Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Abanico veterinario
On-line version ISSN 2448-6132Print version ISSN 2007-428X
Abanico vet vol.11 Tepic Jan./Dec. 2021 Epub May 21, 2021
https://doi.org/10.21929/abavet2021.12
Original Article
Ambystoma mexicanum sperm cryopreservation (Shaw & Nodder, 1798)
1Maestría en Biología de la reproducción animal; Universidad Autónoma Metropolitana-Iztapalapa. San Rafael Atlixco 186, Iztapalapa CDMX, CP, 09340.
2Departamento de Producción Agrícola y Animal; Universidad Autónoma Metropolitana-Xochimilco (UAM-X), Calzada del hueso 1100, Coyoacán CDMX, CP. 04960.
3Departamento de Biología., Universidad Autónoma Metropolitana-Iztapalapa. San Rafael Atlixco 186, Iztapalapa CDMX, CP, 09340.
4Centro de Investigaciones Biológicas y Acuícolas de Cuemanco (CIBAC / UAM-X), Rinconada Cuemanco S/N, Xochimilco, CDMX; CP 16035.
Ambystoma mexicanum is in danger of extinction in free-living, due to anthropogenic actions; sperm cryopreservation for captive breeding can help in its ex-situ conservation. This research aimed to identify the viability of fresh and post-thawing sperm from different spermatophores. During the breeding season, spermatophores releasing was induced in nine specimens by reducing water temperature. The mean concentration per spermatophores was 2.6 ± 0.6 x104 sperm. A reduction of 30 % of living sperms and an increase of 15 % of abnormal morphology were determined in fresh and post-thawing sperm. With the WGA and PNA lectins bounded to FITC, two different fluorescence patterns were determined in each one, which showed the membrane presence and distribution of N-acetyl glucosamine, sialic acid, and mannose respectively. Sperm percentages in each fluorescence pattern showed differences associated with the number of spermatophores in each release. Differences in sperm viability from releases with different numbers of spermatophores were determined. The sperm collection and cryopreservation protocol of A mexicanum were efficient as tools for using cryopreserved semen for ex situ reproduction.
Keywords: Amphibian; conservation; spermatophore; urodele
El Ambystoma mexicanum se encuentra en peligro de extinción en vida libre, debido a efectos antropogénicos; la criopreservación espermática para su reproducción en cautiverio, puede ayudar a su conservación ex situ. El objetivo de esta investigación fue identificar la viabilidad en fresco y post descongelación de espermatozoides provenientes de diferentes espermatóforos. Durante la temporada reproductiva se indujo en nueve ejemplares, la liberación de espermatóforos mediante la reducción de la temperatura del agua. La concentración promedio por espermatóforo fue de 2.6 ± 0.6 X104 espermatozoides. Se determinó en espermatozoides en fresco y post descongelación, una reducción del 30% de espermatozoides vivos y un incremento de 15 % de morfología anormal. Con las lectinas WGA y PNA, unidas a FITC, se determinaron dos patrones de fluorescencia distintos con cada una, lo cual evidencio la presencia y distribución membranal de N-acetil glucosamina, ácido siálico y manosa respectivamente. Los porcentajes de espermatozoides con cada patrón de fluorescencia mostraron diferencias asociadas al número de espermatóforos de cada liberación. Se determinaron diferencias en la viabilidad de espermatozoides obtenidos de liberaciones con diferente número de espermatóforos. El protocolo para la obtención y criopreservación espermática de A mexicanum, fue eficiente como herramienta para utilizar semen criopreservado para su reproducción ex situ.
Palabras clave: Anfibio; conservación; espermatóforo; urodelo
INTRODUCTION
The decrease in amphibian populations has led them to extinction in severe cases, the main causes are pollution, modification of their habitat, the introduction of invasive exotic species and diseases (Catenazzi, 2015; Jiménez et al., 2017; Tietje and Rödel, 2018). Currently, the International Union for the Conservation of Nature (IUCN, 2020). Indicates than more than 85% of amphibian species of Mexico are in risk, due to this situation, most of species of Ambystoma genre in wildlife, at present it has been chosen to reproduce them in laboratory conditions (Mendoza, 2012; Khattak et al., 2014; Jiménez et al., 2017). Specifically Ambystoma mexicanum is included in NOM-059-SEMARNAT- 2010 as an endangered species (NOM-059-ECOL, 2010).
