a. Metabolismo de los carbohidratos. En: Hernández MMT, Bioquímica de Laguna y Piña. Ciudad de México, México: El Manual Moderno. Pp. 591-607. ISBN: 978-607-448-708-4. [ Links ]
Cárabez TA, Sánchez AJ, Riveros RH. 2018b. Vía colateral de oxidación de la glucosa: ciclo de las pentosas. En: Hernández MMT, Bioquímica de Laguna y Piña. Ciudad de México, México: El Manual Moderno. Pp. 636-638. ISBN: 978-607-448-708-4. [ Links ]
Cooper GM. 2019a. The biosynthesis of cell constituents. Carbohydrates, lipids, proteins, adn nucleic acids. In: Cooper GM, The Cell: A Molecular Approach. Oxford, New York: Oxford University Press. Pp. 102-111. ISBN: 978-1-60535-707-2. [ Links ]
Cooper GM. 2019b. The molecules of cells. Chemical bonds, carbohydrates, lipids, nucleic acids y proteins. In: Cooper GM, The Cell: A Molecular Approach. Oxford, New York: Oxford University Press. Pp. 45-60. ISBN: 978-1-60535-707-2. [ Links ]
Cooper GM. 2019c. The origin and evolution of cells. In: Cooper GM, The Cell: A Molecular Approach. Oxford, New York: Oxford University Press. Pp. 4-18. ISBN: 978-1-60535-707-2. [ Links ]
Chavarría KA, Cárabez TA. 2018. Química de los carbohidratos. En: Hernández MMT, Bioquímica de Laguna y Piña. Ciudad de México, México: El Manual Moderno. Pp. 416- 431. ISBN: 978-607-448-708-4. [ Links ]
Dashty M. 2013. A quick look at biocheistry: carbohydrate metabolism. Clinical Biochemistry. 46(1):1339-1352. ISSN: 1873-2933. https://doi.org/10.1016/j.clinbiochem.2013.04.027 [ Links ]
Deemer SE, Plaisance EP, Martins C. 2020. Impact of ketosis on appetite regulation-a review. Nutrition Research. 77(1):1-11. ISSN: 1879-0739. https://doi.org/10.1016/j.nutres.2020.02.010 [ Links ]
Delbianco M, Bharate P, Varela-Aramburu S, Seeberger PH. 2016. Carbohydrates in supramolecular chemistry. Chemical Reviews. 116(4):1693-16752. ISSN: 1520-6890. https://doi.org/10.1021/acs.chemrev.5b00516 [ Links ]
Dowhan W, Bogdanov M. 2016. Functional roles of lipids in membranes. In: Ridway ND, McLeod SR, Bichemistry of lipids, lipoproteins and membranes. Nova Scotia, Canada: Elsevier. Pp.1-40. ISBN: 0444634495. [ Links ]
Dutta BS, Nandi N. 2012. Chirality and protein biosynthesis. Topics in Current Chemistry. 372(1):1-51. ISBN: 2364-8961. http://doi.org/10.1007/128_2012_369 [ Links ]
Engelking LR. 2015. Gluconeogenesis. In: Engelking LR, Textbook of Veterinary Physiological Chemistry. Massachusetts, United States: Academic Press. Pp. 225-230. ISBN: 978-0-12-391909-0. [ Links ]
Fails DA, Magee C. 2018a. Nutrition and metabolism. In: Fails DA , Magee C, Anatomy and physiology of farm animals. Hoboken, United States: John Wiley & Sons. Pp. 413- 419. ISBN: 978-111-923-971-0. [ Links ]
Fails DA, Magee C. 2018b. Transport across cell membranes. In: Fails DA , Magee C, Anatomy and physiology of farm animals. Hoboken, United States: John Wiley & Sons. Pp. 36-43. ISBN: 978-111-923-971-0. [ Links ]
Fernández VDA, Peimbert TM. 2018. Aminoácidos y proteínas. En: Hernández MMT, Bioquímica de Laguna y Piña. Ciudad de México, México: El Manual Moderno. Pp. 217- 260. ISBN: 978-607-448-708-4. [ Links ]
Ferrier RD. 2017a. A introduction to metabolism and glucolysis. In: Shannon M, Biochemistry. Philadelphia, United States: Wolters Kluwer. Pp. 298-326. ISBN: 978-149- 634-449-6. [ Links ]
Ferrier RD. 2017b. Glycolysis and gluconeogenesis. In: Shannon M, Biochemistry. Philadelphia, United States: Wolters Kluwer. Pp. 449-462. ISBN: 1978-149-634-449-6. [ Links ]
