Fouquieriaceae DC (Ericales) consists of a single genus, Fouquieria, which includes eleven arborescent or shrubby species restricted arid and semiarid regions of Mexico and the southwestern United States (Henrickson 1972, Kubitzki 2004). This family is characterized by having placentation that changes from parietal to axial during fruit development, decurrent spines formed from the petioles of primary leaves, and presence of a water-storage parenchyma by means of expansion of rays and axial parenchyma bands, which constitutes a remarkable adaptation to seasonally arid areas (Henrickson 1969a, b, 1972, Carlquist 2000, Kubitzki 2004). Phylogenetic relationships in Ericales have been examined using DNA sequences, which provided strong support for the monophyly of the Fouquieriaceae and the divergence from Polemoniaceae (Schultheis & Baldwin 1999, De Nova et al. 2018).
Previous research on flower development and the embryology have provided characters associated with the development of anthers, ovules and seeds, and have contributed to understand the reproductive biology of Fouquieriaceae (Johansen 1936, Mauritzon 1936, Khan 1943, Amlie 1965, Henrickson 1967, Govil 1970, Schönenberger & Grenhagen 2005, Schönenberger 2009). Regarding development of the ovule and female gametophyte in Fouquieriaceae, the studies are limited to brief and partial descriptions made by Johansen (1936), Mauritzon (1936), Khan (1943), Amlie (1965), and Govil (1970). Johansen (1936) described the Fouquieriaceae ovule as bitegmic and anatropous with a massive nucellus, which gave rise to a nucellar endothelium or epistase. Mauritzon (1936) and Khan (1943) supported the existence of an endothelium, but unlike Johansen (1936) they established that it was of integumentary origin and not nucellar. Johansen (1936) did not observe the tetrad of megaspores, thus he concluded that the development of the female gametophyte is tetrasporic type. However, Mauritzon (1936) observed a triad of megaspores in Fouquieria splendens Engelm. and described the development as monosporic type, which was supported by Khan (1943).
As mentioned above, the data on the first stages of the ovule development, the formation of the functional megaspore and features of the female gametophyte in Fouquieriaceae are incomplete and it is not possible to characterize female embryology. Moreover, there are inconsistencies about the pattern of female gametophyte development and the ontogeny of the ovule. This information is necessary not only for fundamental knowledge about embryological characteristics, but also for a better understanding of the reproductive biology of the family. Therefore, the present study aimed to describe and analyze the ovule development as well as the processes of megasporogenesis and megagametogenesis in Fouquieria fasciculata (Roem. & Schult.) Nash. This species is a caudiciform succulent plant characterized by the large fat swollen stem. Fouquieria fasciculata is restricted to the dry environments in the states of Hidalgo and Querétaro in central Mexico (Henrickson 1972, Zamudio 1995). Because of this limited geographical area, the species is listed in Mexican legislation (NOM-ECOL-059-2001) as threatened to endanger in the near future throughout all or a significant portion of its range (SEMARNAT 2010).
Material and methods
Flower buds at different developmental stages and flowers at pre-anthesis of Fouquieria fasciculata were collected at the Estorax River canyon between Hidalgo and Querétaro in central Mexico (20° 55’ 40” N; 99° 34’ 35” W, 930 m snm). Samples were fixed in situ in FAA (10 % formaldehyde, 5 % glacial acetic acid, 50 % ethanol 96 %, and 35 % distilled water) for 24 hours and later stored in 70 % ethanol (Johansen 1940). Flower buds were measured with a digital caliper and sorted into six size classes based on bud length (1-3.0, 3.1-5.0, 5.1-7.0, 7.1-9.0, 9.1-11, and 11.1-12.0 mm). After, the samples were dehydrated in an ethanol series (70, 85, 96 and 100 %) and embedding in LR-W (London resin white) following Ruzin (1999). Sections (~2 μm) were cut using an ultramicrotome RMC-MT990, stained with 0.05 % Toluidine Blue (O'Brien et al. 1964) and mounted in Entellan (Merck).
Further, samples were also embedded in Paraplast wax following Johansen (1940) and sectioned using an American Optical 810 rotary microtome. Histochemical tests were carried out with Naphthol blue black to detect protein; periodic acid-Schiff for locating insoluble polysaccharides; Lugol iodine to identify starch (Johansen 1940); and oil red “O” for locating lipids. The analysis of section and the acquisition of image were performed using an Olympus Provis AX70 optical microscope.