Ex situ reproduction using cryopreserved semen is an assisted reproduction tool in captivity that can contribute to the Ambystoma mexicanum´s conservation, besides to help the increase its genetic variability since most of the cases the threat is in its own habitat (Clulow et al., 2014; Jiménez et al., 2017). However, before implementing of a spermatic cryopreservation protocol, it is necessary to know reproductive biology and spermatic characteristics of species, to achieve greater success (Chester, 2013; Silla y Byrne, 2019).
Nowadays, techniques for obtaining gametes (sperm and ovules) that are carried out in amphibians are highly invasive, since most of processes require the sacrifice of the specimen to extract the testes and efferent ducts and proceed to macerate them (Chester, 2013; Shishova et al., 2011). Most of cryopreservation protocols used in sperm of diverse amphibian species have been extrapolated from those reported in fish (Comizzoli et al., 2012) showing variable results between each one. Chester (2013) cryopreserved complete spermatophores of A mexicanum using sucrose as the main cryoprotector, reporting 84 % of live spermatozoa after being thawed, however, it was not reported the parameter of live spermatozoa before its freezing.
It is known that in vitro manipulation of sperm causes alterations in their plasma membrane, in which the presence of membrane carbohydrates has been described, which have a role in the recognition between gametes to achieve fertilization (Peláez et al., 2011).
Because of the database on morphological and cryopreservation studies in amphibian spermatozoa, specifically of the order Urodelo, is limited (Browne and Chester, 2011; Chester, 2013; Sunny et al., 2014).
The objective of this research was to identify the basic evaluation parameters and membrane characteristics in spermatozoa from different specimens and spermatophores from each, to evaluate a cryopreservation protocol that allows maintaining their post-thaw fertilizing capacity.
MATERIAL AND METHODS
Care and well-being
The axolotl management was carried out in the Center for Biological and Aquaculture Research of Cuemanco (CIBAC-UAMX) facilities, in accordance with the Management Plan, authorized by the Ministry of Environment and Natural Resources (SEMARNAT) to the Unit of for the Management and Conservation of Wildlife (UMA) CIBAC, with registration DGVS-CR-IN 0952-DF/07/UMA CIBAC.
Accommodation and collection of spermatophores
The axolotls were housed for monitoring for one year individually in 60 L containers, at a temperature of 18 °C, with a photoperiod of 12 h light and 12 h of darkness. To stimulate spermatophore release, the males were transferred to a glass container with a capacity of 700 L of water, which was conditioned with sandy soil and aquatic plants. During darkness hours, using a 0.25 HP chiller (Ártica Resun CL-600), the water temperature was reduced; in the same way for all specimens from 18 to 14 °C, introducing 3 females per male. The spermatophores were recovered from the bottom of the container, at the beginning of the next 12 hours of light.
Spermatophore management and sperm collection
The spermatophores released by each specimen were collected at 5 °C in 2 ml of Simplified Amphibian Ringer (SAR) medium, composed of 113 mM NaCl, 1mM CaCl, 2.0 mM KCl and 3.6 mM NaHCO, with 220 mOsmol kg-1. Spermatozoa were obtained by placing the spermatophores of each specimen in a 4-well NuncMR box with 0.5 ml of SAR medium; in the first well, they were washed to remove organic matter residues; In the second well, the gelatinous material (glycoproteins) was removed to obtain the cap containing the sperm; in the third well, 0.5 ml of 20% Sodium Hydroxide (NaOH) was used to soften the cap for 10 min (Taku et al., 2004); in the fourth well with 0.5 ml of SAR medium, the spermatozoa were extracted by cap maceration. By aspiration, the sperm were extracted from the supernatant, then they were filtered with a 30 µm mesh and the total sperm were recovered from the spermatophores of each release in an Eppendorf tube with 500 µl of SAR medium at 5 °C, to make a pool sperm of each release.
Sperm cryopreservation
Each sperm pool, kept at 5 °C, was adjusted with 6% Dimethylacetamide (DMA), then 0.25 ml straws were filled, to maintain equilibrium for 10 min at 2 °C, then they were placed at 5 cm on steam of Nitrogen at -76 °C for 15 minutes and subsequently submerged in liquid nitrogen at -196 ° C, to be cryopreserved for 30 days until later thawing at 15 ° C for 5 min (Atencio et al., 2013).
Basic sperm evaluation
The percentage of live spermatozoa was determined through a smear that was made with a 1:5 mixture of spermatozoa with eosin-nigrosin staining, in which 100 spermatozoa were counted under the microscope at 40X. Live spermatozoa were considered, those not stained and dead, those that presented staining. Sperm morphology was evaluated in the same staining, to determine percentages of sperm with morphological alterations in the head, neck or flagellum regions (Tanisław et al., 2017).