Ferrier RD. 2017c. Protein structure and function. Amino acids. In: Shannon M, Biochemistry. Philadelphia, United States: Wolters Kluwer. Pp. 13-42. ISBN: 978-149-634-449-6. [ Links ]
Fox SI, Sierra GP, Bari SO. 2017. Respiración y metabolismo celulares. En: Fox SI, Fisiología Humana New York, United States: McGraw-Hill Education / Medical. Pp. 833. ISBN: 978-607-151-413-4. [ Links ]
Friedman JR, Nunnari J. 2014. Mitochondrial form and function. Nature. 505(7483):335- 343. ISSN: 1476-4687. https://doi.org/10.1038/nature12985 [ Links ]
Gadupudi GS, Klingelhutz AJ, Robertson LW. 2016. Diminished Phosphorylation of CREB is a key event in the dysregulation of gluconeogenesis and glycogenolysis in PCB126 hepatotoxicity. Chemical Research in Toxicology. 29(1):1504-1509. ISSN: 0893- 228X. http://doi.org/10.1021/acs.chemrestox.6b00172 [ Links ]
García CAC, Prado ROF, H. PD. 2020. Fisiología del período de transición, posparto y retorno al estro en vacas lecheras: desafíos para la producción sustentable. En: Gutiérrez NNS, Gutiérrez VMC , Ramírez GMJ, Handbook T-II Sustentabilidad, turismo y educación. México, Ciudad de México: ECORFAN-México, S.C. Pp. 63-86. ISBN: 978-607-8695-29-4. [ Links ]
Gundu HRR. 2020. Cardiometabolic diseases: Biochemistry, Pathophysiology and medical innovations. Biochemistry and Modern Applications. 3(1):1-5. ISSN: 2638-7735. http://doi.org/10.33805/2638-7735.126 [ Links ]
Guoyao W. 2017a. Chemistry of lipids. In: Guoyao W, Principles of Animal Nutrition. New York, United States: CRC Press. Pp. 109-142. ISBN: 978-1-4987-2160-8. [ Links ]
Guoyao W. 2017b. Chemistry of protein and amino acids. In: Guoyao W, Principles of Animal Nutrition. New York, United States: CRC Press. Pp. 149-188. ISBN: 978-1-4987-2160-8. [ Links ]
Guoyao W. 2017c. Introduction to metabolism. In: Guoyao W, Principles of Animal Nutrition. New York, United States: CRC Press. Pp. 67-69. ISBN: 978-1-4987-2160-8. [ Links ]
Guoyao W. 2017d. Nutrition and metabolism of lipids. In: Guoyao W, Principles of Animal Nutrition. New York, United States: CRC Press. Pp. 271-338. ISBN: 978-1-4987-2160-8. [ Links ]
Guoyao W. 2017e. Nutrition and metabolism of protein and amino acids. In: Guoyao W, Principles of Animal Nutrition. New York, United States: CRC Press. Pp. 349-411. ISBN: 978-1-4987-2160-8. [ Links ]
Guoyao W. 2017f. Pathway of glycolysis. In: Guoyao W, Principles of Animal Nutrition. New York, United States: CRC Press. Pp. 219. ISBN: 978-1-4987-2160-8. [ Links ]
Ingolia NT. 2014. Ribosome profiling: new views of translation, from single codons to genome scale. Nature Reviews Genetics. 15(3):205-213. ISSN: 1471-0064. https://doi.org/10.1038/nrg3645 [ Links ]
Jameson LJ. 2017. Electrolitos y equilibrio ácido-base. En: Kasper LD, Fauci SA, Hauser LS, Longo LD, Jameson LJ , Loscalzo J, Harrison. Manual de medicina. Ciudad de México, México: McGraw-Hill. Pp.1-23. ISBN: 978-607-15-1409-7. [ Links ]
Jump DB. 2011. Fatty acid regulation of hepatic lipid metabolism. Current Opinion in Clinical Nutrition & Metabolic Care. 14(2):115-120. ISSN: 1473-6519. http://doi.org/10.1097/MCO.0b013e328342991c [ Links ]
Kenneth M, Casey W. 2017. The humoral immune response. In: Toledo M, Janeway’s Immunobiology. New York, United States: Garland Science. Pp. 399-445. ISBN: 978-081-534-505-3. [ Links ]