Scanning electron microscopy was used to describe the ovule development. Flower buds and flowers at pre-anthesis were dehydrated in a graded ethanol series and critical-point dried using a CPD 030 critical-point dryer (Bal-Tec AG, Liechtenstein). The samples were mounted on aluminum stubs using carbon double-sided tape and coated with gold by means of a Denton Vacuum Desk II sputter coater (Denton Vacuum, Moorestown, NJ, USA) (Bozzola & Russell 1992). Then, samples were analyzed on a Jeol JSM5310-LV scanning electron microscope at 15kV.
Results
Ovule development. The ovule primordium appears as dome-shaped protuberances on the inner surface of the ovary (Figure 1A, B). Then, these nucellar protuberances become enlarged and curved towards the placenta forming a finger-like structure. The inner integument is initiated by means of periclinal divisions of nucellar epidermal cells located in the ovule primordium (Figure 1C, D). Slightly later, the outer integument also arises from the periclinal divisions of the dermal cells (Figure 1E). Both the inner and outer integument grows through successive periclinal and anticlinal divisions surrounding the nucellus (Figure 1G, H). Mature ovules are anatropous with a short funicle, bitegmic and tenuinucellate (Figure 2A-C). Lastly, we observed that the ovules are supplied by a vascular bundle in the raphe (Figure 2C).
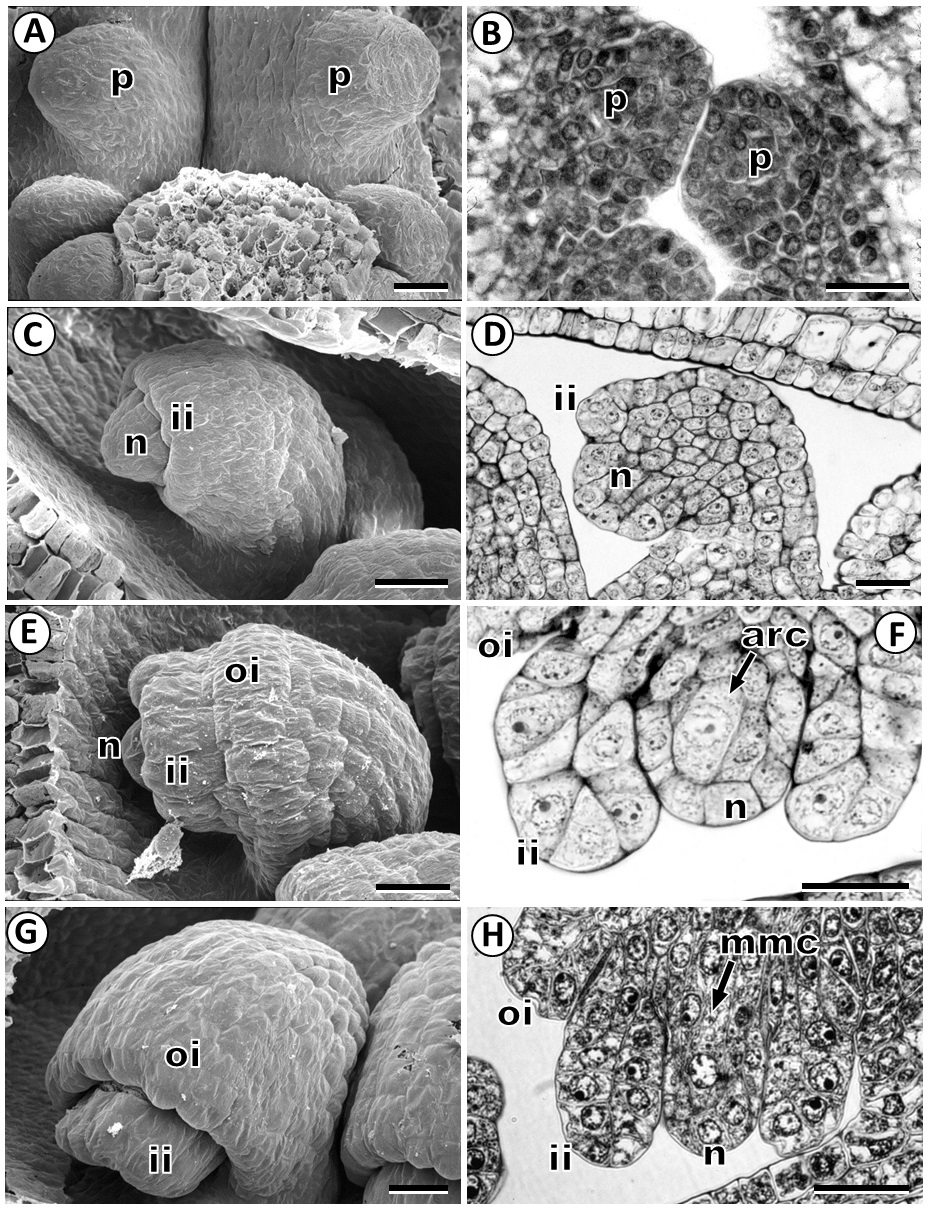
Figure 1 Development of ovule in Fouquieria fasciculata. A-B: Ovule primordium. C-D: Inner integument emerging at the epidermis of the ovule primordium. E: Outer integument developing of the ovule primordium. F: Young ovule with an archesporial cell. G: Young ovule showing two integuments. H: Ovule with megaspore mother cell. arc, archesporial cell; ii, inner integument; mmc, megaspore mother cell; n, nucellus; oi, outer integument; p, ovule primordium. Scale bars = 20 μm.
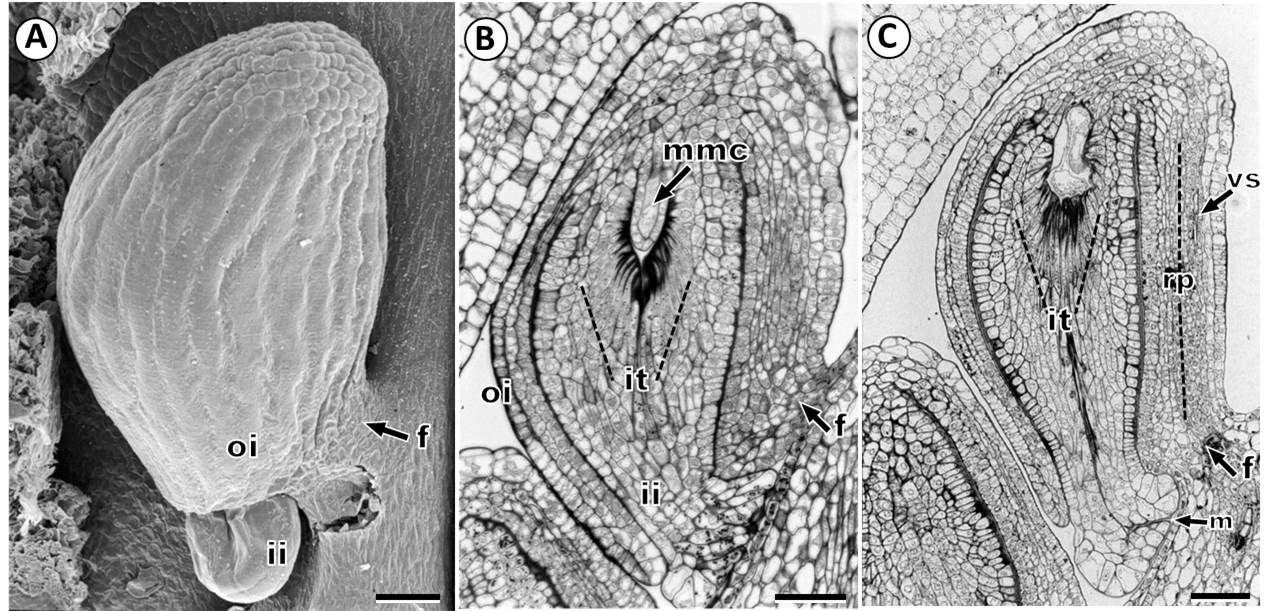
Figure 2 Ovule in Fouquieria fasciculata. A: Mature ovule. B: Bitegmic ovule showing megaspore mother cell. C: Mature ovule showing vascular supply. ii, inner integument; it, integumentary tapetum; mmc, megaspore mother cell; n, nucellus; oi, outer integument; rp, raphe; vs, vascular supply. Scale bars = 50 μm.