Membrane carbohydrate distribution
With the use of lectins from Triticum vulgaris agglutinin (WGA), with affinity to N- Acetylglucosamine residues and Arachis hypogaea (PNA) with affinity to β-galactose, conjugated to fluorescein isothiocyanate (FICT), it was intended to determine the carbohydrate presence that have been reported are receptors for recognition between gametes (Herrera et al., 2017) and as structural parts of the spermatic plasmalemma (Miller, 2015). In a final volume of 40 µl of SAR medium, with 5x106 spermatozoa, 10 µl of WGA-FICT or PNA-FITC was added at a concentration of 15 mg/ml. They were incubated at 25 ºC for 30 minutes, these spermatozoa covering them with light; immediately, preparations were made on object slides, to be observed under the microscope. Each preparation was observed directly under a fluorescence microscope at 260 nm excitation and> 560 nm emission, counting 100 spermatozoa. The presence of membrane carbohydrates was determined by fluorescence patterns and sperm proportion with each determined pattern (Naofumi, 2015).
Statistical analysis
The frequency of live sperm was determined, with normal morphology and with the different fluorescence patterns in the fresh and thawed samples, which were expressed as a proportion with their respective standard error (SE). The different variables were compared between the groups of fresh and thawed spermatozoa with a Xi2 test, with an alpha of 0.05; using the free access statistical package EpiInfo 7.3.
RESULTS
Different quantity of reproductive events and number of spermatophores released by each specimen were determined. A total of 61 spermatophores were obtained. The frequency in the quantity of spermatophores released per specimen was the following: 1:12, 2:10, 1: 8, 1: 6, 3: 4 and 1: 3. The sperm concentration averaged 2.6 ± 0.6 X104 sperm/ml, with a range between 1.0 ± 2.5 to 4.0 ± 3.0. The sperm concentration in releases with six or more spermatophores (2.5X104 spermatozoa/ml), was higher (P <0.05) than the sperm concentration (2.5X104 spermatozoa/ml) in releases with less than six spermatophores.
Sperm evaluation parameters
The percentages of live sperm decreased (P <0.05), approximately 30% in post-thaw; finding averages of 89% in fresh sperm and 58% post-thaw. Live sperm percentages that were determined in releases with different spermatophore numbers showed differences (P <0.05), finding a range of 79% to 100% in fresh sperm and from 45% to 67% in thawed sperm (Table 1).
Table 1 Fresh and post-thaw sperm evaluation parameters of A. mexicanum, in ejaculates of each evaluated specimen
| ID | Spermatophores n= | %Spermatozoa with A pattern ± SE | % Spermatozoa with B pattern ± SE | ||||
|---|---|---|---|---|---|---|---|
| Fresh | Thawed | Xi2 p | Fresh | Thawed | Xi2 p | ||
| A | 12 | 46 ± 3 | 51 ± 7 | 0.3>0.05 | 54 ± 3 | 50 ± 7 | 0.1>0.05 |
| B | 10 | 44 ± 3 | 46 ± 3 | 0.02>0.05 | 48 ± 4 | 53 ± 3 | 0.3>0.05 |
| C | 10 | 52 ± 4 | 56 ± 6 | 0.2>0.05 | 47 ± 4 | 43 ± 6 | 0.2>0.05 |
| D | 8 | 56 ± 5 | 55 ± 8 | 0.01>0.05 | 46 ± 5 | 46 ± 8 | 1.0>0.05 |
| E | 6 | 52 ± 3.5 | 57 ± 9 | 0.3>0.05 | 47 ± 3 | 43 ± 9 | 0.2>0.05 |
| F | 4 | 49 ± 7.7 | 57 ± 7 | 0.9>0.05 | 61 ± 4 | 42 ± 7 | 6.5<0.05 |
| G | 4 | 41 ± 4.2 | 55 ± 6 | 3.9<0.05 | 59 ± 4 a | 45 ± 6 | 3.9<0.05 |
| H | 4 | 45 ± 7.3 | 56 ± 6 | 2<0.05 | 55 ± 7 | 44 ± 6 | 2.0>0.05 |
| I | 3 | 45 ± 2.8 | 55 ± 5 | 1.6<0.05 | 65 ± 3 | 45 ± 5 | 7.3<0.05 |
Different letter in superscript index (a,b,c), indicates difference (P<0.05) when comparing the same variable between columns (Fresh vs Thawed). Different number in superscript (1,2,3), indicates difference (P<0.05) when comparing averages in the same column
Similarly, normal sperm morphology showed a reduction (P <0.05) of approximately 15%, finding percentages of 98% in normal fresh sperm morphology and 83% post-thaw. Spermatozoa percentages with normal morphology that were determined in releases with different spermatophore numbers did not show differences (P> 0.05), finding an average range of 95% to 100% in fresh spermatozoa. However, certain post-thaw was different (P<0.5), with percentages with an average range between 78% and 90% (Table 1).