Kumar S, Chakravarty S. 2018. Amylases. In: Simões NC , Kumar V, Enzymes in Human and Animal Nutrition: Principles and Perspectives. New York, United States: Academic Press. Pp. 163-175. ISBN: 978-012-809-426-6. [ Links ]
Lee MH, Malloy CR, Corbin IR, Li J, Jin ES. 2019. Assessing the pentose phosphate pathway using [2, 3‐13C2] glucose. NMR in Biomedicine. 1(1):1-10. ISSN: 1099-1492. https://doi.org/10.1002/nbm.4096 [ Links ]
Litwack DG. 2018a. Glycogen and glycogenolysis. In: Litwack DG, Human Biochemistry. California, United States: Academic press. Pp. 183-198. ISBN: 978-0-12-383864-3. [ Links ]
Litwack DG. 2018b. Proteins biosynthesis. In: Litwack DG, Human Biochemistry. California, United States: Academic press. Pp. 319-336. ISBN: 978-0-12-383864-3. [ Links ]
Liu X, Bushnell DA, Kornberg RD. 2013. RNA polymerase II transcription: structure and mechanism. Biochimica et Biophysica Acta. 1829(1):2-8. ISSN: 1874-9399. https://doi.org/10.1016/j.bbagrm.2012.09.003 [ Links ]
Longo N, Frigeni M, Pasquali M. 2016. Carnitine transport and fatty acid oxidation. Biochimica et Biophysica Acta. 1863(10):2422-2435. ISSN: 1874-9399. https://doi.org/10.1016/j.bbamcr.2016.01.023 [ Links ]
Madigan TM, Bender SK, Buckley HD, Sattley WM, Stahl AD. 2019a. Biosyntheses. Sugars and polysaccharides. Amino acids and nucleotides. Fatty acids and lipids. En: Madigan TM, Brock Biology of Microorganisms. New York, United States: Pearson. Pp. 130-137. ISBN: 978-1-292-23510-3. [ Links ]
Madigan TM, Bender SK, Buckley HD, Sattley WM, Stahl AD. 2019b. Protein synthesis: translation. In: Madigan TM, Brock Biology of Microorganisms. New York, United States: Pearson. Pp. 156-170. ISBN: 978-1-292-23510-3. [ Links ]
Madigan TM, Bender SK, Buckley HD, Sattley WM, Stahl AD. 2019c. Respiratory processes defined by electron donor. Hydrogen (H2) oxidation. In: Madigan TM, Brock Biology of Microorganisms. New York, United States: Pearson. Pp. 446-449. ISBN: 978-1-292-23510-3. [ Links ]
Madigan TM, Bender SK, Buckley HD, Sattley WM, Stahl AD. 2019d. RNA synthesis: transcription In: Madigan TM, Brock Biology of Microorganisms. New York, United States: Pearson. Pp. 151-155. ISBN: 978-1-292-23510-3. [ Links ]
Marini JC, van Amburgh ME. 2003. Nitrogen metabolism and recycling in Holstein heifers. Journal of Animal Science. 81(2):545-552. ISSN: 0021-8812. https://doi.org/10.2527/2003.812545x [ Links ]
Mas OJ. 2018a. Metabolismo de los lípidos. En: Hernández MMT, Bioquímica de Laguna y Piña. Ciudad de México, México: El Manual Moderno. Pp. 660-785. ISBN: 978-607-448-708-4. [ Links ]
Mas OJ. 2018b. Química de los lípidos. En: Hernández MMT, Bioquímica de Laguna y Piña. Ciudad de México, México: El Manual Moderno. Pp. 456-483. ISBN: 978-607-448-708-4. [ Links ]
McKee T, Mckee JR. 2014a. Carbohidratos. En: De León-Fraga J, Bioquímica. Las bases moleculares de la vida Ciudad de México, México: McGraw-Hil. Pp. 208-212. ISBN: 978-0-19-992046-4. [ Links ]
McKee T, Mckee JR. 2014b. Metabolismo aerobio II: transporte de electrones y fosforilación oxidativa. En: De León-Fraga J, Bioquímica. Las bases moleculares de la vida Ciudad de México, México: McGraw-Hil. Pp. 308. ISBN: 978-0-19-992046-4. [ Links ]
McKee T, Mckee JR. 2014c. Metabolismo de los carbohidratos. En: De León-Fraga J, Bioquímica. Las bases moleculares de la vida Ciudad de México, México: McGraw-Hil. Pp. 240-250. ISBN: 978-0-19-992046-4. [ Links ]