In the mature ovule, the outer integument is composed of 2-3 layers of rectangular cells with a conspicuous nucleus, and the outer epidermis is covering by a thick cuticle, also, a thick cuticle is also present between the inner and outer integuments (Figure 2B). The inner integument has a 4-7 cells thickness and is not fully surrounded by the outer integument, thus, the micropyle is only formed by the inner integument (endostome) (Figure 2B, C). The micropylar end is characterized by elongated swollen cells with grains of insoluble polysaccharides, and shows an appearance of two lips leaving a very narrow and long micropyle (Figure 2B, C).
The innermost layer cells of the inner integument showed morphological and anatomical changes that gave raise a single-layered integumentary tapetum (Figure 3A-F). The cells of integumentary tapetum are characterized by very conspicuous wall thickenings on the inner tangential side, and are radially elongated surrounding the nucellus (Figures 2B, C, 3E, F). Histochemical test revealed the presence of insoluble polysaccharides and lipids on thick walls of the integumentary tapetum cells (Figure 3E, F). Further, when the nucellus epidermis is degraded, the integumentary tapetum is in contact with the female gametophyte.
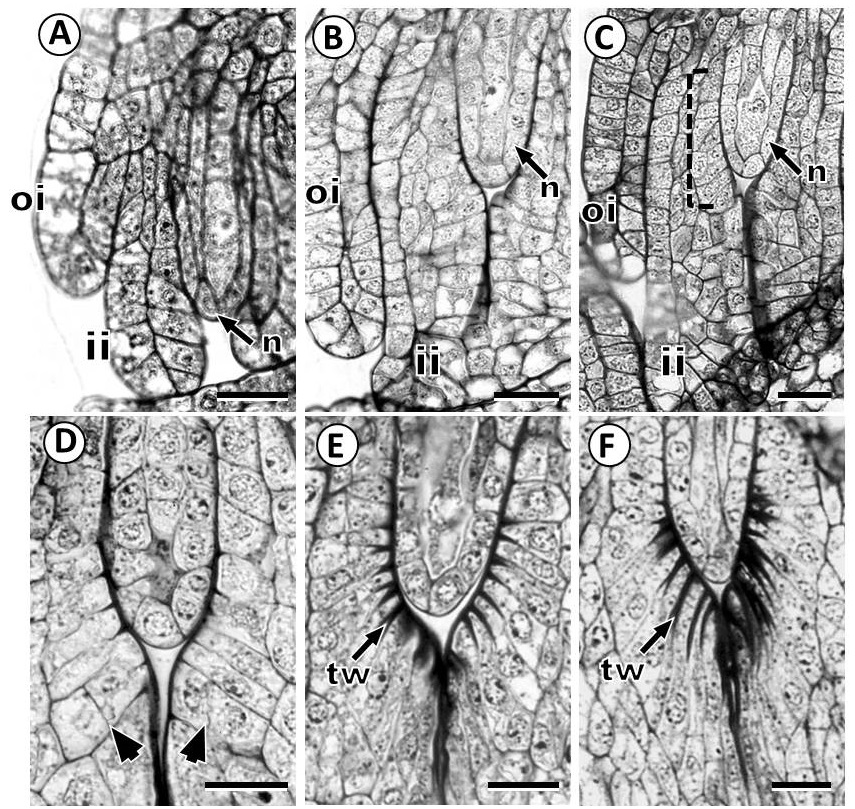
Figure 3 Development of integumentary tapetum in Fouquieria fasciculata. A-D: The innermost layer of the inner integument showed morphological changes to form the integumentary tapetum. F: The cells of integumentary tapetum are radially elongated surrounding the nucellus. Dotted line indicates the innermost layer of the inner integument Arrowheads indicates periclinal divisions. ii, inner integument; n, nucellus; oi, outer integument; tw, thick wall. Scale bars = 20 μm.