Presence and distribution of membrane carbohydrates
With the use of the WGA-FITC lectin: In fresh and post-thaw sperm, the fluorescence intensity emitted by the WGA-FITC lectin on the sperm membrane, evidenced the presence of N-Acetyl glucosamine residues present in the spermatic membrane of A mexicanum. Two fluorescence patterns were determined called: A pattern) with homogeneous intense fluorescence in the flagellum and neck region and with less intensity, but evident in the head one (Figure 1A); and B pattern), with evident homogeneous fluorescence throughout the sperm structure (Figure 1 B).
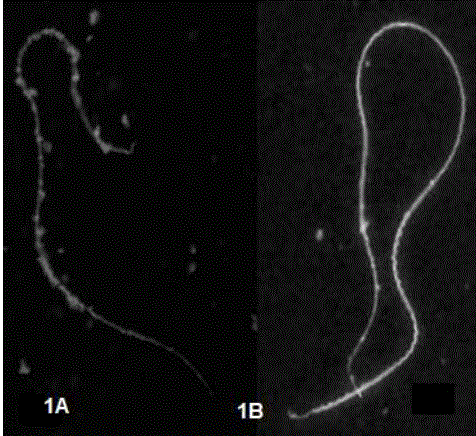
Figure. 1 Sperm fluorescence patterns with WGA-FITC lectin. 1A). Higher fluorescence intensity is observed in the flagellum and neck regions and with less intensity in the head. 1B is observed with homogeneous intensity throughout the spermatic structure
The proportion of patterns determined with WGA-FITC (table 2), showed that:
Table 2 Percentages of fresh and post-thaw sperm, with two fluorescence patterns A and B determined with the use of the WGA-FITC lectin
| ID | Spermatophores n= | % Spermatozoa with pattern A ± SE | % Spermatozoawith pattern B ± SE | ||||
|---|---|---|---|---|---|---|---|
| Fresh | Thawed | Xi2 p | Fresh | Thawed | Xi2 p | ||
| A | 12 | 46 ± 3 | 51 ± 7 | 0.3>0.05 | 54 ± 3 | 50 ± 7 | 0.1>0.05 |
| B | 10 | 44 ± 3 | 46 ± 3 | 0.02>0.05 | 48 ± 4 | 53 ± 3 | 0.3>0.05 |
| C | 10 | 52 ± 4 | 56 ± 6 | 0.2>0.05 | 47 ± 4 | 43 ± 6 | 0.2>0.05 |
| D | 8 | 56 ± 5 | 55 ± 8 | 0.01>0.05 | 46 ± 5 | 46 ± 8 | 1.0>0.05 |
| E | 6 | 52 ± 3.5 | 57 ± 9 | 0.3>0.05 | 47 ± 3 | 43 ± 9 | 0.2>0.05 |
| F | 4 | 49 ± 7.7 | 57 ± 7 | 0.9>0.05 | 61 ± 4 | 42 ± 7 | 6.5<0.05 |
| G | 4 | 41 ± 4.2 | 55 ± 6 | 3.9<0.05 | 59 ± 4 a | 45 ± 6 | 3.9<0.05 |
| H | 4 | 45 ± 7.3 | 56 ± 6 | 2<0.05 | 55 ± 7 | 44 ± 6 | 2.0>0.05 |
| I | 3 | 45 ± 2.8 | 55 ± 5 | 1.6<0.05 | 65 ± 3 | 45 ± 5 | 7.3<0.05 |
Different letter in superscript (a,b,c), indicates difference (P<0.05) when comparing the same variable between columns (Fresh vs Thawed).
The percentage of sperm with A pattern, from releases of twelve to six spermatophores, were similar (P> 0.05) fresh and post-thaw; in comparison with the percentages of sperm from releases between eight and three spermatophores, in which it increased (P <0.05), in thawed sperm. When comparing the percentages of sperm with pattern A, obtained in fresh sperm from each specimen, an average of 48% was determined, with a range between 44% and 56%; without finding difference (P <0.05) between these, in thawed spermatozoa. An average of 54% was determined with a range between 46% to 57%, finding higher percentages (P> 0.05), in sperm with pattern A, from releases with 3 and 4 spermatophores.