Melo V, Cuamatzi OT. 2019. Glucólisis: ruta central del catabolismo de la glucosa. En: Melo V, Bioquímica de los procesos metabólicos. Barcelona, España: Reverté. Pp. 169- 177. ISBN: 978-84-291-9551-4. [ Links ]
Menzies KJ, Zhang H, Katsyuba E, Auwerx J. 2016. Protein acetylation in metabolism - metabolites and cofactors. Nature Reviews Endocrinology. 12(1):43-60. ISSN: 1759- 5037. https://doi.org/10.1038/nrendo.2015.181 [ Links ]
Merritt JL, Norris M, Kanungo S. 2018. Fatty acid oxidation disorders. Annals of Translational Medicine. 6(24):473-475. ISSN: 2305-5839.http://doi.org/10.21037/atm.2018.10.57 [ Links ]
Merritt JL, MacLeod E, Jurecka A, Hainline B. 2020. Clinical manifestations and management of fatty acid oxidation disorders. Reviews in Endocrine and Metabolic Disorders. 21(4):479-493. ISSN:1573-2606. https://doi.org/10.1007/s11154-020-09568-3 [ Links ]
Milani P, França D, Balieiro AG, Faez R. 2017. Polymers and its applications in agriculture. Polímeros. 27(3):256-266. ISSN: 1988-4206. https://doi.org/10.1590/0104-1428.09316 [ Links ]
Nelson LD, Cox MM, Hoskins AA. 2021a. Amino acids, peptides, and proteins. In: Nelson LD , Cox MM, Hoskins AA, Lehninger. Principles of Biochemistry. New York, United States: Macmillan Learning. Pp. 357-392. ISBN: 978-1-319-32234-2. [ Links ]
Nelson LD, Cox MM, Hoskins AA. 2021b. Glycolysis, gluconeogenesis and the pentose phosphate patway. In: Nelson LD , Cox MM, Hoskins AA, Lehninger. Principles of Biochemistry. New York, United States: Macmillan Learning. Pp. 1865-2000. ISBN: 978-1-319-32234-2. [ Links ]
Nelson LD, Cox MM, Hoskins AA. 2021c. Storage lipids. In: Nelson LD , Cox MM, Hoskins AA, Lehninger. Principles of Biochemistry. New York, United States: Macmillan Learning. Pp. 1286-1292. ISBN: 978-1-319-32234-2. [ Links ]
Nelson LD, Cox MM, Hoskins AA. 2021d. RNA metabolism In: Nelson LD , Cox MM, Hoskins AA, Lehninger. Principles of Biochemistry. New York, United States: Macmillan Learning. Pp. 3341-3380. ISBN: 978-1-319-32234-2. [ Links ]
Nelson LD, Cox MM, Hoskins AA. 2021e. ATP synthesis. In: Nelson LD , Cox MM, Hoskins AA, Lehninger. Principles of Biochemistry. New York, United States: Macmillan Learning. 2407-2446 p. ISBN: 978-1-319-32234-2. [ Links ]
Noller HF. 2017. The parable of the caveman and the Ferrari: protein synthesis and the RNA world. Topics in Current Chemistry. 372(1):1-5. ISSN: 1471-2970. https://doi.org/10.1098/rstb.2016.0187 [ Links ]
Nunes-Nesi A, Araujo WL, Obata T, Fernie AR. 2013. Regulation of the mitochondrial tricarboxylic acid cycle. Current Opinion in Plant Biology. 16(3):335-343. ISSN: 1879- 0356. https://doi.org/10.1016/j.pbi.2013.01.004 [ Links ]
OosterVeer MH, Schoonjans K. 2014. Hepatic glucose sensing and integrative pathways in the liver. Cellular and Molecular Life Sciences. 71(8):1453-1467. ISSN: 1420- 9071. https://doi.org/10.1007/s00018-013-1505-z [ Links ]
Pavlinov I, Gerlach EM, Aldrich LN. 2019. Next generation diversity-oriented synthesis: a paradigm shift from chemical diversity to biological diversity. Organic and Biomolecular Chemistry. 17(7):1608-1623. ISSN: 1477-0539. https://doi.org/10.1039/C8OB02327A [ Links ]
Piña GE, Flores HO. 2018. Metabolismo de los compuestos nitrogenados. En: Hernández MMT, Bioquímica de Laguna y Piña. Ciudad de México, México: El Manual Moderno. Pp. 714-763. ISBN: 978-607-448-708-4. [ Links ]