Megasporogenesis and megagametogenesis. Megasporogenesis is initiated when a subepidermal cell of the nucellus develops as an archesporial cell (Figure 1F), which grows and differentiate directly into the megaspore mother cell or megasporocyte (Figure 1H). This cell grows and is characterized by larger volume, prominent spherical nucleus, and distinct nucleolus (Figures 1H, 2B, 4A). The first meiotic division of the megaspore mother cell (Figure 4B) results in a dyad of megaspores with the chalazal cell larger than the micropylar cell (Figure 4C). At the end of meiosis, a linear tetrad of megaspores is formed; where the chalazal cell is the largest megaspore and the three micropylar megaspores that rapidly degenerate (Figure 4D).
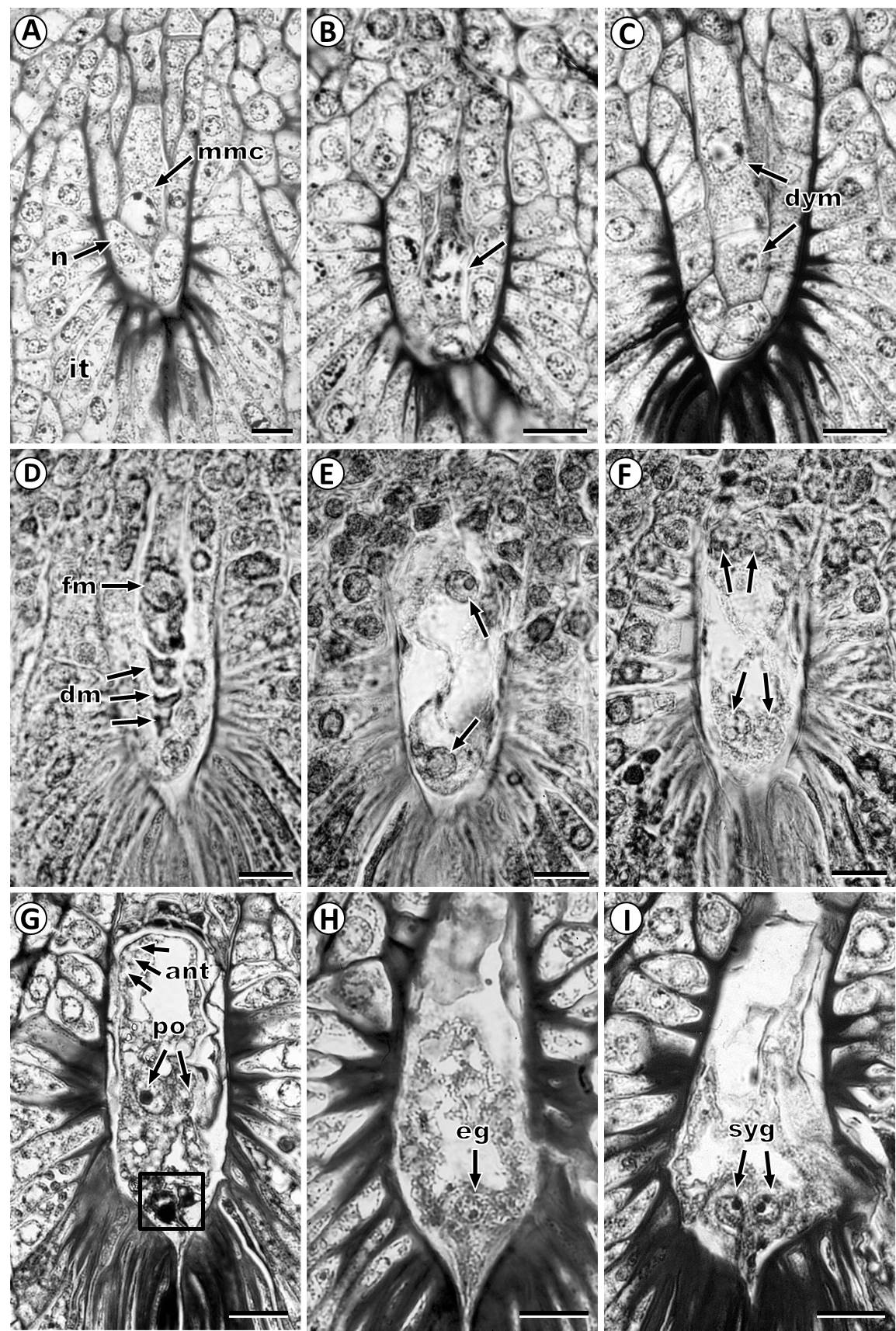
Figure 4 Development of female gametophyte in Fouquieria fasciculata. A: Megaspore mother cell stage. B: Megaspore mother cell at meiosis I (arrowhead). C: Dyad of megaspores with the chalazal cell larger than a micropylar cell. D: Functional megaspore and three degenerating megaspores. E: Two-nucleate female gametophyte. F: Four-nucleate female gametophyte. G: Mature female gametophyte showing two polar nuclei two antipodal cells and the egg apparatus. H: Female gametophyte with egg cell on the micropylar end. I: Female gametophyte showing two synergids on the micropylar side. Arrows indicates nuclei and the square show egg apparatus. ant, antipodal cells; dm, degenerating megaspore; dym, dyad of megaspores; eg, egg cell; fm, functional megaspore; ii, inner integument; mmc, megaspore mother cell; n, nucellus; oi, outer integument; po, polar nucleus; syn, synergid. Scale bars = 20 μm.