The percentages of sperm with B pattern, from releases of twelve to six spermatophores, were similar (P> 0.05) fresh and post-thaw; the percentages of sperm from releases with four and three spermatophores were higher (P <0.05) when fresh, compared to those thawed. In fresh, an average of 53% was determined, with a range between 46 to 65%, without finding a difference (P <0.05) between them; in thawed sperm, an average of 46% was determined with a range between 42% to 53%, finding higher percentages (P> 0.05) in sperm from releases with 10 and 12 spermatophores.
When carrying out the total general comparison (Pool) of the percentages, a difference was evident and inversely the difference by total percentages determined, which were for sperm with A pattern: Xi 2 of 7.21 p <0.05, in fresh semen with 47.7% and 54.3% for thawed semen. Regarding sperm with B pattern: a Xi 2 of 10.8 p <0.05 was determined, in fresh semen with 53.5% and 45.6% in thawed semen.
With the use of the PNA-FITC lectin: in fresh and post-thaw sperm, the intensity of the fluorescence emitted by the PNA-FITC lectin on the sperm membrane, which evidenced the presence of β-galactose glycosidic residues, present in the sperm membrane of A mexicanum. Two fluorescence patterns were determined: C) with intense and homogeneous fluorescence throughout the entire sperm structure (Figure 2A); pattern D) with homogeneous faint fluorescence throughout the sperm structure (Figure 2B).
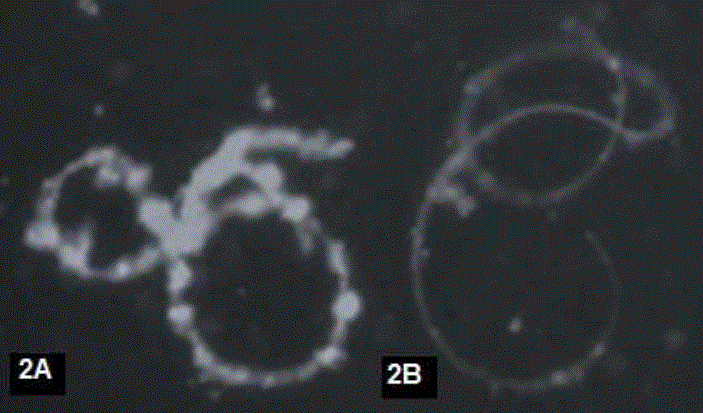
Figure 2 Fluorescence patterns obtained with PNA-FITC lectin: A) With intense homogeneous fluorescence, B) With homogeneous faint fluorescence
The proportion of patterns determined with PNA-FITC, (Table 3), showed that:
Table 3 Percentages of fresh and post-thaw sperm, with two fluorescence C and D patterns determined with PNA-FITC lectin use.
| ID | Spermatophores n= | % ± SE Spermatozoa with pattern C | % ± SE Spermatozoa with pattern D | ||||
|---|---|---|---|---|---|---|---|
| Fresco | Thawed | Xi 2 p | Fresco | Thawed | Xi 2 p | ||
| A | 12 | 51±4 | 47±5 | 0.2>0.05 | 49±4 | 53±5 | 0.2>0.05 |
| B | 10 | 51±5 | 49±5 | 0.02>0.05 | 49±5 | 50±5 | 0.01>0.05 |
| C | 10 | 53±5 | 52±6 | 0.01>0.05 | 48±7 | 47±6 | 0.01>0.05 |
| D | 8 | 49±4 | 66±3 | 5.23<0.05 | 51±5 | 24±6 | 14.4<0.05 |
| E | 6 | 57±4 | 60±7 | 0.08>0.05 | 47±7 | 40±11 | 0.7>0.05 |
| F | 4 | 55±7 | 62±4 | 0.07>0.05 | 45±10 | 37±4 | 1.01>0.05 |
| G | 4 | 55±4 | 68±6 | 0.7>0.05 | 55±6 | 44±7 | 2.0>0.05 |
| H | 4 | 57±3 | 69±4 | 2.6>0.05 | 52±6 | 42±6 | 1.62>0.05 |
| I | 3 | 58±5 | 67±5 | 1.36>0.05 | 42±8 | 43±9 | 0.01>0.05 |
Different letter in superscript (a,b,c), indicates difference (P<0.05) when comparing the same variable between columns (Fresh vs Thawed).
With C and D patterns, sperm percentages from releases with 6 to 12 spermatophores were similar (P> 0.05) fresh and post-thaw, compared to sperm percentages from releases with three and four spermatophores, which were increased (P <0.05), in thawed sperm.