Pol A, Gross SP, Parton RG. 2014. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins and sites. Journal of Cell Biology. 204(5):635-646. ISSN: 1540- 8140. https://doi.org/10.1083/jcb.201311051 [ Links ]
Pratt LR, Chaudhari MI, Rempe SB. 2016. Statistical analyses of hydrophobic interactions. Journal of Physical Chemistry B. 120(27):6455-6460. ISSN: 1520-5207. https://doi.org/10.1021/acs.jpcb.6b04082 [ Links ]
Preethi KA, Sekar D. 2021. Dietary microRNAs: Current status and perspective in food science. Journal of Food Biochemistry. 45(7):e13827-e13832. ISSN: 1745-4514. https://doi.org/10.1111/jfbc.13827 [ Links ]
Quintero FG. 2014. Gliceroneogénesis y el ciclo del triacilglicerol. En: De León-Fraga J, Bioquímica. Las bases moleculares de la vida Ciudad de México, México: McGraw-Hil. Pp. 383-384. ISBN: 978-0-19-992046-4. [ Links ]
Rodnina MV, Wintermeyer W. 2016. Protein elongation, co-translational folding and targeting. Journal of Molecular Biology. 1(1):1-51. ISSN: 0022-2836. https://doi.org/10.1016/j.jmb.2016.03.022 [ Links ]
Rodwell WV. 2018a. Catabolism of proteins and amino acid nitrogen. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. United States: McGraw-Hill Education / Medical. Pp. 661-686. ISBN: 978-1-25-983793-7. [ Links ]
Rodwell WV. 2018b. Metabolism of purine and pyrimidine nucleotides. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. United States: McGraw-Hill Education / Medical. Pp. 801-826. ISBN: 978-1-25-983793-7. [ Links ]
Rui L. 2014. Energy metabolism in the liver. Comprehensive Physiology. 4(1):177-197. ISSN: 20404603. https://doi.org/10.1002/cphy.c130024 [ Links ]
Schoeler M, Caesar R. 2019. Dietary lipids, gut microbiota and lipid metabolism. Reviews in Endocrine and Metabolic Disorders. 1(1):1-12. ISSN: 13899155. https://doi.org/10.1007/s11154-019-09512-0 [ Links ]
Selvaraj S, Kelly DP, Margulies KB. 2020. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation. 141(22):1800-1812. ISSN: 1524-4539. https://doi.org/10.1161/CIRCULATIONAHA.119.045033 [ Links ]
Smith BM. 2020a. Amino acids. En: Smith BM, Biochemistry: An Organic Chemistry Approach. New York, United States: CRC Press. Pp. 253-259. ISBN: 9780815366454. [ Links ]
Smith BM. 2020b. The importance of water in biochemical systems. In: Smith BM, Biochemistry: An Organic Chemistry Approach. New York, United States: CRC Press. Pp. 55-80. ISBN: 9780815366454. [ Links ]
Stincone A, Prigione A, Cramer T, Wamelink MM, Campbell K, Cheung E, Olin- Sandoval V, Gruning NM, Kruger A, Tauqeer AM, Keller MA, Breitenbach M, Brindle KM, Rabinowitz JD, Ralser M. 2015. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biological Reviews of the Cambridge Philosophical Society. 90(3):927-963. ISSN: 1469-185X. https://doi.org/10.1111/brv.12140 [ Links ]
Swiderek K, Marti S, Tunon I, Moliner V, Bertran J. 2015. Peptide bond formation mechanism catalyzed by ribosome. Journal of the American Chemical Society. 137(37):12024-12034. ISSN: 1520-5126. https://doi.org/10.1021/jacs.5b05916 [ Links ]
Tortora JG, Derrickson B. 2018a. Metabolismo de lípidos. En: Rondinone S, Principios de Anatomía y Fisiología. Ciudad de México, México: Médica Panamericana. Pp. 968- 969. ISBN: 978-607-854-611-4. [ Links ]
Tortora JG, Derrickson B. 2018b. Metabolismo y nutrición. En: Rondinone S, Principios de Anatomía y Fisiología. Ciudad de México, México: Médica Panamericana. Pp. 956. ISBN: 978-607-854-611-4. [ Links ]