The functional chalazal megaspore grows and undergoes the first mitotic division forming a two-nucleate stage; during this stage, the nucellus has been completely degraded and the integumentary tapetum surrounds the female gametophyte (Figure 4E). After the second mitotic division is formed a four-nucleate stage (Figure 4F) and, subsequently, an eight-nucleate female gametophyte. After, cytokinesis, female gametophyte has eight nuclei in seven cells consisting of two polar nuclei that form the central binucleate cell, three ephemeral antipodal cells and three cells near the micropyle that form the egg apparatus (Figure 4G) with an egg cell (Figure 4H) and two elongated synergids with evident nucleus (Figure 4I).
The female gametophyte develops a lateral haustorial arm that penetrates the cells of the integumentary tapetum and extends to the inner integument in direction of the raphe side (Figure 5A, B). By the time, in the well-developed haustorium, the two polar nuclei are located near the opening of the haustorial arm, egg apparatus is in the micropylar zone; and the lateral haustorial arm is made up of the vacuolated cellular content of the central cell (Figure 4B).
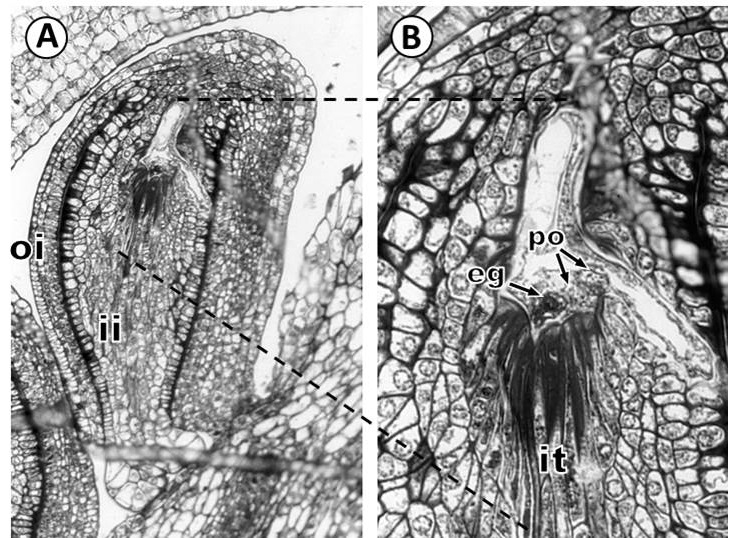
Figure 5 Development of the haustorium in Fouquieria fasciculata. A: Mature ovule showing lateral haustorial arm that penetrates the cells of the integumentary tapetum and extends to the inner integument in the direction of the raphe. B: Detail of haustorium showing the two polar nuclei located near the opening of the haustorial arm, and the egg cell is in the micropylar. eg, egg cell; it, integumentary tapetum; ii, inner integument; oi, outer integument; po, polar nuclei. Scale bars = 50 μm.
Discussion
Our study precisely describes the ovule development of Fouquieria fasciculata, and the results revealed some interesting features, such as the differentiation of an integumentary tapetum, a monosporic Polygonum-type pattern of development and the formation of lateral haustorial arm in the female gametophyte.