Spermatozoa percentages with C and D pattern, determined with the lectin PNA-FITC, only showed a difference (P <0.05) after thawing, when the spermatozoa came from releases with eight spermatophores, observing a higher percentage in spermatozoa with C pattern post thawing and conversely, in sperm with D pattern. The percentage was higher in fresh semen, in the percentages of sperm from releases with twelve, ten, six, four or three spermatophores, When comparing the percentages of sperm with C pattern of each specimen, fresh with an average of 54%, with a range between 49% to 58 % and post thawing with an average of 60% and with a range between 47% to 69%, no differences were found (P <0.05).
When comparing the percentages of sperm with D pattern of each specimen, fresh with an average of 48%, with a range between 42% to 55%, no different percentages were observed (P <0.05). In thawed spermatozoa, an average of 42% was found, with a range 24% to 53%, no difference percentages were observed (P <0.05).
DISCUSSION
Regarding the sperm concentration, the data showed that there are differences between spermatophores of the same specimen and between specimens. These results are similar to those published by Doyle et al. (2011), in spermatozoa number among the spermatophores released from specimens of A maculatum. The quantity and size of spermatophores that can be released varies widely, being related to the physiology and reproductive adaptation of each species (Browne et al., 2019); as well as three physical characteristics: body size, testicle size or age (Uribe and Mejía-Roa, 2014), which was also observed in our study. The sperm viability observed fresh was 80 to 98%, this being the first study to record live sperm percentage extracted from spermatophores. These results are in contrast to those reported by Mansour et al. (2011), who obtained sperm through cloacal massage, reporting 100% live sperm in all samples analyzed.
On the other hand, a study carried out by Chester (2013) mentions that it obtained from 64 to 86% of sperm viability after carrying out spermatophore cryopreservation. These data differ from that obtained in this work, where the viability obtained in thawed spermatozoa was an average of 45 to 68%, so this result indicates that the cap membrane can function as a barrier, which protects the sperm from freezing sudden changes (Chester, 2013; Hall et al., 2016).
The use of WGA-FITC and PNA-FITC lectins proved to be an alternative to identify the presence and distribution of glucosidic residues β-galactose and Acetyl-glucosamine. These results are consistent with the work carried out by Sáez et al., (2004), where it was determined that at spermatogenesis time, different carbohydrates, including β-galactose and acetylglucosamine, are present in the membrane of cells that are found in different developmental stage during spermiogenesis.
The presence and glycosidic residue distribution indicate differences throughout the entire membrane, which was determined with each lectin, which each identified at least two different fluorescence patterns; which may be associated with different metabolic states of the spermatozoa that allow or not the recognition between gametes.
The presence and distribution of glycosidic residues allows to characterize the membrane of spermatozoa that are in different metabolic state, associated with acrosomal training and reaction, and therefore their fertilizing capacity (Browne et al., 2015). This may be useful in assisted reproduction protocols that involve the in vitro sperm handling.
Using assisted reproduction in captivity, it contributes to species conservation; in addition to reducing the extraction of animals from their environment and illegal sale (Jimenez et al., 2017); which can have a sustainable use allocating specimens to conservation, biomedical research and conservation in public and private collections (Prieto et al., 2014), which is in the latter, in which specimens have been reproduced outside their natural habitat; however, amphibian reproduction and breeding in captivity is relatively minimal (Ananjeva et al., 2015).
CONCLUSION
The cryopreservation protocol used proved to be efficient, maintaining parameters of viability and membrane integrity, despite finding sperm differences associated with the number of spermatophores present in each release, for which this study provides tools and knowledge for the assisted reproduction in Ambystoma mexicanum captivity.