Tortora JG, Funke RB, Case LC. 2019a. Organic compounds. Structure and chemistry: carbohydrates, lipids, proteins, nucleic acids. In: Beauparlant S, Microbiology: An Introduction. New York, United States: Pearson. Pp. 33-47. ISBN:978-607-854-611-4. [ Links ]
Tortora JG, Funke RB, Case LC. 2019b. The structure of atoms. How atoms form molecules: chemical bonds. In: Beauparlant S, Microbiology: An Introduction. New York, United States: Pearson. Pp. 25-30. ISBN: 978-607-854-611-4. [ Links ]
Verschueren KHG, Blanchet C, Felix J, Dansercoer A, De Vos D, Bloch Y, Van Beeumen J, Svergun D, Gutsche I, Savvides SN, Verstraete K. 2019. Structure of ATP citrate lyase and the origin of citrate synthase in the Krebs cycle. Nature. 568(7753):571- 575. ISSN: 1476-4687. https://doi.org/10.1038/s41586-019-1095-5 [ Links ]
Voet D, Voet JG, Pratt CW. 2016. Glucose catabolism. In: Ray B, Life at the molecular level New York, United States: Wiley. Pp. 478-495. ISBN: 978-1-118-91840-1. [ Links ]
Wadhera RK, Steen DL, Khan I, Giugliano RP, Foody JM. 2016. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. Journal of Clinical Lipidology. 10(3):472-489. ISSN: 1933-2874. https://doi.org/10.1016/j.jacl.2015.11.010 [ Links ]
Wanders RJA, Visser G, Ferdinandusse S, Vaz FM, Houtkooper RH. 2020. Mitochondrial fatty acid oxidation disorders: laboratory diagnosis, pathogenesis, and the complicated route to treatment. Journal of Lipid and Atherosclerosis. 9(3):313-333. ISSN: 2287-2892. https://doi.org/10.12997/jla.2020.9.3.313 [ Links ]
Wang A, Richhariya A, Gandra SR, Calimlim B, Kim L, Quek RG, Nordyke RJ, Toth PP. 2016. Systematic review of low-density lipoprotein cholesterol apheresis for the treatment of familial hypercholesterolemia. Journal of the American Heart Association. 5(7):1-12. ISSN: 2047-9980. https://doi.org/10.1161/JAHA.116.003294 [ Links ]
Wang W, Zh N, Yan T, Ya-Ning S, Chen J, Chan-Juan Z, Xue-Jiao X, Duan-Fang L, Qin L. 2020. The crosstalk: exosomes and lipid metabolism. Cell Communication and Signaling. 18(3):1-13. ISSN: 1478-811X. https://doi.org/10.1186/s12964-020-00581-2 [ Links ]
Weil AP. 2018a. Protein synthesis and the genetic code. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. United States: McGraw-Hill Education / Medical. Pp. 955-991. ISBN: 978-1-25-983793-7. [ Links ]
Weil AP. 2018b. RNA synthesis, processing and modification. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. United States: McGraw-Hill Education / Medical. Pp. 911-954. ISBN: 978-1-25-983793-7. [ Links ]
Weiner ID, Mitch WE, Sands JM. 2015. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clinical Journal of the American Society of Nephrology. 10(8):1444-1458. ISSN: 1555-905X. https://doi.org/10.2215/CJN.10311013 [ Links ]
Wu T, Jiang Q, Wu D, Hu Y, Chen S, Ding T, Ye X, Liu D, Chen J. 2019. What is new in lysozyme research and its application in food industry? A review. Food Chemistry. 274(1):698-709. ISSN: 1873-7072. https://doi.org/10.1016/j.foodchem.2018.09.017 [ Links ]
Yang Y, Zhang X, Yu B. 2015. O-Glycosylation methods in the total synthesis of complex natural glycosides. Natural Product Reports. 32(9):1331-1355. ISSN: 1460-4752. https://doi.org/10.1039/c5np00033e [ Links ]
Received: August 03, 2021; Accepted: December 15, 2021











 text in
text in