Johansen (1936) observed conspicuously elongated cells around the female gametophyte of Fouquieriaceae, and suggested that these are part of a nucellar epistase. However, Mauritzon (1936) and Khan (1943) referred to these cells as an integumentary tapetum. Our comprehensive study of ovule development supports the observations by Mauritzon (1936) and Khan (1943) and showed that the innermost layer cells of the inner integument were differentiated as an integumentary tapetum with elongate cells characterized by conspicuous wall thickenings. The function of the integumentary tapetum in angiosperms is not completely understood, but it likely plays a role in the protection and nutrition for the female gametophyte and embryo during their development (Kapil & Tiwari 1978, Colombo et al. 1997, Bhojwani & Bhatnagar 1999, Debeaujon et al. 2001, Radchuk & Borisjuk 2014, Friis et al. 2019). In Fouquieria fasciculata, the female gametophyte is surrounded by the integumentary tapetum suggesting that these thick-walled cells are a storage tissue, which is likely an additional source of nutrients necessary for the proper development of the female gametophyte. Further, the confirmation of the presence of an integumentary tapetum in Fouquieriaceae is particularly remarkable because this feature is widespread in Ericales (Endress 2010); therefore, suggesting that this may be symplesiomorphic in the Order (Schönenberger 2009, von Balthazar & Schönenberger 2013).
Other anatomic characteristic of the inner and outer integument includes a cuticular layer of considerable thickness covering the outer epidermal cells. In spermatophytes, the cuticles have an important function in seed coat development (Riederer 2006, Ingram & Nawrath 2017). Particularly, the integument-associated cuticles appear to have a significant role during the fusion between the outer epidermal layer of the inner integument and the inner epidermal layer of the outer integument to form the seed coat (Ingram & Nawrath 2017). However, the establishment of the role of these thick cuticles on integuments of F. fasciculata, still requires of a detailed study of seed development.
Regarding the development of the female gametophyte in Fouquieriaceae, Johansen (1936) interpreted it as tetrasporic, which means that the meiosis is not followed by cytokinesis, and that a megaspore tetrad, of four nuclei, is formed in a shared cytoplasm (Haig 2020). However, observations by Mauritzon (1936) and Khan (1943) supported a monosproric development, which implies that the meiotic divisions are followed by cytokinesis and a linear megaspore tetrad is formed (Haig 2020). In F. fasciculata, a dyad and a linear tetrad of megaspores were observed during the meiosis; thus our study confirms the monosporic development of the female gametophyte and supports the early observations by Mauritzon (1936) and Khan (1943).
Another particular feature of Fouquieriaceae is a lateral haustorial arm present in the mature female gametophyte (Johansen 1936, Mauritzon 1936, Khan 1943). Our investigation showed the development of this structure in F. fasciculata. Johansen (1936) suggested that the haustorial arm in F. burragei Rose and F. splendens has a nutritive function, given the absence of a vascular supply in the raphe. After, Khan (1943) reported also the presence of a haustorium in F. splendens, but he described the presence of a vascular tissue in the raphe. Our observations showed the development of the haustorial arm in the mature female gametophyte and the ovules are supplied by a vascular bundle in the raphe. A haustorium of the female gametophyte has not been previously reported for any family in Ericales (Schönenberger 2009), but an endosperm haustorium has been described in families such as Balsaminaceae and Marcgraviaceae (Anderberg et al. 2002). Therefore, the lateral haustorial arm of the female gametophyte may provide a clear synapomorphy for Fouquieriaceae family (Schönenberger 2009). However, the role of the haustorium during endospermogenesis and embryogenesis has not yet been investigated in the Fouquieriaceae, thus, a broader study of seed development is necessary in order to establish the possible function.
The embryological and morphological research presented here has highlighted the importance of carrying out comprehensive studies of unique structures ontogeny of Fouquieriaceae to understanding their reproductive biology. This detailed study in Fouquieria fasciculata has clarified the origin of the integumentary tapetum, the type of development of the female gametophyte and the presence of the haustorial arm. In addition, development of the endosperm, embryo and seed must be investigated, as well as the development of male structures.











 text new page (beta)
text new page (beta)