LITERATURA CITADA
Ananjeva NB, Uteshev VK, Orlov NL, Gakhova EN. 2015. Strategies for conservation of endangered amphibian and reptile species. Biological Bulletin. 42:432-439. https://link.springer.com/article/10.1134/S1062359015050027 [ Links ]
Atencio V, Pérez E, Espinosa J, Pardo S. 2013. Evaluación de dimetilacetamida como crioprotector para la crioconservación de semen de Bocachico prochilodus magdalenae. Archivos de Medicina Veterinaria. 45(2):151-158. http://dx.doi.org/10.4067/S0301-732X2013000200006 [ Links ]
Browne R, Chester R. Figiel Jr. 2011. Amphibian conservation and cryopreservation of sperm, cells, and tissues. In Cryopreservation in Aquatic Species. 8(3):345-365. http://www.herpconbio.org/Volume_8/Issue_3/Figiel_2013.pdf [ Links ]
Browne RK, Kaurova SA, Uteshev VK, Shishova NV, McGinnity D, Figiel CR, Mansour N, Agnew D, Wu M, Gakhova EN, Dzyuba B, Cosson J. 2015. Sperm motility of externally fertilizing fish ans amphibians. Theriogenology. 83(1):1-13. https://doi.org/10.1016/j.theriogenology.2014.09.018 [ Links ]
Browne RK, Silla AJ, Upton R, Della-Togna G, Marcec-Greaves R, Shishova NV, Uteshev VK, Proaño B, Pérez OD, Mansour N, Kaurova SA, Gakhova EN, Cosson J, Dyzuba B, Kramarova LI, McGinnity D, Gonzalez M, Clulow J, Clulow S. 2019. Sperm collection and storage for the sustainable management of amphibian biodiversity. Theriogenology. 5:133:187-200. https://doi.org/10.1016/j.theriogenology.2019.03.035. PMID: 31155034. [ Links ]
Catenazzi A. 2015. State of the world’s amphibians. Annual Review of Environment and Resources. 40:91-119. https://doi.org/10.1146/annurev-environ-102014-021358 [ Links ]
Clulow J, Trudeau VL, Kouba AJ. 2014. Amphibian declines in the twenty-first century: Why we need assisted reproductive technologies. In Reproductive Sciences in Animal Conservation, W.V. Holt, J.L. Brown, and P. Comizzoli, eds. (New York, NY: Springer New York), pp. 275-316. https://doi.org/10.1007/978-1-4939-0820-2_12 [ Links ]
Comizzoli P, Songsasen N, Hagedorn M, Wildt DE. 2012. Comparative cryobiological traits and requirements for gametes and gonadal tissues collected from wildlife species. Theriogenology. 78(8):1666-1681. https://doi.org/10.1016/j.theriogenology.2012.04.008. [ Links ]
Chester RF. 2013. Cryopreservation of sperm from the axolotl Ambystoma mexicanum: implications for conservation. Herpetological Conservation and Biology. 8(3):748-755. http://www.herpconbio.org/Volume_8/Issue_3/Figiel_2013.pdf [ Links ]
Doyle JM, McCormick CR, De Woody JA. 2011. The quantification of spermatozoa by real-time quantitative PCR, spectrophotometry, and spermatophore cap size: Technical advances. Molecular Ecology Resources. 11(1):101-106. https://doi.org/10.1111/j.1755-0998.2010.02892.x. [ Links ]
EPI. Info R7. 2020. paquete estadístico de libre acceso Epi. Info 7R. https://www.cdc.gov/epiinfo/esp/es_pc.html [ Links ]
Herrera JA, Calderón G, Cruz C, Ávila MA, Quintero GE, Fierro RC. 2017. Changes in the membrane carbohydrates from sperm cryopreserved with dimethylsulfoxide or polyvinylpyrrolidone of red-tailed hawk (Buteo jamaicencis). Cryo Letters. 38(4):257-262. PMID: 29734426. https://pubmed.ncbi.nlm.nih.gov/29734426/ [ Links ]
Hall KW, Eisthen HL, Williams BL. 2016. Proteinaceous pheromone homologs identified from the cloacal gland transcriptome of a male axolotl, Ambystoma mexicanum. PLOS ONE 11, e0146851. https://doi.org/10.1371/journal.pone.0146851 [ Links ]
Jiménez JO, Aviña CR, Ramírez AE, Lucero FG, Andreu CG. 2017. Conservación exsitu de poblaciones en riesgo de ajolotes (Ambystoma spp.) del estado de puebla, Mexico. Revista Latinoamericana el Ambiente y las Ciencias. 8(18):1-10. http://cmas.siu.buap.mx/portal_pprd/work/sites/rlac/resources/LocalContent/90/1/8(18)- 1.pdf [ Links ]
Khattak S, Murawala P, Andreas H, Kappert V, Schuez M, Sandoval-Guzmán T, Crawford K, Tanaka EM. 2014. Optimized axolotl (Ambystoma mexicanum) husbandry, breeding, metamorphosis, transgenesis and tamoxifen-promedioted recombination. Nature Protocols. 9(3):529-540. https://doi.org/10.1038/nprot.2014.040 [ Links ]
NOM-059-SEMARNAT-2010, Norma Oficial Mexicana. Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. https://www.profepa.gob.mx/innovaportal/file/435/1/NOM_059_SEMARNAT_2010.pdf [ Links ]
Mansour N, Lahnsteiner F, Patzner RA. 2011. Collection of gametes from live axolotl, Ambystoma mexicanum, and standardization of in vitro fertilization. Theriogenology. 75(2): 354-361. https://doi.org/10.1016/j.theriogenology.2010.09.006 [ Links ]
Mendoza VT. 2012. Importancia ecológica y cultural de una especie endémica de ajolote (Ambystoma dumerilii) del lago de Patzcuaro Michoacan. Etnobiología. 10(2):40-49. https://revistaetnobiologia.mx/index.php/etno/article/view/212/213 [ Links ]
Miller DJ. 2015. Regulation of Sperm Function by Oviduct Fluid and the Epithelium: Insight into the Role of Glycans. Reproduction in Domestic Animals. 50Suppl (2):31-39. PMID: 26174917 DOI: 10.1111/rda.12570 [ Links ]
Naofumi M. 2015. Protein-carbohydrate interaction between sperm and the egg-coating envelope and its regulation by dicalcin, a Xenopus laevis, zona pellucida protein- associated protein. Molecules. 20(5):9468-9486. https://doi.org/10.3390/molecules20059468 [ Links ]
Prieto MT, Sanchez-Calabuig MJ, Hildebrandt TB, Santiago-Moreno J. Saragusty J. 2014. Sperm cryopreservation in wild animals. European Journal of Wildlife Research. 60: 851-864. https://link.springer.com/article/10.1007%2Fs10344-014-0858-4 [ Links ]
Sáez FJ, Madrid JF, Cardoso S, Gómez L, Hernández F. 2004. Glycoconjugates of the urodele amphibian testis shown by lectin cytochemical methods. Microscopy Research and Technique. 64(1):63-76. https://doi.org/10.1002/jemt.20059 [ Links ]
Shishova NR, Uteshev VK, Kaurova SA, Browne RK, Gakhova EN. 2011. Cryopreservation of hormonally induced sperm for the conservation of threatened amphibians with rana temporaria as a model research species. Theriogenology. 75(2):220-232. https://doi.org/10.1016/j.theriogenology.2010.08.008 [ Links ]
Silla AJ, y Byrne PG. 2019. The Role of Reproductive Technologies in Amphibian Conservation Breeding Programs. Annual Review of Animal Biosciences. 7(1):499-519. https://doi.org/10.1146/annurev-animal-020518-115056 [ Links ]
Sunny A, Monroy-Vilchis O, Fajardo V, Aguilera-Reyes U. 2014. Genetic diversity and structure of an endemic and critically endangered stream river salamander (Caudata: Ambystoma leorae) in México. Conservation Genetics. 15:49-59. https://link.springer.com/article/10.1007/s10592-013-0520-9 [ Links ]
Tanisław K, Anna W, Magdalena K, Krzysztof G. 2017. Application of two staining methods for sperm morphometric evaluation in domestic pigs. Journal of Veterinary Research. 61(3):345-349. https://doi.org/10.1515/jvetres-2017-0045 [ Links ]
Peláez J, Bongalhardo DC, Long JA. 2011. Characterizing the glycocalyx of poultry spermatozoa: III. Semen cryopreservation methods alter the carbohydrate component of rooster sperm membrane glycoconjugates. Poultry Science. 90(2):435-43. https://doi.org/10.3382/ps.2010-00998. [ Links ]
Taku S, Masakazu A, Seiji G. 2004. A new method to extract sperm from spermatophores of the male spiny king crab P αrαlithodes brevipes (Anomura: lithodidae). Crustacean Research. (33):10-14. https://www.jstage.jst.go.jp/article/crustacea/33/0/33_KJ00004479508/_pdf [ Links ]
IUCN the Red List of Threatened Species. 2020. Disponible en línea en: https://www.iucnredlist.org/search?query=AMBYSTOMA%20MEXICANUM&searchType=species [ Links ]
Uribe MC, Mejía-Roa V. 2014. Testicular structure and germ cells morphology in salamanders. Spermatogenesis. 4, e988090. https://doi.org/10.4161/21565562.2014.988090 [ Links ]
Tietje M, Rödel M. 2018. Evaluating the predicted extinction risk of living amphibian species with the fossil record. Ecology Letters. 21(8):1135-1142. https://onlinelibrary.wiley.com/doi/full/10.1111/ele.13080 [ Links ]
Received: September 20, 2020; Accepted: February 05, 2021; Published: February 27, 2021











 text in
text in 



